Abstract
Recent results obtained in our laboratory indicate that paraxanthine, the main metabolite of caffeine in humans, produces a significantly stronger locomotor activation in rats than caffeine. Furthermore, paraxanthine also produced a very significant increase in striatal extracellular concentrations of dopamine. Searching for an additional mechanism other than adenosine antagonism responsible for these psychostimulant-like effects, it was found that paraxanthine, but not caffeine, inhibited cGMP-preferring phosphodiesterases. Furthermore, interrupting nitric oxide neurotransmision (inhibiting nitric oxide synthase) significantly decreased both the locomotor-activating and the dopamine-releasing effects of paraxanthine. These results open up some obvious questions about the role of paraxanthine in the pharmacological effects of caffeine.
Nitric Oxide Neurotransmission
Since nitric oxide (NO) was first identified as an endothelial-derived relaxation factor in peripheral blood vessels,1 its role as a biological messenger, and particularly as a neurotransmitter has been well established. Thus, NO fits within the broad definition of neurotransmitter coined by Snyder and Ferris2–that is, “a molecule, released by neurons or glia, which physiologically influences the electrochemical state of adjacent cells”. Because NO cannot be stored in cells, it depends on new synthesis to exert its functional properties. NO is produced by NO synthase (NOS), which generates NO from the amino acid L-arginine. NOS is a complex heterodimeric protein that is found constitutively in two isoforms, neuronal, and endothelial NO (nNOS and eNOS, respectively). A third inducible form (iNOS) can be expressed by macrophages and microglia upon immunological challenge.3 nNOS is the most abundant isoform found in the brain and the one responsible for NO production in neurons and its enzyme activity is regulated by Ca2+ and calmodulin.4 The subcellular localization of nNOS is mediated by its ability to bind to adapter proteins. For instance, through its PDZ domain it binds to PSD95 (postsynaptic density protein 95), which links nNOS to the N-methyl-D-aspartate (NMDA) receptor and accounts for the efficient activation of nNOS by NMDA receptor stimulation.5
In the central nervous system, nNOS is synthesized by specific populations of neurons (NO neurons). In the cerebellum, nNOS is expressed by local stellate, basket and granule, but not Purkinje cells, although Purkinje are abundant in NO receptors.6 In the brainstem, nNOS is produced by cholinergic neurons of the ascending reticular activating system.6 These cells originate in the latero-dorsal and pedunculo-pontine nuclei (LDT and PPT nuclei), which through a dorsal tegmental pathway project heavily to the thalamus, to the midline-intralaminar and reticular nuclei, which transmit the activity of the ascending reticular activating system to extensive areas of the cerebral cortex (for a recent review see ref. 7). Through a ventral tegmental pathway the cholinergic cells of the LDT and PPT form a extrathalamic cholinergic relay to the cortex and connect with the also cholinergic cells that are the origin of the corticopetal basal forebrain system, the major extrathalamic relay of the ascending reticular activating system to the cortex.7 In the forebrain, cortex, striatum, and hippocampus, nNOS is produced in GABAergic interneurons. In the striatum these interneurons, which also express somatostatin and neuropeptide Y only constitute a little percentage of the striatal population.6,8 Nevertheless they exert an important control of striatal function through its ability to produce NO, which by volume transmission can reach the other major striatal neuronal elements.
The NO receptor is a soluble guanylyl cyclase, and, in common with other receptors, it is composed of a ligand-binding site and a transduction-signaling domain. The ligand-binding site is a heme group similar to the one used by hemoglobin to bind O2, but with a high preference for NO.9 Guanylyl cyclase has an αβ-heterodimeric structure (with two isoforms, α1β1 and α2β1) and comprises three domains: a heme-binding, a dimerizationm, and a catalytic domain, where GTP converts to cGMP.10 The most widespread signaling mechanism used by cGMP is activation of protein kinase G (PKG), which exists in three forms, PKGIα and PKGIβ (splice variants), and PKGII, all present in the brain.11 PKG in the brain is involved in regulation of neurotransmitter release and uptake, neuronal differentiation and gene expression.11 The NO-cGMP-PKG signaling is terminated by hydrolysis of cGMP by means of phosphodiesterases (PDEs). PDEs are classified in three different families: cAMP-preferring PDEs (PDE4, PDE7 and PDE8), cGMP-preferring PDEs (PDE5, PDE6 and PDE9), and nonselective PDEs (which hydrolise both cAMP and cGMP; PDE1, PDE2, PDE3 and PDE10). In the striatum, cGMP hydrolysis depends mostly on PDE2 and PDE9.12
NO and Adenosine in the Striatal Spine Module
We recently introduced the concept of local module, which we defined as ‘the minimal portion of one or more neurons and/or one or more glial cells that operates as an independent integrative unit.13 Conceptually, the local module allows a better understanding of the functional relevance of extrasynaptic receptors, which are activated by volume transmission. Furthermore, the concept of local module provides a rationale for the functional relevance of neurotransmitter receptor heteromers, which can integrate signals that arise from the same or different elements that constitute the local module.13,14 “Striatal spine modules” are striatal local modules which are centered in the dendritic spine of the GABAergic striatal efferent neurons (also called medium spiny neurons or MSN).13 This type of neuron makes up more than 95% of the striatal neuronal population and receives two main extrinsic inputs that converge in the dendritic spine: mesencephalic dopaminergic inputs from the substantia nigra pars compacta and the ventral tegmental area and cortical, limbic, and thalamic glutamatergic inputs.13–15 The main elements of what we called the “striatal spine module I” include the dendritic spine, the glutamatergic and dopaminergic terminals that make synaptic contact with the head and neck of the dendritic spine, respectively, and astroglial processes that wrap the glutamatergic synapse.15 This arrangement allows dopaminergic neurotransmission to control glutamatergic neurotransmission. GABAergic innervation in the striatum predominantly originates from collaterals of projecting neurons and from interneurons.16–18 But GABAergic interneurons, rather than axon collaterals of GABAergic striatal efferent neurons, provide the predominant inhibitory control of striatal neuron excitability.18,19 Like dopaminergic afferents, some GABAergic interneurons make synaptic contact with the neck of the dendritic spines at the same time that glutamatergic terminals make synaptic contact with the head of dendritic spines. In addition, many terminals of GABAergic interneurons make synaptic contact with the dendritic shafts but close to the base of the spine.18 It is quite well established that there is a preponderance of dopaminergic inputs on distal portions of the dendrites, which also contain the greatest density of dopaminergic receptors in the dendritic tree.20,21 On the other hand, in the proximal portion of the dendrites there is a preponderance of GABAergic intrinsic inputs (and also cholinergic, although these mostly constitute asynaptic varicosities).20 Therefore, the striatal spine module I is mostly localized in the distal portion of the dendritic tree. But, there are at least two additional types of striatal spine modules localized in the middle and proximal portions of the dendritic field, which receive GABAergic inputs. One type of striatal module receives both dopamine and GABAergic inputs (striatal spine module II; Figure 1), and the other type receives only GABAergic input (striatal spine module III).15 Since GABAergic interneurons are the predominant source of NO in the striatum, we can speculate that NO place a predominant role in spine modules II and III. As we will see below, in these striatal modules NO released from GABAergic interneurons can potentially modulate glutamate and dopamine release.
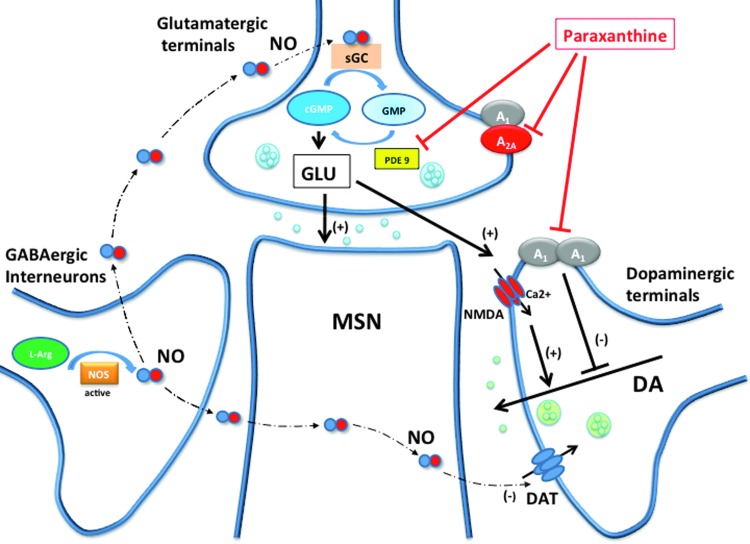
FIG. 1.
Hypothetical mechanisms of actions of glutamate and dopamine release by paraxanthine in the striatal spine module II, which includes a striatal spine from a medium spiny neuron (MSN) receiving glutamatergic, dopaminergic, and GABAergic terminals (from GABA interneurons). NO is produced by GABAergic interneurons and induces glutamate release by acting on soluble guanylyl cyclase (NO receptor or sGC), which converts GMP into cGMP. Phosphodiesterase 9 (PDE9) terminates NO-cGMP signaling by metabolizing cGMP back to GMP. NO produces dopamine release secondarily to glutamate release (that activates ionotropic glutamate receptors localized in dopaminergic terminals) and by directly blocking dopamine transporter (DAT) function. Paraxanthine potentially increases glutamate and dopamine release by blocking A1 receptors (which tonically inhibit glutamate and dopamine release) and by inhibiting PDE9 function, which potentiates NO signaling at the glutamatergic terminal. NO, nitric oxide.
As mentioned above, the local module concept gives a frame for the understanding of the role of volume transmission. In the striatal spine module, glutamate is not only released intrasynaptically to stimulate ionotropic glutamate receptors localized in the glutamatergic synapse, but it can also spillover and stimulate metabotropic glutamate receptors localized perisynaptically and astroglial glutamate receptors, which promote glial glutamate release. This can influence adjacent ionotropic glutamate receptors localized in the dopaminergic terminals, which activation stimulates dopamine release.13–15 This is a mechanism by which a strong glutamatergic input to the striatal spine module facilitates tonic dopamine release.
In the striatal spine module, a main effect of increased NO neurotransmission is an increase in the extracellular concentration of dopamine. Two different mechanisms have been invocated; a direct NO-mediated blocking effect of the dopamine transporter, localized in the dopaminergic terminals8, and an indirect effect through and NO-mediated glutamate release (Fig. 1).22
Adenosine and Caffeine in the Striatal Spine Module
Extracellular adenosine in the striatal spine module mostly comes from ATP released from astroglia, which is immediately converted to adenosine by means of ectonucleotidases.23 But as for glutamate, glial ATP is co-released with glutamate upon stimulation of glial glutamate and ATP receptors, initially stimulated by glutamate and ATP co-released from glutamatergic terminals.23 Therefore, in the striatal spine module, glial-derived extracellular levels of glutamate and adenosine represent an amplification of neuronal-derived glutamate and adenosine.23,13–15 Both adenosine and NO are important and complementary mechanisms that modulate striatal glutamatergic and dopaminergic neurotransmission. Adenosine would be mostly generated by strong excitatory, glutamatergic, input, while NO would be mostly produced by strong inhibitory, GABAergic, input.
Some presynaptic glutamatergic terminals express adenosine A2A receptors, which form heteromers with adenosine A1 receptors.23 These heteromers constitute a concentration dependent switch: low basal concentrations of adenosine produce an inhibition of glutamate release, by mostly activating A1 receptors (with more affinity for adenosine than A2A receptors); while high concentrations of adenosine also activate A2A receptors which in the heteromer shuts down A1 receptor signaling, promoting glutamate release.13,14,24 Selective A2A receptor antagonists counteract striatal glutamate release and therefore, the striatal glutamate-induced dopamine release produced by cortical electrical stimulation.25,26 Preliminary results obtained from our laboratory indicate that the effects of A2A receptor antagonists on glutamate release depend on A1 receptor function, being counteracted by A1 receptor antagonists. It is also important to mention that adenosine can directly modulate dopaminergic neurotransmission by acting on inhibitory A1 receptors localized in some dopaminergic terminals.27
Nonselective competitive blockade of adenosine A1 and A2A receptors is the main mechanism responsible for the central pharmacological effects of caffeine at concentrations reached in the brain after coffee intake.28 A presynaptic dopamine-releasing effect of caffeine has been a matter of debate.27,28 The effect is far from that produced by classical psychostimulants, such as cocaine and amphetamine, and it is more apparent in the dorsal part of the shell of the nucleus accumbens (ventral striatum), where seems to be glutamate-dependent and due to the ability of caffeine to block A1 receptors.27–29 This dopaminergic presynaptic effect correlates with the A1 receptor-mediated locomotor activating effects produced by the acute administration of caffeine.27–29 An extensive qualitative and quantitative comparison of the central pharmacological effects of caffeine with selective A1 and A2A receptor antagonists indicated an important involvement of A1 receptors in the acute locomotor-activating and discriminative-stimulus effects of caffeine.30–32
On the other hand, the locomotor-activating effects of caffeine during its chronic intake seem to depend mostly on its A2A receptor blockade.30 Postsynaptic A2A receptors, localized in GABAergic striatopallidal neurons and which form heteromers with dopamine D2 receptors are the main targets of these locomotor activating effects.26 In the A2A-D2 receptor heteromer, activation of the A2A receptor counteracts D2 receptor-mediated inhibition of the excitability of the sriatopallidal neuron.33 Still another possible target of caffeine are postsynaptic A1 receptors that are localized in a different population of striatal GABAergic efferent neuron, the dopamine D1 receptor-containing striatonigro-striatoentopeduncular neuron, which expresses A1-D1 receptor heteromers.28 In this heteromer, activation of A1 receptor decreases the effects of D1 receptor stimulation. Therefore, a critical aspect of the mechanisms of the psychostimulant effects of caffeine is its ability to release the pre- and post- synaptic inhibition that adenosine imposes on dopaminergic neurotransmission by acting on different adenosine receptor heteromers localized in different elements of the striatal spine module.29
Different Pharmacological Profile of Paraxanthine Compared to Caffeine
In rats, caffeine is mainly demethylated to the dimethylxanthines paraxanthine, theophylline, and theobromine in roughly similar amounts though the catalytic action of cytochrome P450, particularly cytochrome subtype P4501A2 (or CYP1A2).34,35 In humans, caffeine is also rapidly metabolized to the three dimethylxanthines, but with a very different metabolizing rate, with paraxanthine constituting by far the main metabolite (approximately 80% of the three dimethylxanthines).34–36 The first studies which compared the pharmacological effects of caffeine and its main metabolites were reported almost 30 year ago when it was shown that caffeine, paraxanthine, theophylline, but not theobromine, were able to increase locomotor activation in mice.37 Also, in rats trained to discriminate caffeine from saline, both paraxanthine and theophylline, but not theobromine, were able to generalize to the caffeine-cue.38 Moreover, caffeine but not paraxanthine, was able to generalize to theophylline in rats trained to discriminate theophylline from saline.38 Therefore, those studies already suggested more similarities between caffeine and theophylline than with paraxanthine and even less with theobromine, which was consistently the least active methylxanthine. Paraxanthine has also less anxiogenic activity and toxicity in rodents than caffeine.39–41
In a recent study, we demonstrated that, in rats, paraxanthine produces a much stronger locomotor-activating effect than caffeine, theophylline or theobromine. The order of efficacies being paraxanthine >caffeine=theophylline >theobromine. Paraxanthine produced more than a 50% increase in locomotor activity compared to caffeine at the peak dose of both compounds (30 mg/kg, i.p.).42 In drug discrimination experiments, in rats trained to discriminate 30 mg/kg of caffeine, paraxanthine, unlike theophylline, generalized poorly to caffeine.42 Furthermore, with in vivo microdialysis experiments, we found that paraxanthine (30 mg/kg), but not caffeine (30 mg/kg) was able to significantly increase extracellular levels of dopamine in the lateral striatum for about 50% when compared with basal levels.42 By analyzing the differential counteracting effect of the four xanthines on the locomotor-depressant effects of A1 and A2A receptor agonists, we found evidence for a predominant A1 receptor antagonistic profile of not only caffeine, but also paraxanthine, theophylline and theobromine.42 However, although it has also been widely assumed that the main mechanism of action involved in the behavioral effects of paraxanthine, just like caffeine, is its antagonism of adenosine receptors, these two compounds have little differences in their affinities for A1 or A2A receptors,43 which suggests the existence of additional mechanisms to explain their pharmacological differences.
A dopaminergic component for paraxanthine was proposed based on results showing displacement for a low concentration of the labeled dopamine D1 receptor antagonist [3H]SCH-23390 in the rat striatum and the ability of SCH-23390 to partially counteract the motor activating properties of paraxanthine in reserpinized mice.44–45 However, the binding experiments with [3H]SCH-23390 could not be replicated by other authors (K.A. Jacobson, personal communication). In view of the existence of contradictory results, we re-evaluated the possible ability of paraxanthine to displace the selective D1 receptor antagonist [3H]SCH-23390 in striatal membrane preparations. Paraxanthine, and to a lesser extent caffeine, could displace the binding of [3H]SCH-23390, but only partially and at very high, nonpharmacological, concentrations.42 At 3 mM, paraxanthine displaced 27% and caffeine displaced 16% of the specific binding.42 This indicated that the effects of paraxanthine on locomotion could not be mediated by dopamine D1 receptors.
Paraxanthine and NO-cGMP Signaling
We then discovered a role of NO-cGMP signaling in the locomotor activating effects of paraxanthine. First, the NOS inhibitor L-NAME was able to decrease the locomotor activating-effects of paraxanthine, but not the locomotor activating effects of caffeine. Interestingly, after L-NAME administration, the levels of locomotor activity produced by paraxanthine (30 mg/kg) were comparable to those of caffeine (30 mg/kg).42 These results appear to be in contradiction with published results regarding a counteracting effect of L-NAME on locomotor activity induced by caffeine in mice.46 Apart from the difference in species (mice vs. rats), these discrepancies might involve other paradigm variables, such as different doses used for instance. On the other hand, L-arginine (NOS substrate) has been found to potentiate caffeine-induced locomotion in mice, and this effect was counteracted by L-NAME.47 In any case, in our study, we observe a very clear differential effect of L-NAME, indicating at the least that NO-cGMP signaling potentially plays a more relevant role in the locomotor activating effects of paraxanthine than of caffeine.
We then investigated if the NO-cGMP signaling-dependent mechanism involved in the locomotor activating effects of paraxanthine could be due to a selective inhibition of cGMP-preferring PDEs. Several studies had already shown that when comparing the psychostimulant effects of methylxanthines, relatively higher concentrations (100–1000 μM) of caffeine are required to inhibit cAMP-preferring PDE activity than those concentrations required for the interaction with adenosine receptors (10–100 μM).48–50 However, although this property of caffeine has been known for decades, the ability of caffeine and its main metabolites to modify cGMP-preferring PDE activity had not been investigated. We found that the cGMP-preferring PDE (PDE9) inhibitor BAY 73-6691, potentiated locomotor activation induced by caffeine (Fig. 2) but not by paraxanthine locomotor.42 Our behavioral experiments with L-NAME and BAY 73-6691 suggested that activation of NO-cGMP pathway could potentiate the locomotor activating effects induced by blockade of adenosine receptors. Activation of NO-cGMP pathway would then underlie the stronger locomotor-activating of paraxanthine compared to caffeine. In fact, we found that BAY 73-6691 potentiated the locomotor activation induced by the A1 receptor antagonist CPT, but not by the A2A receptor antagonist KW-6002.42 Finally, ex vivo experiments confirmed the ability of paraxanthine, but not caffeine, to induce a significant increase in the striatal concentration of cGMP.42
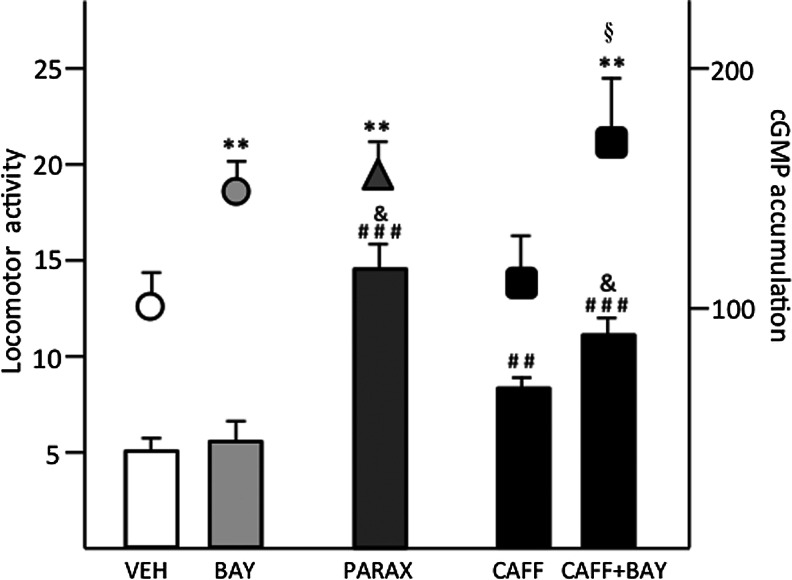
FIG. 2.
Effect of paraxanthine (PARAX) (30 mg/kg, i.p.), caffeine (CAFF) (30 mg/kg, i.p.) and the PDE9 inhibitor BAY 73-6691 (BAY) (3 mg/kg, i.p.) on locomotor activation and striatal cGMP accumulation. Paraxanthine produces stronger locomotor activation than caffeine and the same cGMP accumulation than BAY. Bars represent locomotor activity (mean±SEM) of the 10-min period transformed values during the first 60 min of recording. ## and ###p<0.01 and p<0.001 compared to vehicle, respectively; &p<0.05 compared to CAFF; analysis of variance (ANOVA) with Neuwman-Keuls post hoc test. Symbols represent cGMP accumulation (mean±SEM) from striatal homogenates after 30 min of systemic administration of compounds expressed in % of the vehicle (VEH). **p<0.01 compared to vehicle; §p<0.05 compared to CAFF; ANOVA with Neuwman-Keuls post hoc test.
We have previously shown in experiments with in vivo microdialysis that the A1 receptor-mediated locomotor-activating effects of caffeine correlate with dopamine release in the striatum, particularly in ventral compartments.29,51,52 This effect, however, is milder when compared to classical psychostimulants, which produce a large increase in the extracellular striatal concentrations of dopamine.53 In our recent study, we show that paraxanthine is more efficient than caffeine at increasing the extracellular striatal concentration of dopamine. Paraxanthine increased dopamine in a lateral striatal compartment, where caffeine was ineffective.42 Importantly, L-NAME, at a dose that was ineffective on its own, completely counteracted the dopamine-releasing effect of paraxanthine,42 demonstrating the involvement of NO-cGMP signaling in this classical psychostimulant-like effect of paraxanthine. The interaction between both mechanisms of paraxanthine in the striatal spine module, which ends up with a prominent dopamine release, could take place in the same element, either glutamatergic or dopaminergic terminals or both (Fig. 1). Experiments are in progress to determine these possibilities. In the meantime, we favor the glutamatergic terminals as the main target, in view of the evidence for a substantial role of glutamate in striatal NO-induced dopamine release.22
Final Remarks and Future Directions
When considering the classical psychostimulants-like pharmacological profile of paraxanthine, a series of questions arise. Does paraxanthine have reinforcing properties? Does it contribute to the reinforcing properties of caffeine? To answer these questions we should first know if after caffeine consumption, paraxanthine reaches the CNS at sufficient concentrations to elicit its psychostimulants effects. At least two factors support this possibility. First, that paraxanthine is the main metabolite of caffeine in humans (see Introduction) and, second, that the plasma levels of caffeine metabolites are known to increase with its chronic consumption, due to induction of caffeine metabolization. It has been shown that paraxanthine metabolism is dose-dependent, which results in nonlinear accumulation of paraxanthine in the body.54 Thus, with repetitive caffeine consumption, steady-state plasma paraxanthine levels reach about two thirds those of caffeine.54 In the experimental animal, during chronic caffeine consumption, the metabolites can even surpass caffeine plasma levels.55 Definitively, more clinical research is needed to undoubtedly demonstrate a role of paraxanthine in the psychostimulants effects of caffeine. Factors that alter metabolism of caffeine by CYP1A2 will have to be taken into account. CYP1A2 plays an important role in the metabolism of several clinically used drugs and it accounts for approximately 13% of the total content of P450 enzymes in the human liver.56 Importantly, CYP1A2 activity shows a pronounced intra- and inter-individual variability, which depends on genetic factors (gene polymorphisms) and environmental, such as exposure to smoking, several drugs, and dietary factors.56 Interestingly, the salivary or plasma ratio of paraxanthine to caffeine is commonly used as a valid marker of CYP1A2 activity.56 Finally, one more question is if paraxanthine could be used as a substitutive for classical psychostimulants, such as cocaine or amphetamine. Further preclinical studies with cGMP-preferring PDE inhibitors alone and in combination with A1R antagonists could also provide a new therapeutic approach for drug addiction or other basal ganglia disorders.
Acknowledgments
Work supported by NIDA IRP funds.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Palmer RM. Ferrige AG. Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;27:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 2.Snyder SH. Ferris CD. Novel neurotransmitters and their neuropsychiatric relevance. Am J Psychiatry. 2000;157:1738–1751. doi: 10.1176/appi.ajp.157.11.1738. [DOI] [PubMed] [Google Scholar]
- 3.Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou L. Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 2009;20:223–230. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Sattler R. Xiong Z. Lu WY. Hafner M. MacDonald JF. Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- 6.Vincent SR. Nitric oxide neurons and neurotransmission. Prog Neurobiol. 2010;90:246–255. doi: 10.1016/j.pneurobio.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Ferré S. Role of the central ascending neurotransmitter systems in the psychostimulant effects of caffeine. J Alzheimers Dis. 2010;(Suppl 1):S35–S49. doi: 10.3233/JAD-2010-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiss JP. Vizi ES. Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends Neurosci. 2001;24:211–215. doi: 10.1016/s0166-2236(00)01745-8. [DOI] [PubMed] [Google Scholar]
- 9.Martin E. Berka V. Tsai AL. Murad F. Soluble guanylyl cyclase: the nitric oxide receptor. Methods Enzymol. 2005;396:478–492. doi: 10.1016/S0076-6879(05)96040-0. [DOI] [PubMed] [Google Scholar]
- 10.Sunahara RK. Beuve A. Tesmer JJ. Sprang SR. Garbers DL. Gilman AG. Exchange of substrate and inhibitor specificities between adenylyl and guanylyl cyclases. J Biol Chem. 1998;273:16332–16338. doi: 10.1074/jbc.273.26.16332. [DOI] [PubMed] [Google Scholar]
- 11.Domek-Lopacinska K. Strosznajder JB. Cyclic GMP metabolism and its role in brain physiology. J Physiol Pharmacol. 2005;56(Suppl 2):15–34. [PubMed] [Google Scholar]
- 12.Van Staveren WC. Steinbusch HW. Markerink-Van Ittersum M. Repaske DR. Goy MF. Kotera J. Omori K. Beavo JA. De Vente J. mRNA expression patterns of the cGMP-hydrolyzing phosphodiesterases types 2, 5, and 9 during development of the rat brain. J Comp Neurol. 2003;467:566–580. doi: 10.1002/cne.10955. [DOI] [PubMed] [Google Scholar]
- 13.Ferré S. Agnati LF. Ciruela F. Lluis C. Woods AS. Fuxe K. Franco R. Neurotransmitter receptor heteromers and their integrative role in “local modules”: the striatal spine module. Brain Res Rev. 2007;55:55–67. doi: 10.1016/j.brainresrev.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferré S. Ciruela F. Woods AS. Lluis C. Franco R. Functional relevance of neurotransmitter receptor heteromers in the central nervous system. Trends Neurosci. 2007;30:440–446. doi: 10.1016/j.tins.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Ferré S. Goldberg SR. Lluis C. Franco R. Looking for the role of cannabinoid receptor heteromers in striatal function. Neuropharmacology. 2009;56(Suppl 1):226–234. doi: 10.1016/j.neuropharm.2008.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yung KK. Smith AD. Levey AI. Bolam JP. Synaptic connections between spiny neurons of the direct and indirect pathways in the neostriatum of the rat: evidence from dopamine receptor and neuropeptide immunostaining. Eur J Neurosci. 1996;8:861–869. doi: 10.1111/j.1460-9568.1996.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 17.Kawaguchi Y. Wilson CJ. Augood SJ. Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- 18.Kubota Y. Kawaguchi Y. Dependence of GABAergic synaptic areas on the interneuron type and target size. J Neurosci. 2000;20:375–386. doi: 10.1523/JNEUROSCI.20-01-00375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koos T. Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- 20.Smith AD. Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- 21.Sesack SR. Aoki C. Pickel VM. Ultrastructural localization of D2 receptor- like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West AR. Galloway MP. Grace AA. Regulation of striatal dopamine neurotransmission by nitric oxide: effector pathways and signaling mechanisms. Synapse. 2002;44:227–245. doi: 10.1002/syn.10076. [DOI] [PubMed] [Google Scholar]
- 23.Halassa MM. Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciruela F. Casadó V. Rodrigues RJ. Luján R. Burgueño J. Canals M. Borycz J. Rebola N. Goldberg SR. Mallol J. Cortés A. Canela EI. López-Giménez JF. Milligan G. Lluis C. Cunha RA. Ferré S. Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quiroz C. Luján R. Uchigashima M. Simoes AP. Lerner TN. Borycz J. Kachroo A. Canas PM. Orru M. Schwarzschild MA. Rosin DL. Kreitzer AC. Cunha RA. Watanabe M. Ferré S. Key modulatory role of presynaptic adenosine A2A receptors in cortical neurotransmission to the striatal direct pathway. Sci World J. 2009;9:1321–1344. doi: 10.1100/tsw.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orru M. Bakešová J. Brugarolas M. Quiroz C. Beaumont V. Goldberg SR. Lluís C. Cortés A. Franco R. Casadó V. Canela EI. Ferré S. Striatal pre- and postsynaptic profile of adenosine A(2A) receptor antagonists. PLoS One. 2011;6:e16088. doi: 10.1371/journal.pone.0016088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borycz J. Pereira MF. Melani A. Rodrigues RJ. Köfalvi A. Panlilio L. Pedata F. Goldberg SR. Cunha RA. Ferré S. Differential glutamate-dependent and glutamate-independent adenosine A1 receptor-mediated modulation of dopamine release in different striatal compartments. J Neurochem. 2007;101:355–363. doi: 10.1111/j.1471-4159.2006.04386.x. [DOI] [PubMed] [Google Scholar]
- 28.Ferré S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008;105:1067–79. doi: 10.1111/j.1471-4159.2007.05196.x. [DOI] [PubMed] [Google Scholar]
- 29.Solinas M. Ferré S. You ZB. Karcz-Kubicha M. Popoli P. Goldberg SR. Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J Neurosci. 2002;22:6321–6324. doi: 10.1523/JNEUROSCI.22-15-06321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karcz-Kubicha M. Antoniou K. Terasmaa A. Quarta D. Solinas M. Justinova Z. Pezzola A. Reggio R. Müller CE. Fuxe K. Goldberg SR. Popoli P. Ferré S. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology. 2003;28:1281–1291. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- 31.Antoniou K. Papadopoulou-Daifoti Z. Hyphantis T. Papathanasiou G. Bekris E. Marselos M. Panlilio L. Müller CE. Goldberg SR. Ferré S. A detailed behavioral analysis of the acute motor effects of caffeine in the rat: involvement of adenosine A1 and A2A receptors. Psychopharmacology. 2005;183:154–162. doi: 10.1007/s00213-005-0173-6. [DOI] [PubMed] [Google Scholar]
- 32.Solinas M. Ferré S. Antoniou K. Quarta D. Justinova Z. Hockemeyer J. Pappas LA. Segal PN. Wertheim C. Müller CE. Goldberg SR. Involvement of adenosine A1 receptors in the discriminative-stimulus effects of caffeine in rats. Psychopharmacology. 2005;179:576–586. doi: 10.1007/s00213-004-2081-6. [DOI] [PubMed] [Google Scholar]
- 33.Azdad K. Gall D. Woods AS. Ledent C. Ferré S. Schiffmann SN. Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology. 2009;34:972–986. doi: 10.1038/npp.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnaud MJ. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. Handb Exp Pharmacol. 2011;200:33–91. doi: 10.1007/978-3-642-13443-2_3. [DOI] [PubMed] [Google Scholar]
- 35.Berthou F. Guillois B. Riche C. Dreano Y. Jacqz-Aigrain E. Beaune PH. Interspecies variations in caffeine metabolism related to cytochrome P4501A enzymes. Xenobiotica. 1992;22:71–80. doi: 10.3109/00498259209053129. [DOI] [PubMed] [Google Scholar]
- 36.Lelo A. Kjellen G. Birkett DJ. Miners JO. Paraxanthine metabolism in humans: determination of metabolic partial clearances and effects of allopurinol and cimetidine. J Pharmacol Exp Ther. 1989;248:315–319. [PubMed] [Google Scholar]
- 37.Seale TW. Johnson P. Carney JM. Rennert OM. Interstrain variation in acute toxic response to caffeine among inbred mice. Pharmacol Biochem Behav. 1984;20:567–573. doi: 10.1016/0091-3057(84)90306-x. [DOI] [PubMed] [Google Scholar]
- 38.Carney JM. Seale TW. Logan L. McMaster SB. Sensitivity of inbred mice to methylxanthines is not determined by plasma xanthine concentration. Neu- rosci Lett. 1985;56:27–31. doi: 10.1016/0304-3940(85)90435-5. [DOI] [PubMed] [Google Scholar]
- 39.Stavric B. Methylxanthines: toxicity to humans. 3. Theobromine, para- xanthine and the combined effects of methylxanthines. Food Chem Toxicol. 1988;26:725–733. doi: 10.1016/0278-6915(88)90073-7. [DOI] [PubMed] [Google Scholar]
- 40.Benowitz NL. Jacob P., 3rd Mayan H. Denaro C. Sympathomimetic effects of paraxanthine and caffeine in humans. Clin Pharmacol Ther. 1995;58:684–691. doi: 10.1016/0009-9236(95)90025-X. [DOI] [PubMed] [Google Scholar]
- 41.Okuro M. Fujiki N. Kotorii N. Ishimaru Y. Sokoloff P. Nishino S. Effects of paraxanthine and caffeine on sleep, locomotor activity, and body temperature in orexin/ataxin-3 transgenic narcoleptic mice. Sleep. 2010;33:930–942. doi: 10.1093/sleep/33.7.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orrú M. Guitart X. Karcz-Kubicha M. Solinas M. Justinova Z. Barodia SK. Zanoveli J. Cortes A. Lluis C. Casado V. Moeller FG. Ferré S. Psychostimulant pharmacological profile of paraxanthine, the main metabolite of caffeine in humans. Neuropharmacology. 2012;67C:476–484. doi: 10.1016/j.neuropharm.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snyder SH. Katims JJ. Annau Z. Bruns RF. Daly JW. Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci USA. 1981;78:3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferré S. Herrera-Marschitz M. Grabowska-Andén M. Ungerstedt U. Casas M. Andén N-E. Postsynaptic dopamine/adenosine interaction: II. Post- synaptic dopamine agonism and adenosine antagonism of methylxanthines in short-term reserpinized mice. Eur J Pharmacol. 1991;192:36–42. doi: 10.1016/0014-2999(91)90065-x. [DOI] [PubMed] [Google Scholar]
- 45.Ferré S. Guix T. Sallés J. Badia A. Parra P. Jané F. Herrera-Marschitz M. Ungerstedt U. Casas M. Paraxanthine displaces the binding of [3H]SCH 23390 from rat striatal membranes. Eur J Pharmacol. 1990;179:295–299. doi: 10.1016/0014-2999(90)90168-6. [DOI] [PubMed] [Google Scholar]
- 46.Kayir H. Uzbay IT. Evidence for the role of nitric oxide in caffeine-induced locomotor activity in mice. Psychopharmacology. 2004;172:11–15. doi: 10.1007/s00213-003-1625-5. [DOI] [PubMed] [Google Scholar]
- 47.Kimura M. Ushijima I. Hiraki M. Kimura M. Ono N. Enhancement of caffeine-induced locomotor hyperactivity produced by the combination with L-arginine or taurine in mice: possible involvement of nitric oxide. Methods Find Exp Clin Pharmacol. 2009;31:585–589. doi: 10.1358/mf.2009.31.9.1435462. [DOI] [PubMed] [Google Scholar]
- 48.Sutherland EW. Rall TW. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem. 1958;232:1077–1091. [PubMed] [Google Scholar]
- 49.Butcher RW. Sutherland EW. Adenosine 3′,5′-phosphate in biological materials. I. Purification and properties of cyclic 30,50-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3′,5′-phosphate in human urine. J Biol Chem. 1962;237:1244–1250. [PubMed] [Google Scholar]
- 50.Francis SH. Blount MA. Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev. 2011;91:651–690. doi: 10.1152/physrev.00030.2010. [DOI] [PubMed] [Google Scholar]
- 51.Quarta D. Ferré S. Solinas M. You ZB. Hockemeyer J. Popoli P. Goldberg SR. Opposite modulatory roles for adenosine A1 and A2A receptors on glutamate and dopamine release in the shell of the nucleus accumbens. Effects of chronic caffeine exposure. J Neurochem. 2004;88:1151–1158. doi: 10.1046/j.1471-4159.2003.02245.x. [DOI] [PubMed] [Google Scholar]
- 52.Quarta D. Borycz J. Solinas M. Patkar K. Hockemeyer J. Ciruela F. Lluis C. Franco R. Woods AS. Goldberg SR. Ferré S. Adenosine receptor-mediated modulation of dopamine release in the nucleus accumbens depends on glutamate neurotransmission and N-methyl-D-aspartate receptor stimulation. J Neurochem. 2004;91:873–880. doi: 10.1111/j.1471-4159.2004.02761.x. [DOI] [PubMed] [Google Scholar]
- 53.Di Chiara G. Bassareo V. Fenu S. De Luca MA. Spina L. Cadoni C. Acquas E. Carboni E. Valentini V. Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 54.Denaro CP. Brown CR. Wilson M. Jacob P., 3rd Benowitz NL. Dose-dependency of caffeine metabolism with repeated dosing. Clin Pharmacol Ther. 1990;48:277–285. doi: 10.1038/clpt.1990.150. [DOI] [PubMed] [Google Scholar]
- 55.Gasior M. Jaszyna M. Munzar P. Witkin JM. Goldberg SR. Caffeine potentiates the discriminative-stimulus effects of nicotine in rats. Psychopharmacology. 2002;162:385–395. doi: 10.1007/s00213-002-1113-3. [DOI] [PubMed] [Google Scholar]
- 56.Faber MS. Jetter A. Fuhr U. Assessment of CYP1A2 activity in clinical practice: why, how, and when? Basic Clin Pharmacol Toxicol. 2005;97:125–134. doi: 10.1111/j.1742-7843.2005.pto_973160.x. [DOI] [PubMed] [Google Scholar]