Abstract
The denatonium cation, as a benzoate salt, is the most bitter cation known to modern society and is frequently added to consumer products to reduce accidental and intentional consumption by humans and animals. Denatonium can enter the environment by accidental discharges, potentially rendering water supplies undrinkable. Interactions of denatonium with soil components (i.e., smectite minerals) ultimately control the environmental fate of denatonium, but the current literature is devoid of studies that evaluate denatonium sorption to smectite minerals. This study investigated the mechanism and kinetics of denatonium sorption to smectite clay minerals as a function of smectite type, temperature, pH and ionic strength. Uptake by synthetic mica montmorillonite (Syn-1), Wyoming montmorillonite (SWy-2), and Texas montmorillonite (STx-1b) at 305K was rapid, with equilibrium being reached within 2 min for all clays. Complete removal of denatonium was observed for STx-1b at pH 6.9, while partial removal was observed for Syn-1 and SWy-2. Kinetic behavior of SWy-2 and Syn-1 is consistent with a pseudo–second-order model at 305K. An activation energy of +25.9 kJ/mol was obtained for sorption to Syn-1 and was independent of temperature between 286K and 338K. Activation-free energy (ΔG*), activation enthalpy (ΔH*), and activation entropy (ΔS*) for Syn-1 were found to be +62.91 kJ/mol, +23.36 kJ/mol, and −0.130 kJ/(K·mol), respectively. Sorption capacities at pH 3.6, 6.9, and 8.2 were constant at 1.3×10−2 g denatonium/g clay; however, the kinetic rate constant increased by 56%, going from acidic to basic solution conditions. Distribution coefficients were negatively correlated with ionic strength, suggesting cation exchange. Collectively, results suggested that smectite minerals can serve as efficient sinks for denatonium cations. This is much-needed information for agencies developing regulations regarding denatonium usage and for water treatment professionals who may ultimately have to treat denatonium-impacted water supplies.
Key words: drinking-water quality, groundwater quality, sorption, water chemistry
Introduction
Denatonium benzoate (DB) is a water-soluble quaternary ammonium salt (4.5 g/100 mL), containing denatonium, the most bitter cation known to modern society (Fig. 1) (Kaukeinen and Buckle, 1992). DB is a white crystalline solid, melts between 163°C and 171°C, produces nearly neutral solutions (pH 6.5–7.5), and has an oral median lethal dose (rat) of 612 mg/kg. At present, the nontoxic compound is used as a chemical denaturant and is voluntarily added to a number of industrial and consumer products to discourage or prevent ingestion of potentially fatal doses of toxic substances (Damon and Pettitt, 1980). For example, manufacturers have routinely added 6 ppm DB to rubbing alcohol since the early 1970s (Kaukeinen and Buckle, 1992). Many pesticide formulations contain the compound, but the intended outcome is preventing consumption by animals (Payne, 1988). Since the mid-1990s, Oregon and California have mandated the addition of DB to products such as automobile antifreeze containing >10% (v/v) ethylene glycol (Henderson et al., 1998).
FIG. 1.
Structure of denatonium benzoate with the quaternary ammonium denatonium cation and the benzoate anion.
Automobile antifreeze can be ingested by animals (Doty et al., 2006) and children (White et al., 2009) alike because of its attractive color and sweet taste. Given the toxicity of antifreeze, federal lawmakers have proposed legislation mandating that DB be added to all formulations of antifreeze sold in the United States (U.S. House, 2006). Although well-intentioned, any legislation mandating that a substance be added to consumer products must be informed by a discussion of potentially negative implications. In this case, a possible ramification of widespread use of DB in antifreeze is contamination of drinking-water supplies. Generally, an environmental assessment of chemical fate and transport is determined by the speciation, phase (i.e., solid, liquid, or gas), and interaction(s) of the material with environmental matrices (air, soil, and ground water). Since concerns exist about DB contaminating ground water, the starting point in any discussion must include a study of DB–soil solution interactions (Gorman-Lewis and Fein, 2004; Hari et al., 2005).
Smectite minerals are ubiquitous constituents of soil in many areas of the United States and are known to strongly influence the transport properties of organic cations because of large mineral surface areas, high cation exchange capacities, and variable interlayer spacings (Brownawell et al., 1990). Table 1 shows selected properties of the clay minerals chosen for this study. The sorption of organic cations to smectite surfaces has been previously studied, and cation sorption was inferred based on an inverse dependence of Kd on the ionic strength of the supporting electrolyte (Wang et al., 2009). In the case of a large, permanently charged cation such as denatonium, we postulate that sorption to smectite minerals occurs via attractive interactions with negatively charged sorption sites within the interlayer space, occuring through an ion-exchange process whereby the weakly hydrated denatonium cation replaces hydrated, charge-balancing inorganic cations in the smectite interlayer space.
Table 1.
Selected Physical Properties for Each Clay
| Clay type | CEC (mEq/100 g) | SSA (m2/g) |
|---|---|---|
| Syn-1 | 140 | 133.66±0.72 |
| STx-1b | 84.4 | 83.79±0.22 |
| SWy-2 | 76.4 | 31.82±0.22 |
Data were obtained from Olphen and Fripiat (1997).
Syn-1, synthetic mica montmorillonite; SWy-2, Wyoming montmorillonite; STx-1b, Texas montmorillonite.
Previous work has clearly established the inherent ability for clay minerals to impact chemical mobility in the subsurface environment; thus, it should not be surprising that concerns exist about DB escaping into the environment. Accordingly, the objectives of this initial contribution are to (1) evaluate the kinetics of denatonium sorption to three smectite clay minerals, (2) determine the sorption capacity, and (3) characterize the activation parameters of sorption. To the best of our knowledge, this contribution represents the first published study of denatonium sorption to smectite minerals.
Experimental
Materials
DB was obtained from MP Biomedicals and used without further purification. The mineral sorbents are Wyoming montmorillonite (SWy-2), Texas montmorillonite (STx-1b), and synthetic montmorillonite (Syn-1). All minerals were obtained from the Clay Minerals Society and used as received to better represent natural materials. For the analyses, high-performance liquid chromatography (HPLC)–grade acetonitrile (ACN) and phosphoric acid were purchased from Fisher Scientific.
Sorption kinetics
Kinetic experiments utilized a 200 ppm initial concentration of DB in 10 mM CaCl2. Each suspension contained 125 mg clay/30 mL solution in 50-mL polypropylene conical centrifuge tubes. Before each kinetic experiment, clay and background electrolyte were vortexed for at least 15 s to disperse the clay. Following vortexing, DB was added to the tubes and rotated end-over-end at 25 rpm in a hybridization oven (Boekel Scientific, Big SHOT III, Model 230402) at the set temperature. The experiments were completed using times ranging from 15 s to 60 min. Supernatants were recovered after centrifuging for 30 min at 4150 g. The impact of centrifuging versus filtering on the concentrations of DB after 15 s was evaluated and showed that although the solution was in contact with the solid-phase sorbent for a time far in excess of the lowest experimental sorption time, the concentration differences were negligible (Wang et al., 2012).
Detection of aqueous denatonium concentrations were determined in 2-mL aliquots of supernatant via HPLC (Perkin Elmer, Series 275 HRes UV/VIS Liquid Chromatography System with autosampler) equipped with a C-18 analytical column (LiChrospher, 100 μm×4.6 μm, 5 μm). The mobile phase consisted of ACN and Milli-Q water adjusted to pH 2.2 with H3PO4. The column flow rate was 1.0 mL/min with a total analysis time of 59 min per sample. The mobile-phase composition was initially held constant at 70% water (solvent A) from 0 to 8 min, linearly ramped to 60% water from 8 to 14 min, held constant at 60% water from 14 to 23 min, linearly ramped to 30% water from 23 to 28 min, held constant at 30% water from 28 to 43 min, and finally, linearly ramped back to 70% water. The retention time of DB was 15.5 min in standards, but varied from 14.9 to 15.8 min in supernatant solutions, suggesting that dissolved solutes from the clay mineral were present.
Denatonium was detected via absorption at 205 nm with 600 nm as the reference wavelength. Linear calibration curves (R2=0.999) were consistently obtained up to 100 ppm DB in 0.01 M CaCl2. Control and blank experiments were run for each denatonium solution. Control samples consisted of DB solutions without clay minerals, and experiment blanks were composed of clay suspensions without DB. At the lowest denatonium concentration, no changes in the aqueous concentration were detected in controls. Thus, aqueous DB concentrations were determined by calculating the difference between the initial and final concentrations.
Results
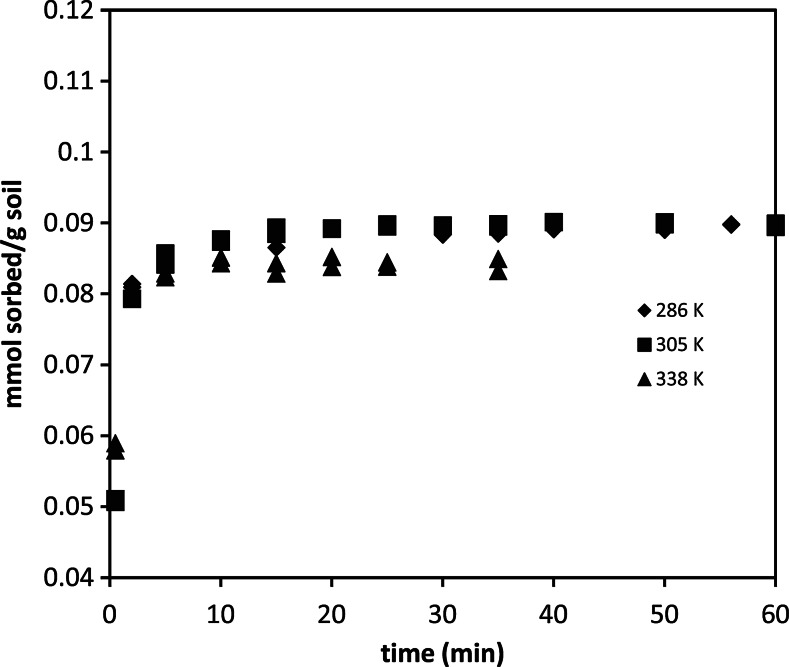
The sorption kinetics for SWy-2 and Syn-1 clays studied is shown in Fig. 2; data from STx-1 are not shown since complete removal occurred in less than a minute. At a 200 ppm initial concentration, equilibrium is reached for each clay in <30 s. Additionally, for each clay, except the synthetic montmorillonite (Syn-1), 100% removal of denatonium is achieved. For the synthetic clay, an average removal of 75% was achieved. Short-term, single-cycle desorption experiments for each clay (data not shown) were performed and were shown to be completely hysteretic on the time scale of the experiment. To gain insight into potential sorption mechanisms and to evaluate the Arrhenius parameters, Syn-1 kinetic profiles were evaluated at multiple temperatures as shown in Fig. 3. The sorption rate is positively correlated with temperature.
FIG. 2.
Kinetic profile of denatonium sorption onto Wyoming montmorillonite (SWy-2) and synthetic mica montmorillonite (Syn-1) at pH 6.9 and 305K.
FIG. 3.
Effect of temperature on the kinetic profile of denatonium sorption onto synthetic mica at pH 6.9.
Discussion
Kinetic analyses
Smectites were chosen as DB sorbents because of their ubiquity and because previous studies of organic cation sorption in soil systems established that smectite minerals are effective sorbents. An important consideration in evaluating the mineral sorption potential of any chemical must necessarily include some discussion of the capacity of the soil phase to concentrate the substance under equilibrium conditions. Accordingly, our kinetic data were interrogated using different models to estimate sorption capacities, calculate the kinetic rate constant, and to gain insight into potential sorption mechanisms. Our team evaluated the Lagergren and Ho-McKay models. Lagergren's linearized model is given by Eq. (1):
 |
(1) |
where qe represents the equilibrium sorbed concentration (mmol DB/g soil), qt the sorbed concentration (mmol DB/g soil) at time t (min), k1 the pseudo-first order rate constant (min−1), and t time (min). If a plot of ln (qe−qt) versus t yielded a straight line, one could be reasonably conclude that kinetics obeyed the Lagergren model. An essential constraint for successfully using the model is the equilibrium sorption capacity, qe, which is determined by allowing sorption experiments to run for extended time periods until no change in the aqueous concentration is observed. In our case, the sorption process was rapid (<2 min) for all clays and conditions studied, so qe could be reasonably estimated. When applied to our experimental results, the data (not shown) could not be satisfactorily modeled (r2<0.01).
Ho and McKay's model for data displaying pseudo-second kinetics behavior is given by Eq. (2):
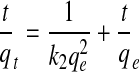 |
(2) |
where qe represents the equilibrium sorbed concentration (mmol DB/g soil), qt the sorbed concentration (mmol DB/g soil) at time t (min), k2 the pseudo-second-order rate constant (g clay/mmol DB-min), and t time (min). The results, as shown in Fig. 4, suggested that the sorption kinetics for Syn-1 and SWy-2 studied (under all conditions) could be adequately modeled using the pseudo–second-order model (r2=0.9991) which, in turn, allowed for an estimation of qe and k2. The values for rate constants, sorption capacities, and corresponding regression coefficients are provided in Table 2.
FIG. 4.
Pseudo–second-order kinetic model of denatonium sorption to Syn-1 and SWy-2 clay minerals at pH 6.9 and 305K.
Table 2.
Sorption Capacities and Rate Constants for Synthetic Mica and Wyoming Montmorillonite Clays
| Clay type | T (°C) | qe (g solute/g clay) | qs (g solute/m2 clay) | Rate constant (g clay/g solute) | R2 |
|---|---|---|---|---|---|
| Syn-1 | 13 | 1.31×10−2 | 9.77×10−5 | 1.90×102 | 0.9993 |
| 32 | 1.31×10−2 | 9.81×10−5 | 2.65×102 | 0.9991 | |
| 65 | 1.27×10−2 | 9.50×10−5 | 1.29×103 | 0.9992 | |
| SWy-2 | 32 | 1.57×10−2 | 4.94×10−4 | 4.67×102 | 0.9997 |
Effect of temperature
The magnitude of the sorption activation energy can be used to ascertain whether the rate of the solute sorption process is limited by the time it takes solute molecules to diffuse to the surface site or if sorption is limited by the energy barrier to sorption (Dogan et al., 2006). Ideally, the activation energy of a reaction is constant over a given temperature range with the kinetic rate constant expected to exhibit Arrhenius behavior according to the linearized form of Arrhenius' relationship as given below:
 |
(3) |
where k represents a kinetic rate constant (a pseudo-second-order constant in our case), A is the frequency factor, Ea is the activation energy in kJ/mol, and R is the molar gas constant in kJ/(mol·K). Essentially, Ea represents the amount of energy a mole of solute particles must attain for sorption to occur. The activation energy can be calculated using Equation (3) after determining the rate constant at multiple temperatures (Fig. 5), and plotting ln k versus 1/T as shown in Fig. 6. Hasan et al. (2008) found the activation energy associated with reactive blue dye sorption to crosslinked chitosan/oil palm ash composite beads to be 12.9 kJ/mol. In a study of a cationic dye onto sepiolite clay, the activation energy was determined to be +39.3 kJ/mol (Rodríguez et al., 2010), while for maxilon blue GRL sorption onto sepiolite clay, Ea was +33.96 kJ/mol (Dogan et al., 2006). When methylene blue sorption to montmorillonite clay was studied, a kinetic potential barrier of +28.5 kJ/mol was obtained (Almeida et al., 2009); however, Ea was +49.1 kJ/mol for removing a PCB from contaminated wastewater by fly ash sorption (Nollet et al., 2003). Generally, when activation energies fall between 5 and 40 kJ/mol, physisorption is inferred (Dogan et al., 2006). However, when sorption activation energies are 40–800 kJ/mol, chemisorption is posited. In our study, an activation energy of +25.9 kJ/mol was calculated from which, we hypothesized that sorption proceeded via physisorption in agreement with others' work (Dogan et al., 2006; Gürses et al., 2006; Rodríguez et al., 2010), with denatonium sorbing to the clay surface in a manner similar to other hydrophobic quaternary cations (Jaynes and Boyd, 1991). Experimental support for a physisorptive or cation exchange mechanism was provided via triplicate single-point sorption experiments as a function of background electrolyte ionic strength. Kd (L/g) increased by 76% to 1.26 L/g from 0.30 L/g as the ionic strength decreased from 733 mM to 2.66 mM. An abundance of literature exists supporting the contention that ion exchange processes are strongly influenced by ionic strength (Sparks, 1995; Wang et al., 2009; Yang et al., 2009). Consequently, we conclude that denatonium sorbs to the smectite surfaces in a cation exchange process, where inorganic cations are replaced by denatonium ions.
FIG. 5.
Effect of temperature on pseudo–second-order model kinetics of denatonium sorption to Syn-1 at pH 6.9.
FIG. 6.
Plot of kinetic rate constant calculated using the pseudo–second-order kinetic model as a function of 1/T.
To further investigate kinetics and thermodynamics of sorption, thermodynamic activation parameters (ΔH*, and ΔS*) were calculated using the Eyring equation (Chowdhury and Saha, 2011):
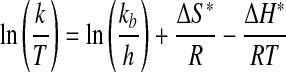 |
(4) |
where k is the second-order rate constant, kb is the Boltzmann's constant, and T is the absolute temperature. The activation Gibbs energy, ΔG*, was calculated from Equation (5):
 |
(5) |
Equation (4) shows that ln (k/T) versus (1/T) plots will be linear when the enthalpy of activation is independent of temperature. Accordingly, ΔS* and ΔH* were determined to be −0.130 kJ/(K·mol) and +23.36 kJ/mol, respectively. ΔG* (305K) was calculated as +62.91 kJ/mol. The negative ΔS* indicates that the reaction proceeds through a transition state less entropic than the reactants and suggests an associative process. Thermodynamically speaking, the result implies that denatonium cations are less mobile after binding occurs, which one would expect if intercalation occurred. The positive ΔH* signifies that sorption is endothermic, which is plausible since the reaction was not barrierless; that ΔH* and Ea are similar in magnitude also points toward a condensed-phase reaction as known from the transition state theory. From the magnitude and sign of ΔG*, we conclude that sorption is not spontaneous at 305K. It can be further concluded that the process is enthalpy driven (Chowdhury and Saha, 2011).
Denatonium is a permanent-charge ion lacking any ionizable functional groups. As such, 100% of dissolved denatonium is expected to be present as a cation. Consequently absent of any significant changes in surface charge density, the sorption capacity should be independent of pH. However, for permanently charged clays, the surface potential should decrease with pH (Kraepiel et al., 1998). Data presented in Table 3 for Syn-1 suggest a sorption capacity that is independent of pH. Although the specific origin of this contradictory behavior is unknown for our quaternary ammonium system, similar results have been observed in absorption studies of different quaternary ammonium cations to two heterogeneous solids—a high-organic content aquifer soil and an EPA reference soil (Brownawell et al., 1990)—over a similar pH range. The question of why the sorption capacity is independent of pH remains an open question, however. As can be further seen in Table 3, decreasing the hydrogen ion concentration results in a 56% net increase in the reaction rate at the most alkaline pH value. This suggests that the hydrogen ion concentration plays a role in the sorption process although the role is yet to be elucidated.
Table 3.
Impact of pH on Sorption Rate (k2) and Sorption Capacity (qe)
| pH | k2 (g sorbent/mmol DB/min) | qe (g solute/g sorbent) |
|---|---|---|
| 3.6 | (3.51±0.18)×102 | (1.31±1.0)×10−2 |
| 6.9 | (2.65±0.29)×102 | (1.31±2.0)×10−2 |
| 8.2 | (5.49±0.38)×102 | (1.28±1.0)×10−2 |
Conclusions
The sorption kinetics of denatonium to three source clays was studied under a variety of conditions and each sorbent was shown to effectively remove DB. We demonstrated that when DB is added in concentrations in excess of those proposed for industrial applications, rapid removal occurs at near neutral pH values for three commercially available clay minerals. Equilibrium is reached in all cases in less than 2 min. Sorption kinetics were best described with a pseudo-second-order kinetic model with surface area normalized sorption capacities for Syn-1 and SWy-2 calculated as 9.81×105 and 4.94×104 g/m2, respectively. An associative, physisorptive process was inferred and reaction kinetics was impacted by clay identity, temperature to small degree, and pH.
Our study suggests that under the conditions tested, DB would not likely present a problem if directly released to soils rich in smectites. Additionally, the results suggest that DB in waterways could be efficiently removed by treating impacted water with a high surface area clay mineral such as those utilized here or, perhaps, some other high surface area sorbent. The major implication of the latter observation is that water treatment facilities may be able to properly treat impacted waters, thus mitigating calls to the treatment facilities about poor water quality concerns.
Acknowledgments
This research was funded, in part, by the United States Geological Survey, State Water Resources Research Institute Program of Ohio through grant 2010OH169B. Department of Chemistry and Biochemistry and University of Dayton Research Institute provided support for this work through faculty start-up funds provided to G.S.C.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- Almeida C.A.P. Debacher N.A. Downs A.J. Cottet L. Mello C.A.D. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J. Colloid Interface Sci. 2009;332:46. doi: 10.1016/j.jcis.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Brownawell B.J. Chen H. Collier J.M. Westall J.C. Adsorption of organic cations to natural materials. Environ. Sci. Technol. 1990;24:1234. [Google Scholar]
- Chowdhury S. Saha P. Adsorption thermodynamics and kinetics of malachite green onto Ca(OH)2-treated fly ash. J. Environ. Eng.-ASCE. 2011;137:388. [Google Scholar]
- Damon C.E. Pettitt B.C. High-performance liquid-chromatographic determination of denatonium benzoate in rapeseed oil. J. Chromatogr. 1980;195:243. [Google Scholar]
- Dogan M. Alkan M. Demirbas Ö. Özdemir Y. Özmetin C. Adsorption kinetics of maxilon blue GRL onto sepiolite from aqueous solutions. Chem. Eng. J. 2006;124:89. [Google Scholar]
- Doty R.L. Dziewit J.A. Marshall D.A. Antifreeze ingestion by dogs and rats: Influence of stimulus concentration. Can. Vet. J.-Rev. Vet. Can. 2006;47:363. [PMC free article] [PubMed] [Google Scholar]
- Gorman-Lewis D.J. Fein J.B. Experimental study of the adsorption of an ionic liquid onto bacterial and mineral surfaces. Environ. Sci. Technol. 2004;38:2491. doi: 10.1021/es0350841. [DOI] [PubMed] [Google Scholar]
- Gürses A. Doğar C. Yalçın M. Bayrak R. Karaca S. The adsorption kinetics of the cationic dye, methylene blue, onto clay. J. Hazard. Mater. 2006;131:217. doi: 10.1016/j.jhazmat.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Hari A.C. Paruchuri R.A. Sabatini D.A. Kibbey T.C.G. Effects of pH and cationic and nonionic surfactants on the adsorption of pharmaceuticals to a natural aquifer material. Environ. Sci. Technol. 2005;39:2592. doi: 10.1021/es048992m. [DOI] [PubMed] [Google Scholar]
- Hasan M. Ahmad A.L. Hameed B.H. Adsorption of reactive dye onto cross-linked chitosan/oil palm ash composite beads. Chem. Eng. J. 2008;136:164. [Google Scholar]
- Henderson M.C. Neumann C.M. Buhler D.R. Analysis of denatonium benzoate in oregon consumer products by HPLC. Chemosphere. 1998;36:203. doi: 10.1016/s0045-6535(97)10033-9. [DOI] [PubMed] [Google Scholar]
- Jaynes W.F. Boyd S.A. Hydrophobicity of siloxane surfaces in smectites as revealed by aromatic hydrocarbon adsorption from water. Clay Clay Min. 1991;39:428. [Google Scholar]
- Kaukeinen D.E. Buckle A.P. Evaluations of aversive agents to increase the selectivity of rodenticides with emphasis on denatonium benzoate (Bitrex) bittering agent. Fifteenth Vertebrate Pest Conference: Proceedings. Vertebrate Pest Conference; Davis, CA. 1992. p. 192. [Google Scholar]
- Kraepiel A.M.L. Keller K. Morel F.M.M. On the acid–base chemistry of permanently charged minerals. Environ. Sci. Technol. 1998;33:516. [Google Scholar]
- Nollet H. Roels M. Lutgen P. Van der Meeren P. Verstraete W. Removal of PCBs from wastewater using fly ash. Chemosphere. 2003;53:655. doi: 10.1016/S0045-6535(03)00517-4. [DOI] [PubMed] [Google Scholar]
- Payne H.A.S. Bitrex–—A solution to safety. Chem. Ind. 1988;21:721. [Google Scholar]
- Rodríguez A. Ovejero G. Mestanza M.A. García J. Removal of dyes from wastewaters by adsorption on sepiolite and pansil. Ind. Eng. Chem. Res. 2010;49:3207. [Google Scholar]
- Sparks D.L. Environmental Soil Chemistry. San Diego, CA: Academic Press; 1995. [Google Scholar]
- U.S. House Committee on Energy and Commerce. Washington, DC: Government Printing Office; 2006. H.R. 2567, The Antifreeze Bittering Act of 2005 Hearing, 23 May 2006. [Google Scholar]
- Wang C. Ding Y. Teppen B.J. Boyd S.A. Song C. Li H. Role of interlayer hydration in lincomycin sorption by smectite clays. Environ. Sci. Technol. 2009;43:6171. doi: 10.1021/es900760m. [DOI] [PubMed] [Google Scholar]
- Wang C. Liu J. Zhang Z. Wang B. Sun H. Adsorption of Cd(II), Ni(II), and Zn(II) by tourmaline at acidic conditions: kinetics, thermodynamics, and mechanisms. Ind. Eng. Chem. Res. 2012;51:4397. [Google Scholar]
- White N.C. Litovitz T. Benson B.E. Horowitz B.Z. Marr-Lyon L. White M.K. The impact of bittering agents on pediatric ingestions of antifreeze. Clin. Pediatr. 2009;48:913. doi: 10.1177/0009922809339522. [DOI] [PubMed] [Google Scholar]
- Yang S. Li J. Lu Y. Chen Y. Wang X. Sorption of Ni(II) on GMZ bentonite: Effects of pH, ionic strength, foreign ions, humic acid and temperature. Appl. Radiat. Isot. 2009;67:1600. doi: 10.1016/j.apradiso.2009.03.118. [DOI] [PubMed] [Google Scholar]