Abstract
The symptoms of tethered cord syndrome (TCS) cases mostly appear during infancy and childhood. Though the adolescent presentation of TCS is well-recognized, it continues to pose significant diagnostic and management controversies. In this report, we describe two cases of adolescent onset TCS associated with two different etiologies. Our first case, an 18-year-old girl who presented due to overflow incontinence in association with TCS was diagnosed to have lumbar meningocele. The second case, a 19-year-girl presenting with perianal anesthesia and bowel and bladder incontinence had lipomyelomeningocele as the cause of TCS. Both of them underwent untethering surgery. The clinical charts and follow-up data were studied in respect to the clinical manifestation, surgical intervention and outcome with a brief review of pertinent literature.
Keywords: Adolescent, lipomyelomeningocele, meningocele, tethered cord syndrome
Introduction
Tethered cord syndrome (TCS) is a developmental abnormality of the neuroaxis, which is usually diagnosed in childhood. Adolescents with TCS are arguably the most neglected individuals in the population with a neurosurgical disease. However, sufficient differences in the mode of onset, clinical manifestations, and outcome exist between pediatric and adolescent patients with tethered cord to warrant a more detailed analysis of the adolescent syndrome. TCS is a stretch-induced functional disorder of the spinal cord due to the fact that its caudal portion is anchored by an inelastic structure. The functional lesion of TCS is generally situated in the lumbosacral cord, and many authors have shown that the syndrome is reversible via surgical untethering of the cord. The most problematic technical consideration in surgery for the release of the tethered cord is how to preserve functioning neural elements and rebuild the dural sac to maintain normal cerebrospinal fluid circulation.
Case Reports
Case 1
A case of overflow incontinence in an 18-year-old girl was referred from the Department of Urology. She was having a progressively increasing right gluteal swelling with the urinary frequency and urgency for last 5 years. On examination, she had decreased power around the ankle with absent reflex. Her sensation of pain and touch was lost in lateral side of foot with saddle type of perianal anesthesia. Local examination revealed a large non tender, soft, cystic, globular, fluctuating mass of size about 15 cm × 15 cm over her right gluteal area with positive impulse on coughing [Figure 1]. Lumbosacral bony defect was evident in X-ray spine [Figure 2]. Magnetic resonance imaging (MRI) of the lumbo-sacral spine demonstrated meningocele with pulled out tuft of cord from S1 level exiting through right inferior sacral dysraphic defect causing tethered cord [Figure 3]. Incidental finding was bilateral hydronephrosis [Figure 4]. Urodynamic study revealed neurogenic bladder with non-functioning internal sphincter and under active detrusor contractility. Following meningocele repair and untethering of the cord, she had marked neurological improvement with delayed return of urological function.
Figure 1.
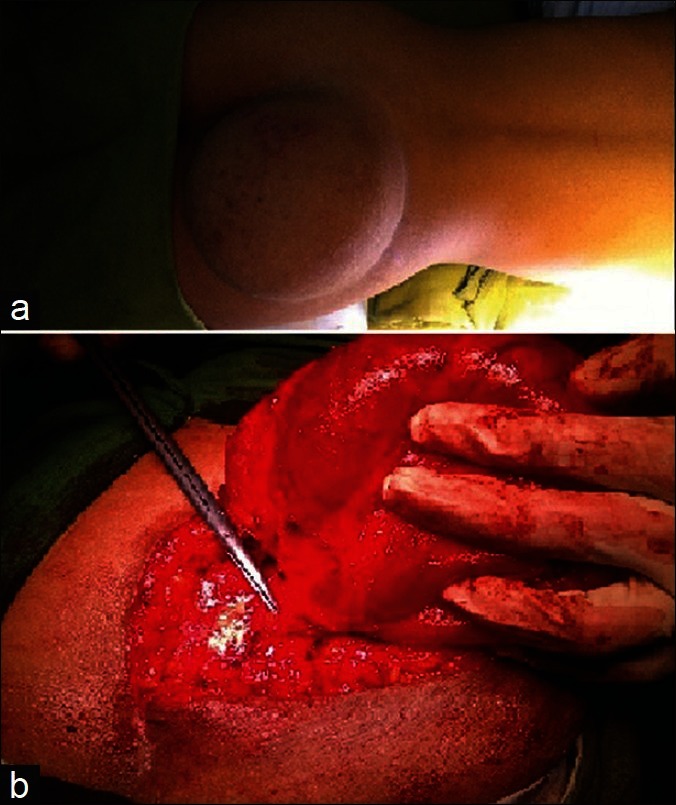
Clinical photograph showing right gluteal globular cystic mass of size about 15 cm × 15 cm (a). Intraoperative picture shows the meningocele exiting through the sacral defect (b)
Figure 2.
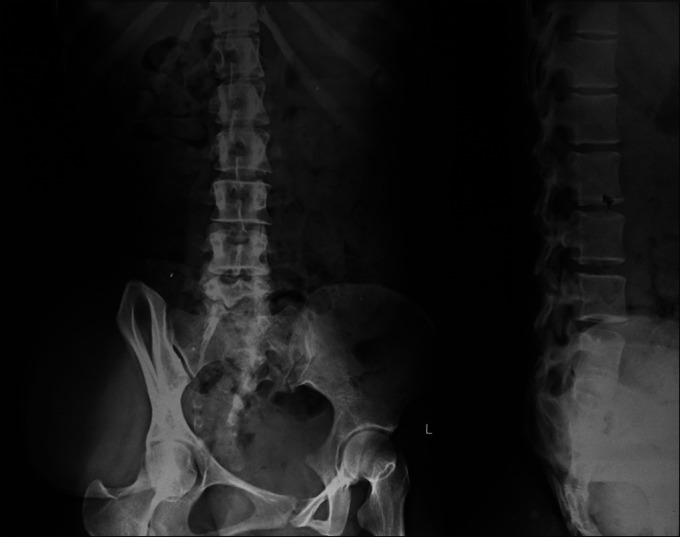
X-ray spine revealing lumbosacral bony defect along with right gluteal soft tissue shadow
Figure 3.
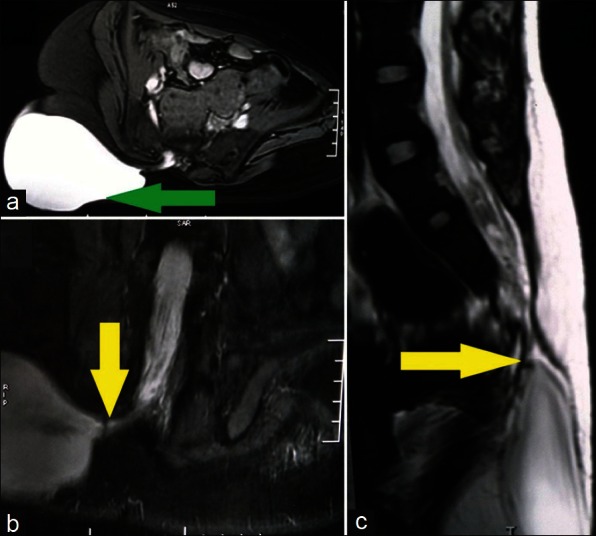
Magnetic resonance imaging axial (a), coronal (b), and sagittal (c) view demonstrating meningocele (green arrow) with pulled out tuft of spinal cord causing tethered cord (yellow arrow)
Figure 4.
Magnetic resonance imaging showing incidental finding of bilateral hydronephrosis
Case 2
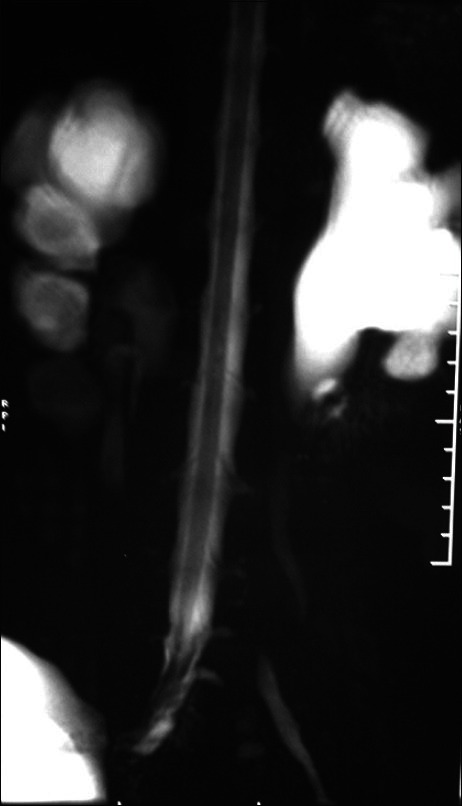
A 19-year-old girl presented with perianal sensory loss with bowel and bladder incontinence for about 4 years. Following excision of a lipoma in her right gluteal region 2 years back she developed low back pain radiating down the legs. Her examination findings revealed bilateral small muscle atrophy of feet with weak plantar flexion. She had perianal sensory loss with patulous anal sphincter. Low lying conus and sacral lipomyelomeningocele causing cord tethering was encountered in the MRI of the lumbosacral spine [Figure 5]. Her urodynamic study showed underactive detrusor contractility. On operation, an intradural lipomatous mass attached to the distal end of thick, dilated filum terminale with entangled lower sacral roots was found. Untethering of the cord following excision of the lipomatous mass was carried out. Post-operatively she gained gradual self-control over her bladder in a period of over 6 months.
Figure 5.
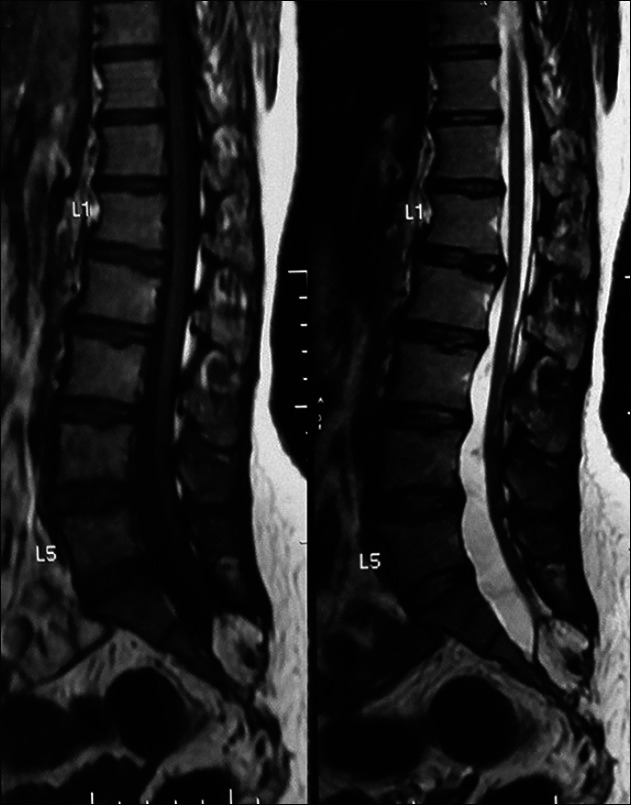
Magnetic resonance image of spine sagittal view showing T1 and T2 hyperintense soft tissue mass pulling the cord up to S1 level
Discussion
The difference in growth rates between the spinal cord and musculoskeletal elements accounts for the fact that the conus medullaris may terminate anywhere from the T12 to the L2-L3 interspace.[1] A conus below the L2-L3 interspace is abnormal, and has been termed a “tethered conus.”[2] The syndrome is usually diagnosed in childhood, and although the presentation is variable and may be insidious, a tethered conus should be suspected in patients of any age with an unexpected spastic gait, neurogenic bladder, bowel dysfunction, lower extremity weakness, scoliosis, or foot deformities.[2,3]
The symptoms and signs of TCS may be related to traction upon the conus and cauda equina by an intradural mass and tethering band and may grow worse with age owing to accelerated skeletal growth in relation to the neuroaxis during puberty. Since, the spinal cord grows more slowly than the spinal column; the cord becomes stretched and stressed over time, causing neurological damage. Bending and stretching movements of the body put additional tension on the tethered spinal cord. Study conducted by Kang et al.[4] supports the hypothesis that spinal cord dysfunction in the early stage of tethering results from mechanical and vascular damage to a spinal cord segment, which results in widespread ischemic involvement of the cord in a prolonged tethering effect during growth. Adolescents with TCS are arguably the most neglected individuals in the population with a neurosurgical disease. There are several reported cases of adolescent presentations of tethered conus, and it is unclear why these patients should have been symptom-free and the diagnosis overlooked for so long. Pool[5] theorized that the tethered conus eventually became symptomatic in one patient because local stretching and ischemia occurred from the repetitive and insidious low back trauma associated with the patient′s occupation. These clinical and experimental reports, indicating a delicate balance between skeletal maturation and traction upon the tethered conus, may explain why some patients remain symptom-free until adolescence.
The actual cord tethering has been attributed to a variety of pathologic entities, including a thickened tight filum terminale,[2,6,7] intradural lipomas with or without a connecting extradural component,[6,8] intradural fibrous adhesions,[6,9] myelomeningocele etc. Though lipomyelomeningocele (LMMC) and meningocele causing TCS are not rare, delay in diagnosis (as it has occurred in our cases) may lead to significant management problem.
Pain is an uncommon presenting complaint in children with TCS[6,7] as opposed to 50% incidence in our group of adolescents. In our case, the pain is located in the lumbosacral region, with variable radiation down the legs. Progressive foot deformities are one of the most common presenting features in children with TCS.[6,10] None of our patients developed this problem. This suggests that the insult to the adolescent conus causes flank sensorimotor deficits or even atrophy, but does not result in any type of muscle imbalance. Progressive scoliosis was noted in 33% of a series of children with TCS,[7] but progressive spinal deformities have not been described by any of our patients. The incidence of urologic symptoms appears to be similar in childhood and adolescent syndromes.[6,8] Both of our cases had incontinence with one of them showing features of hydronephrosis in radiological imaging. This might be due to chronic urinary retention exerting back pressure due to the development of neurogenic bladder.
Pre-operative studies are necessary to plan the treatment in order to avoid operative morbidity or complications. Magnetic resonance imaging (MRI) is a useful technique for evaluation of patients with spinal lesions producing TCS. While MRI is a superior diagnostic tool for verifying the cystic lesion in the sagittal plane, its relation with the spinal cord and associated spinal cord anomalies, direct X-ray and computed tomography scan may give information about the associated bone defects.[11] Pre-operative urodynamic investigation is strongly recommended, especially if the patient seems continent.[12] Urodynamic study of our cases demonstrated non-functioning internal sphincter and detrusor hyporeflexia indicating flaccid neurogenic bladder.
In case of adolescent patients having TCS with impending neurologic deficits and/or progression of existing neurologic deficits, it is reasonable to operate as soon as possible. Adequate surgical release of the tethered cord, and reforming the dural sac (water tight dural closure) might be the keys to successful surgical outcome. The operative results for pain are most gratifying.[13] In our patients, pain relief after surgery was marked, and sensorimotor deficits also responded favorably with surgical release of the conus. Many authors with experience in the childhood TCS have commented on the poor operative results in terms of urinary dysfunction.[14] Though, delayed for over 6 months, our results for bladder dysfunction are not very unsatisfactory.
Conclusion
In adolescents with radiologically confirmed cord tethering, untethering is an appropriate procedure when the neurologic symptoms and signs are suggestive of TCS. Urological function must be evaluated in late cases of TCS. Early diagnosis, adequate surgical release, and reforming the dural sac might be the keys to successful management in such cases.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Barson AJ. The vertebral level of termination of the spinal cord during normal and abnormal development. J Anat. 1970;106:489–97. [PMC free article] [PubMed] [Google Scholar]
- 2.Fitz CR, Harwood Nash DC. The tethered conus. Am J Roentgenol Radium Ther Nucl Med. 1975;125:515–23. doi: 10.2214/ajr.125.3.515. [DOI] [PubMed] [Google Scholar]
- 3.Steinbok P, Garton HJ, Gupta N. Occult tethered cord syndrome: A survey of practice patterns. J Neurosurg. 2006;104:309–13. doi: 10.3171/ped.2006.104.5.309. [DOI] [PubMed] [Google Scholar]
- 4.Kang JK, Kim MC, Kim DS, Song JU. Effects of tethering on regional spinal cord blood flow and sensory-evoked potentials in growing cats. Childs Nerv Syst. 1987;3:35–9. doi: 10.1007/BF00707191. [DOI] [PubMed] [Google Scholar]
- 5.Pool JL. Spinal cord and local signs secondary to occult sacral meningoceles in adults. Bull N Y Acad Med. 1952;28:655–63. [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson FM. Occult spinal dysraphism: A series of 73 cases. Pediatrics. 1975;55:826–35. [PubMed] [Google Scholar]
- 7.Hoffman HJ, Hendrick EB, Humphreys RP. The tethered spinal cord: Its protean manifestations, diagnosis and surgical correction. Childs Brain. 1976;2:145–55. doi: 10.1159/000119610. [DOI] [PubMed] [Google Scholar]
- 8.Bruce DA, Schut L. Spinal lipomas in infancy and childhood. Childs Brain. 1979;5:192–203. doi: 10.1159/000119818. [DOI] [PubMed] [Google Scholar]
- 9.Yashon D, Beatty RA. Tethering of the conus medullaris within the sacrum. J Neurol Neurosurg Psychiatry. 1966;29:244–50. doi: 10.1136/jnnp.29.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selden NR. Occult tethered cord syndrome: The case for surgery. J Neurosurg. 2006;104:302–4. doi: 10.3171/ped.2006.104.5.302. [DOI] [PubMed] [Google Scholar]
- 11.Raghavan N, Barkovich AJ, Edwards M, Norman D. MR imaging in the tethered spinal cord syndrome. AJR Am J Roentgenol. 1989;152:843–52. doi: 10.2214/ajr.152.4.843. [DOI] [PubMed] [Google Scholar]
- 12.Kondo A, Kato K, Kanai S, Sakakibara T. Bladder dysfunction secondary to tethered cord syndrome in adults: Is it curable? J Urol. 1986;135:313–6. doi: 10.1016/s0022-5347(17)45622-9. [DOI] [PubMed] [Google Scholar]
- 13.Paradiso G, Lee GY, Sarjeant R, Hoang L, Massicotte EM, Fehlings MG. Multimodality intraoperative neurophysiologic monitoring findings during surgery for adult tethered cord syndrome: Analysis of a series of 44 patients with long-term follow-up. Spine (Phila Pa 1976) 2006;31:2095–102. doi: 10.1097/01.brs.0000231687.02271.b6. [DOI] [PubMed] [Google Scholar]
- 14.Dubowitz V, Lorber J, Zachary RB. Lipoma of the cauda equina. Arch Dis Child. 1965;40:207–13. doi: 10.1136/adc.40.210.207. [DOI] [PMC free article] [PubMed] [Google Scholar]


