Abstract
Background
Although motor impairments in Parkinson disease are attributed to nigrostriatal dopaminergic denervation, postural instability and gait difficulty (PIGD) features are less responsive to dopaminergic medications. PIGD features are a risk factor also for development of dementia in Parkinson disease. These observations suggest that non-dopaminergic mechanisms may contribute to axial motor impairments. The objective was to perform a correlative positron emission tomography study to examine the relationship between neocortical β-amyloid deposition ([11C]-Pittsburgh Compound-B), nigrostriatal dopaminergic denervation ([11C]-dihydrotetrabenazine), and PIGD feature severity in Parkinson disease patients at risk for dementia.
Methods
Cross-sectional study of 44 Parkinson disease patients (11 Female / 33 Male; 69.5 ± 6.6 years; 7.0 ± 4.8 years motor disease duration; mean Hoehn and Yahr stage 2.7 ± 0.5) who underwent positron emission tomography, motor feature severity assessment using the Movement Disorder Society revised Unified Parkinson's Disease Rating Scale, and the Dementia Rating Scale.
Results
Linear regression (R2adj=0.147, F4,39=2.85, p=0.036) showed that increased PIGD feature severity was associated with increased neocortical [11C]-Pittsburgh Compound-B binding (β=0.346, t39=2.13, p=0.039), while controlling for striatal [11C]-dihydrotetrabenazine binding, age, and Dementia Rating Scale total score.
Conclusion
Increased neocortical β-amyloid deposition, even at low range levels, is associated with higher PIGD feature severity in Parkinson disease patients at risk for dementia. This finding may explain why the PIGD motor phenotype is a risk factor for development of dementia in Parkinson disease.
Keywords: Parkinson disease, β-amyloid, dopamine, PET, MDS-UPDRS
Introduction
Postural instability and gait difficulty (PIGD) features are among the most disabling motor features of Parkinson disease (PD) and are least responsive to dopaminergic medications.1, 2 There is a need to explore non-dopaminergic mechanisms of PIGD since the presence of PIGD features is a critical determinant of quality of life in PD.3, 4
PD is a multisystem neurodegeneration syndrome manifesting with both motor and cognitive morbidity. There are several lines of evidence showing an intrinsic association between cognition and mobility in PD. First, the PIGD motor subtype is a risk factor for development of dementia in PD.5–7 Second, walking and/or maintaining upright balance is affected by concurrent cognitive tasks.8 Third, cognitive impairment is a risk factor for falls.9 Although some aspects of cognitive dysfunction are related to striatal dopaminergic deficits,10 dopamine replacement therapy has mixed effects on cognition in PD.11, 12 Given the ambiguous role of dopamine in the etiology of both PIGD features and cognitive impairment, non-dopaminergic processes may underlie the relationship between cognition and PIGD features in PD.
In Alzheimer disease (AD) deposition of β-amyloid (Aβ) plaques occurs early in disease13 and is associated with cognitive impairment.14 Post-mortem findings of Alzheimer pathology are found also in brains of PD patients, typically reflecting late stage (and age-related) pathology.15 In vivo imaging studies of Aβ, however, using 11C-Pittsburgh compound-B ([11C]-PiB) positron emission tomography (PET) generally show lower and more variable levels of Aβ in the neocortex of subjects with PD or PD with dementia when compared to AD.16–20 The effect of neocortical Aβ on clinical features of PD, particularly with respect to dopamine non-responsive motor symptoms, has not been studied well. Given the severe striatal dopaminergic deficits of PD, it is possible that comorbid Aβ pathology may aggravate specific clinical features of PD.
The goal of this study was to examine the relationship between neocortical Aβ burden, estimated in vivo with [11C]-PiB, and ratings of PIGD features in PD patients with mild cognitive impairment or with other dementia risk factors. The effect of nigrostriatal dopaminergic denervation on these features was taken into account by in vivo [11C]-DTBZ PET imaging of the vesicular monoaminergic transporter – type 2. We hypothesized that increased neocortical [11C]-PiB retention is associated with increased PIGD feature severity.
Methods
Subjects
This cross-sectional study included 44 PD patients with either mild cognitive impairment symptoms or known risk factors for developing PD associated dementia, specifically older age, longer disease duration, or evidence of PIGD features.6, 21, 22 These patients underwent a [11C]-PiB scan as part of a larger ongoing cohort study (NIH P01 NS015655). Detailed demographic and disease severity information is provided in Table 1.
Table 1.
Detailed demographic information, modified Hoehn & Yahr staging,46 and medication use including levodopa equivalent dose47 in milligram (mg). Values represent M ± SD (range). CL = carbidopa-levodopa.
| Gender | ||
| Female | N = 11 | |
| Male | N = 33 | |
| Age (years) | 69.5 ± 6.6 (55–84) | |
| Motor disease duration (years) | 7.0 ± 4.8 (1–20) | |
| Modified Hoehn & Yahr staging | 2.7 ± 0.5 | |
| Stage 1.5 | N = 1 | |
| Stage 2.0 | N = 3 | |
| Stage 2.5 | N = 23 | |
| Stage 3.0 | N = 14 | |
| Stage 4.0 | N = 3 | |
| Dopaminergic medication use | ||
| CL | N = 23 | |
| Dopamine agonist | N = 6 | |
| Combination CL & dopamine agonist | N = 15 | |
| Levodopa equivalent dose (mg) | 812.4 ± 630.3 (100–3180) | |
Patients met the UK Parkinson's Disease Society Brain Bank clinical diagnostic criteria.23 The diagnosis of PD was confirmed by the presence of a typical pattern of nigrostriatal dopaminergic denervation on [11C]-DTBZ PET imaging.24
All subjects were on dopamine replacement therapy (see also Table 1). None of the subjects were on anti-cholinergic or cholinesterase inhibitor drugs. Subjects were clinically examined and imaged in the morning after overnight withholding of their dopaminergic drugs.
Written informed consent was obtained from all subjects prior to research procedures. The University of Michigan Medical School Institutional Review Board for human studies approved the study.
Clinical assessments
Clinical evaluations included PD motor feature assessment using the Movement Disorder Society revised Unified Parkinson's Disease Rating Scale (MDS-UPDRS).25, 26 The PIGD score was calculated as the sum of items 2.11-2.13, 3.9-3.13 and a non-PIGD score was calculated as the sum of the non-PIGD items of parts II & III of the MDS-UPDRS. Cognitive capacity was assessed with the Dementia Rating Scale (DRS)27 with subjects on their regular dopaminergic medications.
Magnetic resonance imaging (MRI)
All subjects underwent brain MRI for anatomic co-registration with PET. MRI was performed on a 3 Tesla Philips Achieva system (Philips, Best, The Netherlands) utilizing an eight-channel head coil and the ‘ISOVOX’ exam card protocol primarily designed to yield isotropic spatial resolution. A standard T1-weighted series of a 3D inversion recovery-prepared turbo field echo was performed in the sagittal plane using repetition time/echo time/inversion time = 9.8/4.6/1041 ms; turbo factor = 200; single average; field of view = 240 × 200 × 160 mm; acquired matrix = 240 × 200. One hundred and sixty slices were reconstructed to 1 mm isotropic resolution.
PET imaging
PET imaging was performed in 3-D imaging mode using an ECAT HR+ tomograph (Siemens Molecular Imaging, Inc., Knoxville, TN), which acquires 63 transaxial slices (slice thickness = 2.4 mm; intrinsic in-plane resolution = 4.1 mm full width at half maximum over a 15.2 cm axial field of view). A NeuroShield (Scanwell Systems, Montreal, Canada) head-holder/shielding unit was attached to the patient bed to reduce the contribution of detected photon events originating from the body outside the scanner field of view. Prior to the radioligand injections, a 5 min transmission scan was acquired using rotating 68Ge rods for attenuation correction of emission data using the standard vendor-supplied segmentation and re-projection routines.
[11C]-DTBZ (no-carrier-added (+)-α-[11C]-dihydrotetrabenazine) was prepared as reported previously.28 [11C]-DTBZ PET scans were performed using a bolus/infusion protocol acquiring 15 emission scans over 60 minutes (4 × 30 sec; 3 ×1 min; 2 × 2.5 min; 2 × 5 min; 4 × 10 min), with a priming bolus of 55% followed by continuous infusion of the remaining 45% over the study duration using a dose of 555 megabecquerel (MBq).
[11C]-PiB (N-methyl-[11C]2-(4’-methylaminophenyl)-6-hydroxybenzothiazole; Pittsburgh Compound-B) was synthesized following published methods.29, 30 [11C]-PiB PET scans were performed using a bolus/infusion protocol acquiring 17 emission scans over 80 minutes (same as [11C]-DTBZ scan sequence plus 2 additional 10 min scans), with a priming bolus of 40% followed by continuous infusion of the remaining 60% over the study duration using a dose of 666 MBq.
All subjects were studied supine, with eyes and ears unoccluded, resting quietly in a dimly lit room.
PET analysis
All dynamic PET imaging frames were spatially co-registered within subjects with a rigid body transformation to reduce the effects of subject motion during the imaging session.31 These motion corrected PET frames were spatially co-registered to the MRI using SPM8 software (Wellcome Trust Centre for Neuroimaging, London, UK). IDL image analysis software (Research systems, Inc., Boulder, CO) was used to manually trace volumes of interest (VOI) on the MRI scan. Traced VOIs included the striatum (caudate and putamen), thalamus, cerebellum, and the neocortex. Neocortical VOI definition used semi-automated thresholding delineation of the neocortical gray matter signal on the MRI images.
Time activity curves for each VOI were generated from the spatially aligned PET frames. [11C]-PiB and [11C]-DTBZ PET distribution volume ratio (DVR), a measure of binding, was estimated by using the Logan plot graphical analysis method32 with the time activity curves as the input function and the cerebellar gray matter as reference tissue for [11C]-PiB and the neocortex as reference tissue for [11C]-DTBZ.
Statistical analysis
Variables were rank-order transformed to mitigate the effect of possible outliers. Linear regression analysis was performed to assess the association between PIGD sub-score and neocortical [11C]-PiB DVR while controlling for striatal [11C]-DTBZ DVR, age, and DRS total score. Post-hoc analyses were performed to assess the effects of sub-cortical [11C]-PiB DVR on PIGD sub-score. Analyses were performed using PASW Statistics 18 (IBM, Chicago, Ill).
Results
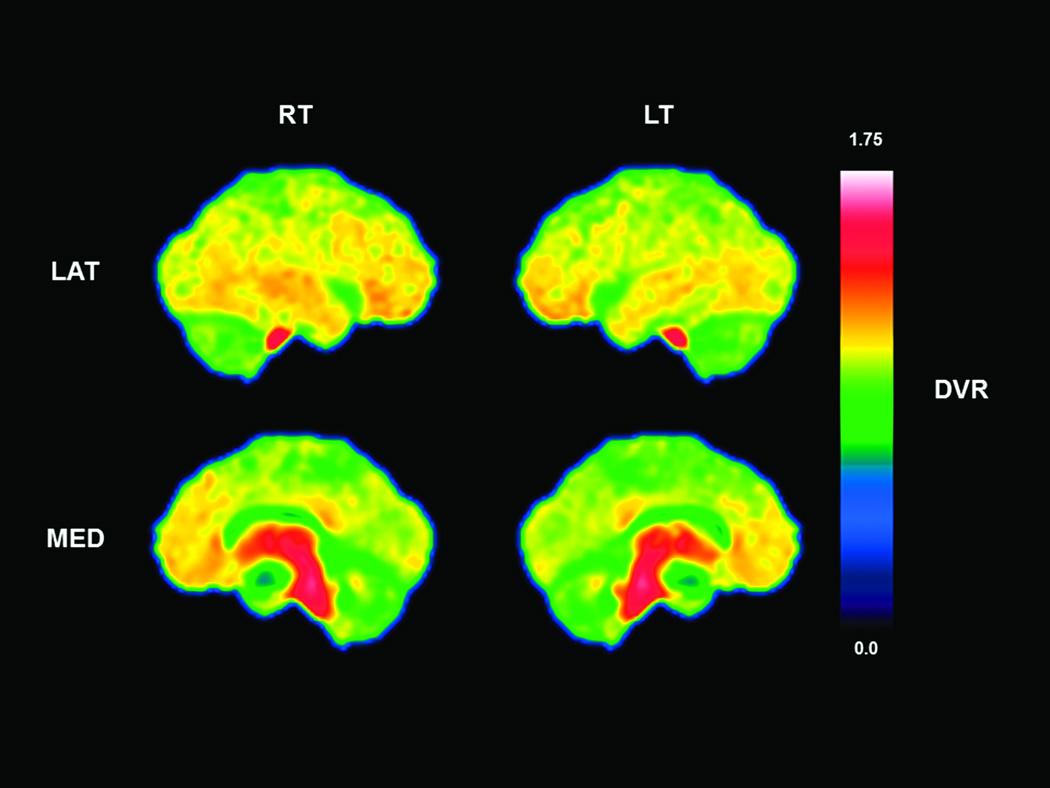
Descriptive results for the MDS-UPDRS, DRS, and PET ligands are presented in Table 2. Subjects had mild to moderate motor disease severity and a mean DRS total score that was within the mildly impaired range.33 Across all subjects, neocortical [11C]-PiB binding was generally in the low range but regionally more elevated in the frontal and temporal lobes and the cingulate gyrus (Figure 1).
Table 2.
M ± SD (range) for Dementia Rating Scale, MDS-UPDRS summed PIGD and non-PIGD sub-scores, and PET ligands. DVR = distribution volume ratio.
| Dementia Rating Scale (0–144) | 136.4 ± 5.0 (123–144) | |
| MDS-UPDRS | ||
| Motor Aspects of Experiences of Daily Living (0–52) | 10.6 ± 5.9 (2–26) | |
| Motor Examination (0–132) | 37.8 ± 14.6 (14.5–69.5) | |
| PIGD sub-score (0–32) | 9.0 ± 4.9 (1.5–27) | |
| non-PIGD sub-score (0–152) | 39.4 ± 14.2 (18–72.5) | |
| PET | ||
| Striatal [11C]-DBTZ DVR | 1.90 ± 0.31 (1.50–2.94) | |
| Neocortical [11C]-PiB DVR | 1.16 ± 0.16 (0.93–1.78) | |
| Thalamic [11C]-PiB DVR | 1.54 ± 0.13 (1.19–1.80) | |
| Striatal [11C]-PiB DVR | 1.37 ± 0.19 (1.07–2.10) | |
Figure 1.
Parametric lateral and medial projections of [11C]-PiB binding averaged across all 44 subjects. Overall there was low range binding, however, regionally increased binding is seen in the cingulate gyrus, and temporal and frontal lobes. There was high non-specific white matter [11C]-PiB binding in the brainstem and thalamus. RT = right; LT = left; LAT = lateral; MED = medial; DVR = distribution volume ratio.
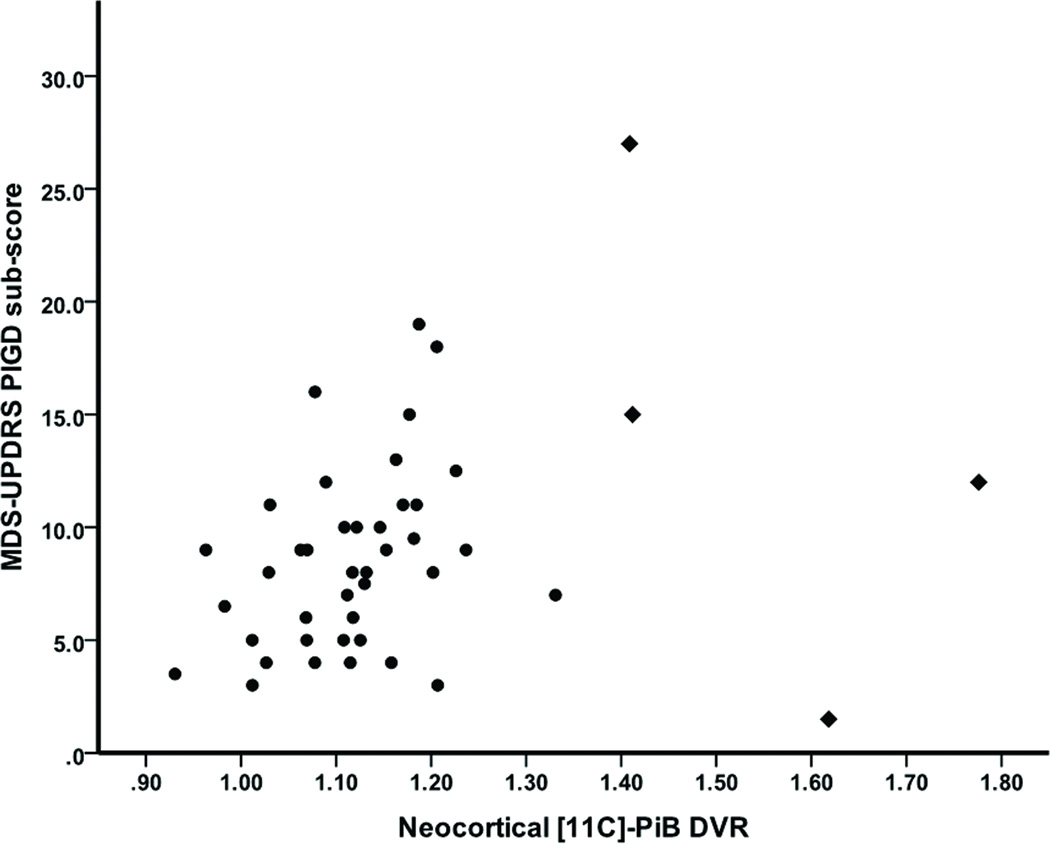
Linear regression analysis showed that neocortical [11C]-PiB binding (β=0.346, t39=2.13, p=0.039) significantly predicted MDS-UPDRS PIGD sub-score (R2adj=0.147, F4,39=2.85, p=0.036; Figure 2), while there were no significant effects of striatal [11C]-DTBZ binding (β=−0.232, t39=−1.52, p=0.136), age (β=0.125, t39=0.85, p=0.400), or DRS total score (β=0.100, t39=0.62, p=0.538). Additional linear regression analysis showed that the non-PIGD sub-score could not be significantly predicted by the same independent variables (R2adj=0.072, F4,39=1.84, p=0.142). Reanalysis with possible influential outliers removed (see also Figure 2) resulted in the same findings (results not shown).
Figure 2.
Scatterplot of neocortical [11C]-PiB binding against PIGD sub-score across all subjects. Possible influential outliers are identified by diamonds.
Post-hoc linear regression analyses were performed to examine possible effects of subcortical [11C]-PiB binding on PIGD sub-score. These analyses showed that neither striatal [11C]-PiB binding nor thalamic [11C]-PiB binding significantly predicted PIGD sub-scores while controlling for striatal [11C]-DTBZ binding, age, and DRS total score (results not shown).
Discussion
We examined the relationship between PIGD feature severity and neocortical Aβ burden in PD patients with mild cognitive impairment or at risk for development of dementia. We found that increased PIGD feature severity was associated with increased neocortical Aβ burden while controlling for the effect of possible confounding variables such as the degree of striatal dopaminergic denervation, age, or the degree of cognitive capacity impairment. This was a PIGD feature specific effect as neocortical Aβ burden did not have an association with non-PIGD feature severity. Furthermore, the results remained significant even after exclusion of subjects with more severe neocortical amyloidopathy. Subcortical Aβ burden did not affect PIGD feature severity.
Dopaminergic denervation is severe in PD, even at the onset of the disease.24, 34 For example, Frey and colleagues have reported reductions of striatal dopaminergic [11C]-DTBZ binding of 61% in the putamen and 43% in the caudate nucleus, with no overlap in putamen [11C]-DTBZ binding between individual elderly controls and PD patients.34 Striatal [11C]-DTBZ DVR in our study was in the same range. The results of this study support our hypothesis that in the presence of severe striatal dopaminergic denervation, comorbid neocortical amyloidopathy may have exacerbating effects on gait and postural stability, even when the amyloidopathy is at below-AD level of Aβ binding levels.35 This suggests that relatively low levels of comorbid neocortical Aβ plaques are of pathophysiological significance in PD and may aggravate motor impairments of PD patients.
Further evidence for a possible role of cortical amyloidopathy on the etiology of balance and gait impairments can be found in AD patients. Subtle changes in balance and gait occur early in the course of AD.36 Several studies have shown that gait and static standing balance characteristics of AD patients are different from normal subjects.37–39 These changes are especially evident when there are additional cognitive task demands while walking.40–42 These observations support an intrinsic relationship between cognition and motor control functions and suggest a possible pathogenic role for neocortical amyloidopathy in gait dysfunction.
The cross-sectional study design and regression analyses were limitations of our study, as these do not allow for causal assessment of the relationship between PIDG feature severity and the degree of neocortical amyloidopathy. Prospective studies are needed to confirm that amyloidopathy is an important mechanism underlying both progression of PIGD motor features and cognitive decline in PD.
PD is a multisystem neurodegenerative disorder.43 Our study shows that in the presence of severe nigrostriatal dopaminergic denervation even relatively low levels of comorbid neocortical amyloidopathy may augment gait and postural impairments in PD. These results and our recent findings of an association between cortical amyloidopathy and the degree of cognitive impairment in PD patients44 suggest that cortical amyloidopathy may provide a common mechanism for PIGD features and cognitive impairment. This may explain why the PIGD motor phenotype is a risk factor for development of dementia in PD. Several promising therapies have been tested in AD patients that target Aβ production, aggregation, or accumulation, which likely are most effective when applied to asymptomatic patients with very early signs of AD pathology.45 Future studies could evaluate whether early treatment of amyloidopathy may modify the progression of PIGD features in PD.
Acknowledgements
The authors thank Christine Minderovic, Kristine Wernette, Virginia Rogers, the PET technologists, cyclotron operators, and chemists for their assistance.
Funding agencies: This work was supported by National Institutes of Health grants P01 NS015655 & R01 NS070856, the Michael J. Fox Foundation, and the Department of Veterans Affairs.
Abbreviations
- [11C]-DTBZ
[11C]-dihydrotetrabenazine
- [11C]-PiB
[11C]-Pittsburgh compound-B
- Aβ
β-amyloid
- AD
Alzheimer disease
- DVR
distribution volume ratio
- MDS-UPDRS
Movement Disorder Society revised Unified Parkinson's Disease Rating Scale
- DRS
Dementia Rating Scale
- PD
Parkinson disease
- PET
positron emission tomography
- PIGD
postural instability and gait difficulty
- VOI
volume of interest
Footnotes
Relevant conflicts of interest/financial disclosures: The authors have no relevant financial or conflict of interest to disclose.
Financial Disclosures
Müller: Research support from the National Institutes of Health (NIH) and the Department of Veteran Affairs (VA).
Frey: Research support from the NIH, GE Healthcare, and AVID Radiopharmaceuticals. Consultant for AVID Radiopharmaceuticals, MIMVista, Bayer-Schering, and GE healthcare. Holds equity (common stock) in GE, Bristol-Myers, Merck, and Novo-Nordisk.
Petrou: No disclosures.
Kotagal: Research support from the American Academy of Neurology Clinical Research Training Fellowship.
Koeppe: Research support from the NIH. Consultant for Johnson & Johnson, Merck, and AVID Radiopharmaceuticals. Serves on the board of the International Society of Cerebral Blood Flow and Metabolism.
Albin: Research support from the NIH and the VA. Serves on the editorial boards of Neurology, Experimental Neurology, and Neurobiology of Disease. Served on the Data Safety and Monitoring Boards for the QE3 and HORIZON trials.
Bohnen: Research support from the NIH and the VA.
- Research Project: A. Conception, B. Organization, C. Execution
- Statistical Analysis: A. Design, B. Execution, C. Review and Critique
- Manuscript: A. Writing of the First Draft, B. Review and Critique
Müller: 1A, 1B, 1C, 2A, 2B, 3A; Frey: 1A, 1B, 1C, 2A, 2C, 3B; Petrou: 2C, 3B; Kotagal: 2C, 3B; Koeppe: 1A, 1B, 1C, 2C, 3B; Albin: 1A, 1B, 1C, 2C, 3B; Bohnen: 1A, 1B, 1C, 2A, 2C, 3B.
References
- 1.Bohnen NI, Cham R. Postural control, gait, and dopamine functions in parkinsonian movement disorders. Clin Geriatr Med. 2006;22:797–812. doi: 10.1016/j.cger.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Bloem BR, Beckley DJ, van Dijk JG, Zwinderman AH, Remler MP, Roos RA. Influence of dopaminergic medication on automatic postural responses and balance impairment in Parkinson's disease. Mov Disord. 1996;11:509–521. doi: 10.1002/mds.870110506. [DOI] [PubMed] [Google Scholar]
- 3.Muslimovic D, Post B, Speelman JD, Schmand B, de Haan RJ. Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology. 2008;70:2241–2247. doi: 10.1212/01.wnl.0000313835.33830.80. [DOI] [PubMed] [Google Scholar]
- 4.Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson's disease? J Neurol Neurosurg Psychiatry. 2000;69:308–312. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Post B, Merkus MP, de Haan RJ, Speelman JD. Prognostic factors for the progression of Parkinson's disease: a systematic review. Mov Disord. 2007;22:1839–1851. doi: 10.1002/mds.21537. [DOI] [PubMed] [Google Scholar]
- 6.Burn DJ, Rowan EN, Allan LM, Molloy S, O'Brien JT, McKeith IG. Motor subtype and cognitive decline in Parkinson's disease, Parkinson's disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2006;77:585–589. doi: 10.1136/jnnp.2005.081711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov Disord. 2006;21:1123–1130. doi: 10.1002/mds.20897. [DOI] [PubMed] [Google Scholar]
- 8.Hausdorff JM, Balash J, Giladi N. Effects of cognitive challenge on gait variability in patients with Parkinson's disease. J Geriatr Psychiatry Neurol. 2003;16:53–58. doi: 10.1177/0891988702250580. [DOI] [PubMed] [Google Scholar]
- 9.Hausdorff JM, Doniger GM, Springer S, Yogev G, Simon ES, Giladi N. A common cognitive profile in elderly fallers and in patients with Parkinson's disease: the prominence of impaired executive function and attention. Exp Aging Res. 2006;32:411–429. doi: 10.1080/03610730600875817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cools R. Dopaminergic control of the striatum for high-level cognition. Curr Opin Neurobiol. 2011;21:402–407. doi: 10.1016/j.conb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald PA, Monchi O. Differential effects of dopaminergic therapies on dorsal and ventral striatum in Parkinson's disease: implications for cognitive function. Parkinsons Dis. 2011;2011 doi: 10.4061/2011/572743. 572743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 14.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halliday G, Hely M, Reid W, Morris J. The progression of pathology in longitudinally followed patients with Parkinson's disease. Acta Neuropathol. 2008;115:409–415. doi: 10.1007/s00401-008-0344-8. [DOI] [PubMed] [Google Scholar]
- 16.Foster ER, Campbell MC, Burack MA, et al. Amyloid imaging of Lewy body-associated disorders. Mov Disord. 2010;25:2516–2523. doi: 10.1002/mds.23393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villemagne VL, Okamura N, Pejoska S, et al. In vivo assessment of vesicular monoamine transporter type 2 in dementia with lewy bodies and Alzheimer disease. Arch Neurol. 2011;68:905–912. doi: 10.1001/archneurol.2011.142. [DOI] [PubMed] [Google Scholar]
- 18.Maetzler W, Reimold M, Liepelt I, et al. [11C]PIB binding in Parkinson's disease dementia. Neuroimage. 2008;39:1027–1033. doi: 10.1016/j.neuroimage.2007.09.072. [DOI] [PubMed] [Google Scholar]
- 19.Edison P, Rowe CC, Rinne JO, et al. Amyloid load in Parkinson's disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry. 2008;79:1331–1338. doi: 10.1136/jnnp.2007.127878. [DOI] [PubMed] [Google Scholar]
- 20.Gomperts SN, Rentz DM, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71:903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riedel O, Klotsche J, Spottke A, et al. Cognitive impairment in 873 patients with idiopathic Parkinson's disease. Results from the German Study on Epidemiology of Parkinson's Disease with Dementia (GEPAD) J Neurol. 2008;255:255–264. doi: 10.1007/s00415-008-0720-2. [DOI] [PubMed] [Google Scholar]
- 22.Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2010;289:18–22. doi: 10.1016/j.jns.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 23.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohnen NI, Albin RL, Koeppe RA, et al. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab. 2006;26:1198–1212. doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
- 25.Goetz CG, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov Disord. 2007;22:41–47. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- 26.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 27.Mattis S. Dementia Rating Scale. Odessa, FL: Psychological Assessment Resources; 1998. [Google Scholar]
- 28.Jewett DM, Kilbourn MR, Lee LC. A simple synthesis of [11C]dihydrotetrabenazine (DTBZ) Nucl Med Biol. 1997;24:197–199. doi: 10.1016/s0969-8051(96)00213-2. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Klunk WE, Debnath ML, et al. Development of a PET/SPECT agent for amyloid imaging in Alzheimer's disease. J Mol Neurosci. 2004;24:55–62. doi: 10.1385/JMN:24:1:055. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Klunk WE, Huang GF, Debnath ML, Holt DP, Mathis CA. Synthesis and evaluation of 2-(3'-iodo-4'-aminophenyl)-6-hydroxybenzothiazole for in vivo quantitation of amyloid deposits in Alzheimer's disease. J Mol Neurosci. 2002;19:11–16. doi: 10.1007/s12031-002-0004-8. [DOI] [PubMed] [Google Scholar]
- 31.Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer's disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995;36:1238–1248. [PubMed] [Google Scholar]
- 32.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Villeneuve S, Rodrigues-Brazete J, Joncas S, Postuma RB, Latreille V, Gagnon JF. Validity of the Mattis Dementia Rating Scale to detect mild cognitive impairment in Parkinson's disease and REM sleep behavior disorder. Dement Geriatr Cogn Disord. 2011;31:210–217. doi: 10.1159/000326212. [DOI] [PubMed] [Google Scholar]
- 34.Frey KA, Koeppe RA, Kilbourn MR, et al. Presynaptic monoaminergic vesicles in Parkinson’s disease and normal aging. Ann Neurol. 1996;40:873–884. doi: 10.1002/ana.410400609. [DOI] [PubMed] [Google Scholar]
- 35.Pike KE, Ellis KA, Villemagne VL, et al. Cognition and beta-amyloid in preclinical Alzheimer's disease: data from the AIBL study. Neuropsychologia. 2011;49:2384–2390. doi: 10.1016/j.neuropsychologia.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Scherder E, Eggermont L, Swaab D, et al. Gait in ageing and associated dementias; its relationship with cognition. Neurosci Biobehav Rev. 2007;31:485–497. doi: 10.1016/j.neubiorev.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Suttanon P, Hill KD, Said CM, Logiudice D, Lautenschlager NT, Dodd KJ. Balance and mobility dysfunction and falls risk in older people with mild to moderate Alzheimer disease. Am J Phys Med Rehabil. 2012;91:12–23. doi: 10.1097/PHM.0b013e31823caeea. [DOI] [PubMed] [Google Scholar]
- 38.Beauchet O, Allali G, Berrut G, Hommet C, Dubost V, Assal F. Gait analysis in demented subjects: Interests and perspectives. Neuropsychiatr Dis Treat. 2008;4:155–160. doi: 10.2147/ndt.s2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheridan PL, Hausdorff JM. The role of higher-level cognitive function in gait: executive dysfunction contributes to fall risk in Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;24:125–137. doi: 10.1159/000105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheridan PL, Solomont J, Kowall N, Hausdorff JM. Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer's disease. J Am Geriatr Soc. 2003;51:1633–1637. doi: 10.1046/j.1532-5415.2003.51516.x. [DOI] [PubMed] [Google Scholar]
- 41.Montero-Odasso M, Muir SW, Speechley M. Dual-task complexity affects gait in people with mild cognitive impairment: the interplay between gait variability, dual tasking, and risk of falls. Arch Phys Med Rehabil. 2012;93:293–299. doi: 10.1016/j.apmr.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 42.Muir SW, Speechley M, Wells J, Borrie M, Gopaul K, Montero-Odasso M. Gait assessment in mild cognitive impairment and Alzheimer's disease: The effect of dual-task challenges across the cognitive spectrum. Gait Posture. 2012;35:96–100. doi: 10.1016/j.gaitpost.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Langston JW. The Parkinson's complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59:591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- 44.Petrou M, Bohnen N, Muller M, Koeppe R, Albin R, Frey K. Aβ amyloid deposition in Parkinson disease patients at risk for development of dementia. Neurology. 2012;79:1161–1167. doi: 10.1212/WNL.0b013e3182698d4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golde TE, Schneider LS, Koo EH. Anti-abeta therapeutics in Alzheimer's disease: the need for a paradigm shift. Neuron. 2011;69:203–213. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 47.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]