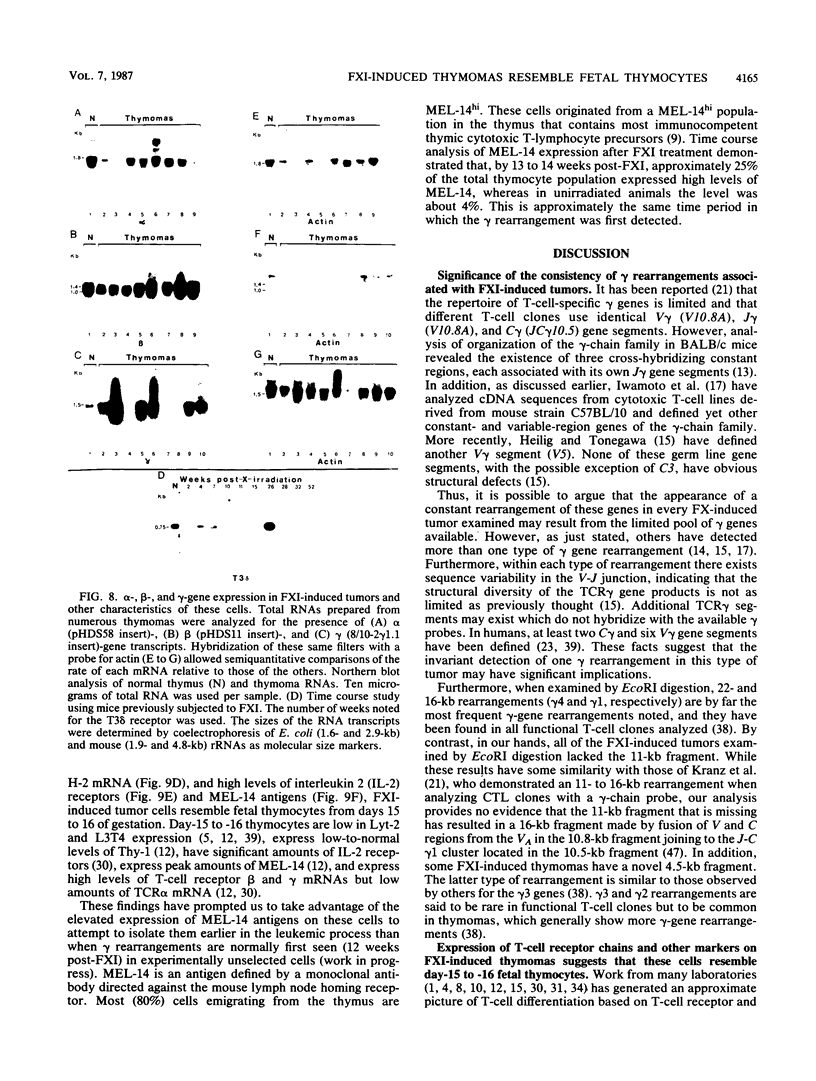
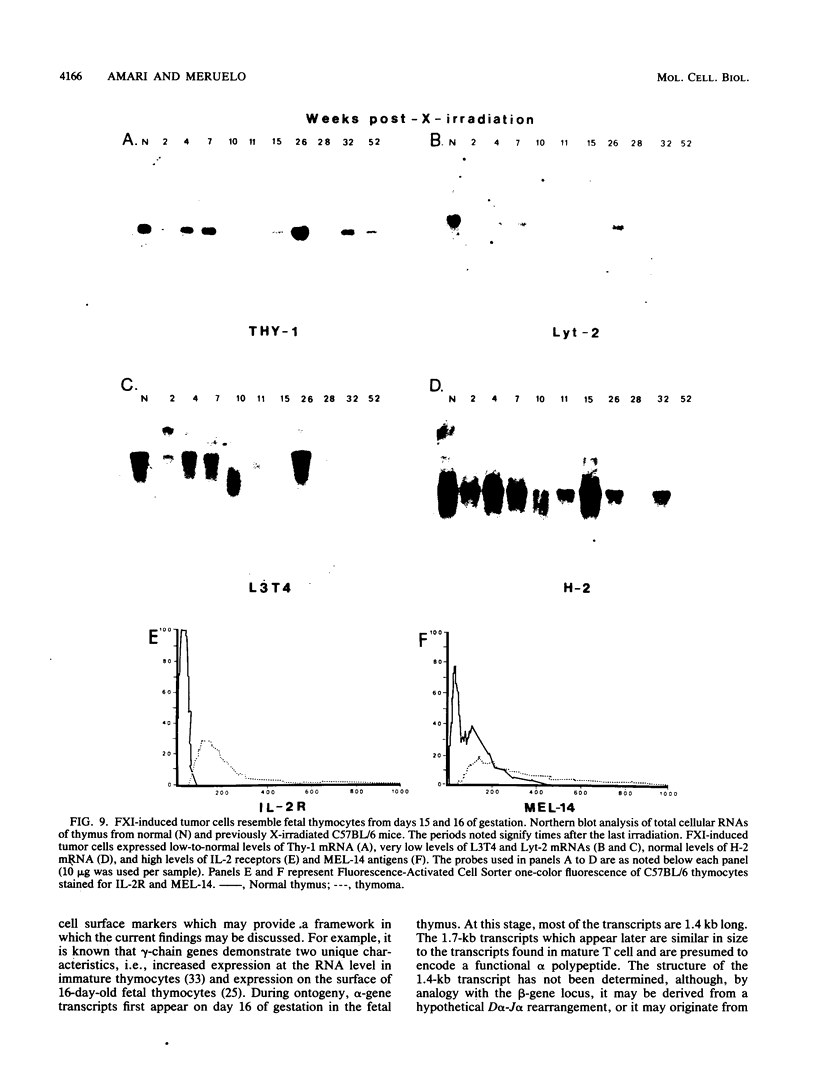
Abstract
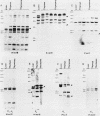
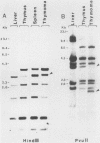
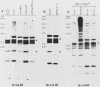
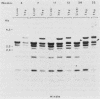
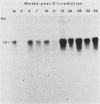
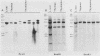
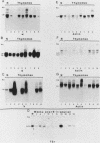
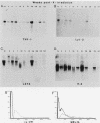
We report here that specific T-cell receptor rearrangements were observed in fractionated-X-irradiation-induced murine leukemias. Consistent gamma-chain rearrangements, limited beta-chain rearrangements, and no detectable alpha-chain rearrangements were observed. Gene expression studies revealed that, in comparison with normal thymus tissue, expression of alpha T-cell receptor genes was lower in the thymomas, beta expression was much higher but approximately equal to that of normal thymocytes, and gamma expression was significantly increased. After coupling these data with those from analyses using reagents against other surface markers, such as Lyt-2, L3T4, H-2, IL-2R and MEL-14, we concluded that the target T cells for fractionated-X-irradiation-induced transformation resemble fetal thymocytes from days 15 and 16 of gestation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuto O., Hussey R. E., Fitzgerald K. A., Protentis J. P., Meuer S. C., Schlossman S. F., Reinherz E. L. The human T cell receptor: appearance in ontogeny and biochemical relationship of alpha and beta subunits on IL-2 dependent clones and T cell tumors. Cell. 1983 Oct;34(3):717–726. doi: 10.1016/0092-8674(83)90528-7. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniver J., Houben-Defresne M. P., Goffinet G., Lenaerts P., Betz E. H. Target cells and thymus microenvironment in the pathogenesis of thymic lymphomas in C57BL/Ka mice. Int J Radiat Oncol Biol Phys. 1985 Jan;11(1):65–69. doi: 10.1016/0360-3016(85)90363-3. [DOI] [PubMed] [Google Scholar]
- Born W., Yagüe J., Palmer E., Kappler J., Marrack P. Rearrangement of T-cell receptor beta-chain genes during T-cell development. Proc Natl Acad Sci U S A. 1985 May;82(9):2925–2929. doi: 10.1073/pnas.82.9.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M. Molecular genetics of the T cell-receptor beta chain. Annu Rev Immunol. 1985;3:537–560. doi: 10.1146/annurev.iy.03.040185.002541. [DOI] [PubMed] [Google Scholar]
- Defresne M. P., Rongy A. M., Greimers R., Boniver J. Cellular aspects of radiation leukemogenesis in C57 BL/Ka mice: alterations to thymic microenvironment and lymphopoiesis. Leuk Res. 1986;10(7):783–789. doi: 10.1016/0145-2126(86)90298-5. [DOI] [PubMed] [Google Scholar]
- Furley A. J., Mizutani S., Weilbaecher K., Dhaliwal H. S., Ford A. M., Chan L. C., Molgaard H. V., Toyonaga B., Mak T., van den Elsen P. Developmentally regulated rearrangement and expression of genes encoding the T cell receptor-T3 complex. Cell. 1986 Jul 4;46(1):75–87. doi: 10.1016/0092-8674(86)90861-5. [DOI] [PubMed] [Google Scholar]
- Gallatin M., St John T. P., Siegelman M., Reichert R., Butcher E. C., Weissman I. L. Lymphocyte homing receptors. Cell. 1986 Mar 14;44(5):673–680. doi: 10.1016/0092-8674(86)90832-9. [DOI] [PubMed] [Google Scholar]
- Garman R. D., Doherty P. J., Raulet D. H. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell. 1986 Jun 6;45(5):733–742. doi: 10.1016/0092-8674(86)90787-7. [DOI] [PubMed] [Google Scholar]
- Greenberg J. M., Quertermous T., Seidman J. G., Kersey J. H. Human T cell gamma-chain gene rearrangements in acute lymphoid and nonlymphoid leukemia: comparison with the T cell receptor beta-chain gene. J Immunol. 1986 Sep 15;137(6):2043–2049. [PubMed] [Google Scholar]
- Haars R., Kronenberg M., Gallatin W. M., Weissman I. L., Owen F. L., Hood L. Rearrangement and expression of T cell antigen receptor and gamma genes during thymic development. J Exp Med. 1986 Jul 1;164(1):1–24. doi: 10.1084/jem.164.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayday A. C., Diamond D. J., Tanigawa G., Heilig J. S., Folsom V., Saito H., Tonegawa S. Unusual organization and diversity of T-cell receptor alpha-chain genes. 1985 Aug 29-Sep 4Nature. 316(6031):828–832. doi: 10.1038/316828a0. [DOI] [PubMed] [Google Scholar]
- Hayday A. C., Saito H., Gillies S. D., Kranz D. M., Tanigawa G., Eisen H. N., Tonegawa S. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell. 1985 Feb;40(2):259–269. doi: 10.1016/0092-8674(85)90140-0. [DOI] [PubMed] [Google Scholar]
- Heilig J. S., Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. 1986 Aug 28-Sep 3Nature. 322(6082):836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- Ihle J. N. Experimental models and conceptual approaches to studies of lymphomas and leukemia: etiology, biology, and control. Semin Hematol. 1978 Apr;15(2):95–115. [PubMed] [Google Scholar]
- Iwamoto A., Rupp F., Ohashi P. S., Walker C. L., Pircher H., Joho R., Hengartner H., Mak T. W. T cell-specific gamma genes in C57BL/10 mice. Sequence and expression of new constant and variable region genes. J Exp Med. 1986 May 1;163(5):1203–1212. doi: 10.1084/jem.163.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. S. On the natural history of the murine leukemias: presidential address. Cancer Res. 1967 Aug;27(8):1325–1340. [PubMed] [Google Scholar]
- Kavaler J., Davis M. M., Chien Y. Localization of a T-cell receptor diversity-region element. Nature. 1984 Aug 2;310(5976):421–423. doi: 10.1038/310421a0. [DOI] [PubMed] [Google Scholar]
- Kranz D. M., Saito H., Disteche C. M., Swisshelm K., Pravtcheva D., Ruddle F. H., Eisen H. N., Tonegawa S. Chromosomal locations of the murine T-cell receptor alpha-chain gene and the T-cell gamma gene. Science. 1985 Feb 22;227(4689):941–945. doi: 10.1126/science.3918347. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Goverman J., Haars R., Malissen M., Kraig E., Phillips L., Delovitch T., Suciu-Foca N., Hood L. Rearrangement and transcription of the beta-chain genes of the T-cell antigen receptor in different types of murine lymphocytes. Nature. 1985 Feb 21;313(6004):647–653. doi: 10.1038/313647a0. [DOI] [PubMed] [Google Scholar]
- Lefranc M. P., Rabbitts T. H. Two tandemly organized human genes encoding the T-cell gamma constant-region sequences show multiple rearrangement in different T-cell types. Nature. 1985 Aug 1;316(6027):464–466. doi: 10.1038/316464a0. [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Maeda K., Ito K., Heller M., Tonegawa S. T gamma protein is expressed on murine fetal thymocytes as a disulphide-linked heterodimer. Nature. 1987 Feb 19;325(6106):720–723. doi: 10.1038/325720a0. [DOI] [PubMed] [Google Scholar]
- Nowell P. C., Croce C. M. Chromosomes, genes, and cancer. Am J Pathol. 1986 Oct;125(1):7–15. [PMC free article] [PubMed] [Google Scholar]
- Owen F. L., Strauss W. M., Murre C., Duby A. D., Hiai H., Seidman J. G. AKR murine thymic leukemias are from a distinct thymic cell lineage and do not express the beta chain of the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7434–7437. doi: 10.1073/pnas.83.19.7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicci P. G., Knowles D. M., 2nd, Magrath I., Dalla-Favera R. Chromosomal breakpoints and structural alterations of the c-myc locus differ in endemic and sporadic forms of Burkitt lymphoma. Proc Natl Acad Sci U S A. 1986 May;83(9):2984–2988. doi: 10.1073/pnas.83.9.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quertermous T., Murre C., Dialynas D., Duby A. D., Strominger J. L., Waldman T. A., Seidman J. G. Human T-cell gamma chain genes: organization, diversity, and rearrangement. Science. 1986 Jan 17;231(4735):252–255. doi: 10.1126/science.3079918. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Royer H. D., Ramarli D., Acuto O., Campen T. J., Reinherz E. L. Genes encoding the T-cell receptor alpha and beta subunits are transcribed in an ordered manner during intrathymic ontogeny. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5510–5514. doi: 10.1073/pnas.82.16.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984 Nov 1;312(5989):36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. 1984 Jun 28-Jul 4Nature. 309(5971):757–762. doi: 10.1038/309757a0. [DOI] [PubMed] [Google Scholar]
- Snodgrass H. R., Kisielow P., Kiefer M., Steinmetz M., von Boehmer H. Ontogeny of the T-cell antigen receptor within the thymus. Nature. 1985 Feb 14;313(6003):592–595. doi: 10.1038/313592a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Frelinger J. G., Fisher D., Hunkapiller T., Pereira D., Weissman S. M., Uehara H., Nathenson S., Hood L. Three cDNA clones encoding mouse transplantation antigens: homology to immunoglobulin genes. Cell. 1981 Apr;24(1):125–134. doi: 10.1016/0092-8674(81)90508-0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Traunecker A., Oliveri F., Allen N., Karjalainen K. Normal T cell development is possible without 'functional' gamma chain genes. EMBO J. 1986 Jul;5(7):1589–1593. doi: 10.1002/j.1460-2075.1986.tb04400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winoto A., Mjolsness S., Hood L. Genomic organization of the genes encoding mouse T-cell receptor alpha-chain. 1985 Aug 29-Sep 4Nature. 316(6031):832–836. doi: 10.1038/316832a0. [DOI] [PubMed] [Google Scholar]
- Zauderer M., Iwamoto A., Mak T. W. Gamma gene rearrangement and expression in autoreactive helper T cells. J Exp Med. 1986 May 1;163(5):1314–1318. doi: 10.1084/jem.163.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zech L., Gahrton G., Hammarström L., Juliusson G., Mellstedt H., Robèrt K. H., Smith C. I. Inversion of chromosome 14 marks human T-cell chronic lymphocytic leukaemia. 1984 Apr 26-May 2Nature. 308(5962):858–860. doi: 10.1038/308858a0. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Crisanti A., Kisielow P., Haas W. Absence of growth by most receptor-expressing fetal thymocytes in the presence of interleukin-2. Nature. 1985 Apr 11;314(6011):539–540. doi: 10.1038/314539a0. [DOI] [PubMed] [Google Scholar]