Abstract
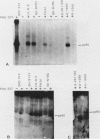
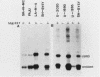
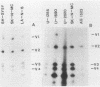
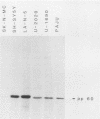
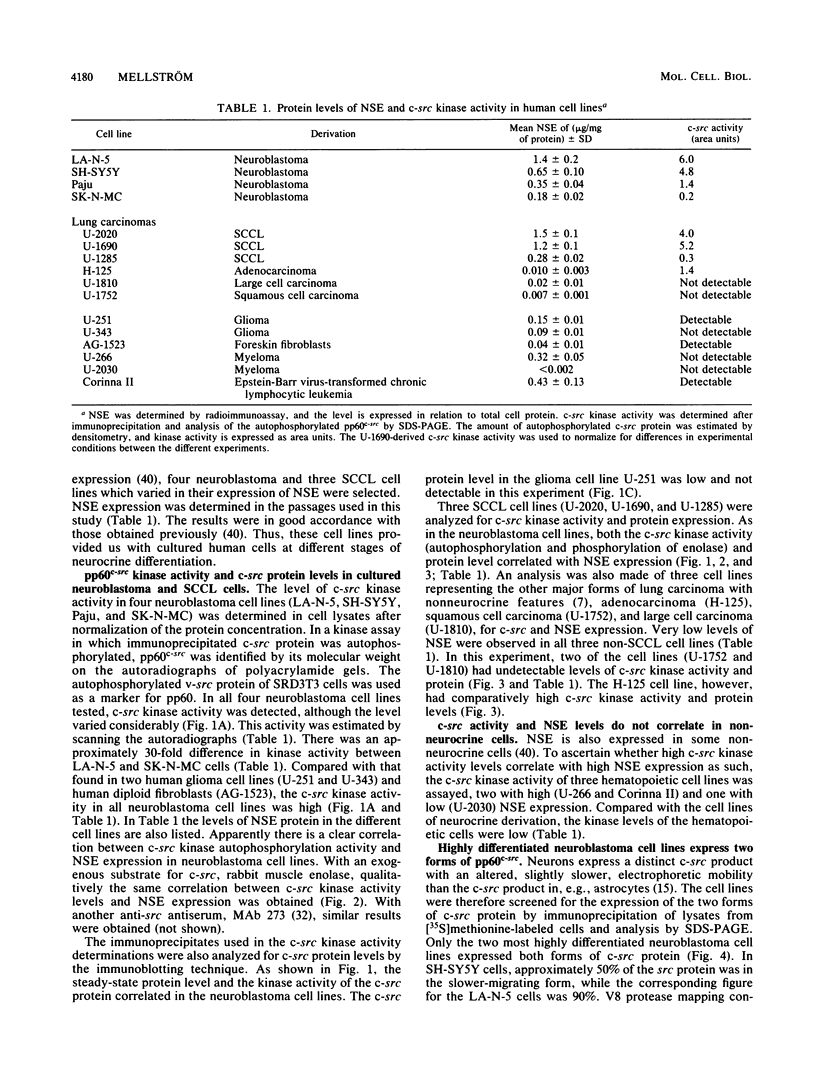
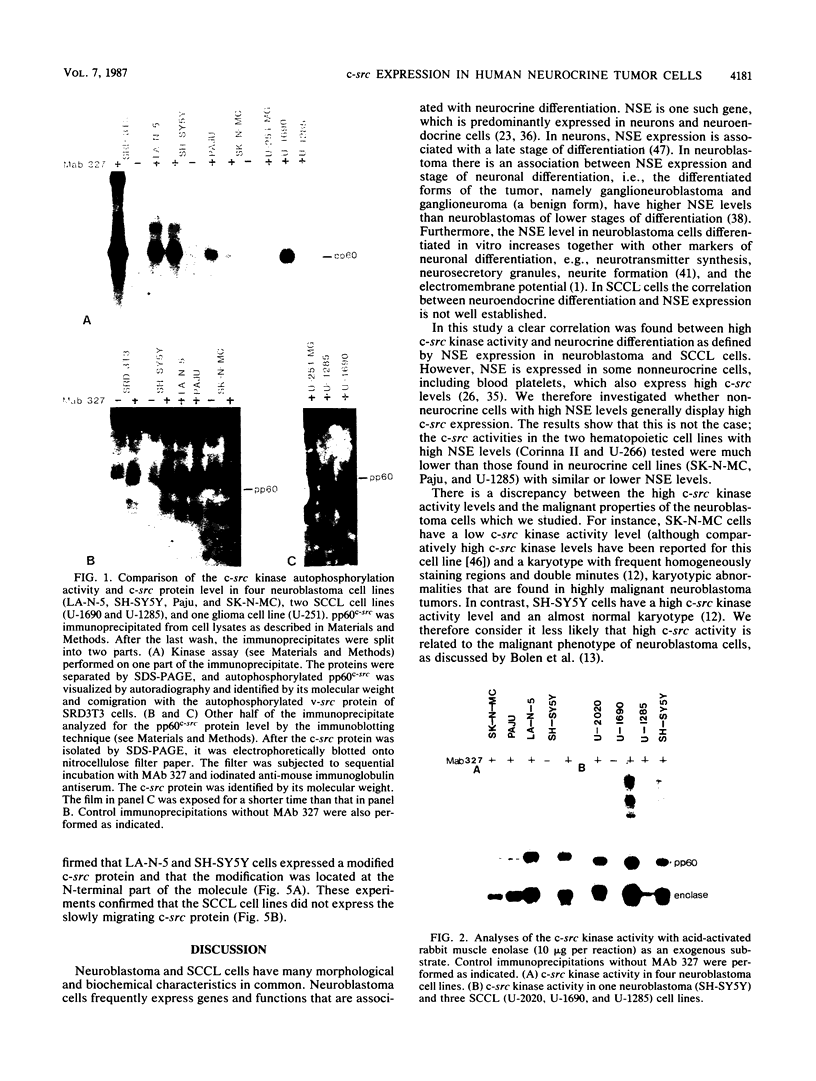
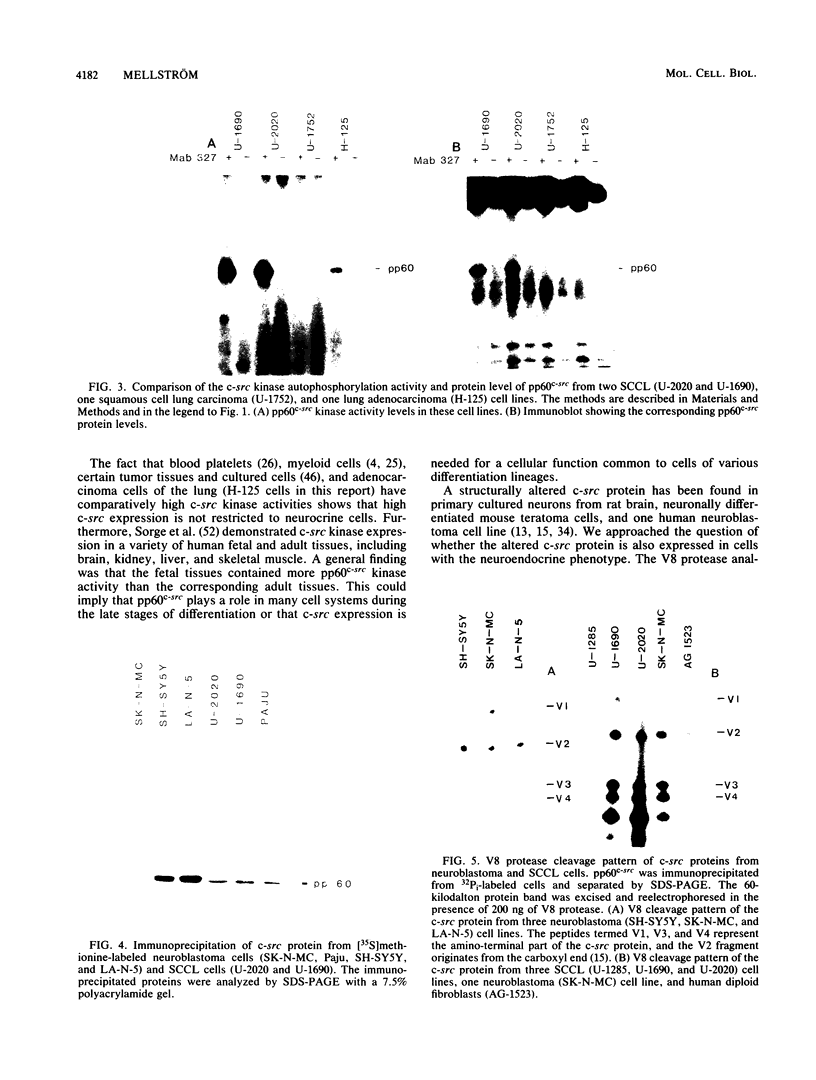
Human cell lines with neuronal and neuroendocrine features were examined for their expression of pp60c-src, the cellular homolog of the transforming gene product pp60v-src of Rous sarcoma virus. Four neuroblastoma (LA-N-5, SH-SY5Y, Paju, and SK-N-MC) and three small-cell lung carcinoma (U-2020, U-1690, and U-1285) cell lines were selected on the basis of their stage of neurocrine differentiation, as determined by the expression of neuron-specific enolase. In an immune complex protein kinase assay, all seven cell lines displayed c-src kinase activity which was considerably higher than that found in nonneurocrine cells (human diploid fibroblasts, glioma, and non-small cell lung carcinoma cell lines). Furthermore, the c-src kinase activity, as determined by autophosphorylation or phosphorylation of an exogenous substrate, enolase, correlated with the stage of neurocrine differentiation. There was an approximately 30-fold difference in c-src kinase autophosphorylation activity between the cell lines representing the highest and lowest stages of neurocrine differentiation. A similar variation was found in the steady-state levels of the c-src protein of these cell lines. Highly differentiated neuroblastoma cells expressed two forms of the src protein. Digestion by Staphylococcus aureus V8 protease did reveal structural diversity in the amino-terminal ends of these c-src molecules. In summary, we found a clear correlation between c-src kinase activity and the stage of neuronal and neuroendocrine differentiation. Thus, the phenotypic similarity between neurons and neuroendocrine cells includes high c-src expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alema S., Tato F., Boettiger D. myc and src oncogenes have complementary effects on cell proliferation and expression of specific extracellular matrix components in definitive chondroblasts. Mol Cell Biol. 1985 Mar;5(3):538–544. doi: 10.1128/mcb.5.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemà S., Casalbore P., Agostini E., Tatò F. Differentiation of PC12 phaeochromocytoma cells induced by v-src oncogene. Nature. 1985 Aug 8;316(6028):557–559. doi: 10.1038/316557a0. [DOI] [PubMed] [Google Scholar]
- Barnekow A., Gessler M. Activation of the pp60c-src kinase during differentiation of monomyelocytic cells in vitro. EMBO J. 1986 Apr;5(4):701–705. doi: 10.1002/j.1460-2075.1986.tb04270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh J., Larsson E., Zech L., Nilsson K. Establishment and characterization of two neoplastic cell lines (U-1285 and U-1568) derived from small cell carcinoma of the lung. Acta Pathol Microbiol Immunol Scand A. 1982 May;90(3):149–158. doi: 10.1111/j.1699-0463.1982.tb00076_90a.x. [DOI] [PubMed] [Google Scholar]
- Bergh J., Nilsson K., Dahl D., Andersson L., Virtanen I., Lehto V. P. Expression of intermediate filaments in established human lung cancer cell lines. An indicator of differentiation and derivation. Lab Invest. 1984 Sep;51(3):307–316. [PubMed] [Google Scholar]
- Bergh J., Nilsson K., Ekman R., Giovanella B. Establishment and characterization of cell lines from human small cell and large cell carcinomas of the lung. Acta Pathol Microbiol Immunol Scand A. 1985 May;93(3):133–147. doi: 10.1111/j.1699-0463.1985.tb03932.x. [DOI] [PubMed] [Google Scholar]
- Bergh J., Nilsson K., Zech L., Giovanella B. Establishment and characterization of a continuous lung squamous cell carcinoma cell line (U-1752). Anticancer Res. 1981;1(6):317–322. [PubMed] [Google Scholar]
- Biedler J. L., Helson L., Spengler B. A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973 Nov;33(11):2643–2652. [PubMed] [Google Scholar]
- Biedler J. L., Roffler-Tarlov S., Schachner M., Freedman L. S. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978 Nov;38(11 Pt 1):3751–3757. [PubMed] [Google Scholar]
- Bolen J. B., Rosen N., Israel M. A. Increased pp60c-src tyrosyl kinase activity in human neuroblastomas is associated with amino-terminal tyrosine phosphorylation of the src gene product. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7275–7279. doi: 10.1073/pnas.82.21.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Cotton P. C., Queral A. E., Barrett J. N., Nonner D., Keane R. W. Neurones express high levels of a structurally modified, activated form of pp60c-src. Nature. 1985 Aug 8;316(6028):554–557. doi: 10.1038/316554a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Purchio A. F., Brugge J. S., Erikson R. L. A normal cell protein similar in structure and function to the avian sarcoma virus transforming gene product. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3159–3163. doi: 10.1073/pnas.76.7.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Esch F. S., Taylor S. S., Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984 Jun 25;259(12):7835–7841. [PubMed] [Google Scholar]
- Cotton P. C., Brugge J. S. Neural tissues express high levels of the cellular src gene product pp60c-src. Mol Cell Biol. 1983 Jun;3(6):1157–1162. doi: 10.1128/mcb.3.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. The complex of polyoma virus middle-T antigen and pp60c-src. EMBO J. 1984 Mar;3(3):585–591. doi: 10.1002/j.1460-2075.1984.tb01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher L., Rider C. C., Taylor C. B. Enolase isoenzymes. III. Chromatographic and immunological characteristics of rat brain enolase. Biochim Biophys Acta. 1976 Nov 8;452(1):245–252. doi: 10.1016/0005-2744(76)90077-2. [DOI] [PubMed] [Google Scholar]
- Gee C. E., Griffin J., Sastre L., Miller L. J., Springer T. A., Piwnica-Worms H., Roberts T. M. Differentiation of myeloid cells is accompanied by increased levels of pp60c-src protein and kinase activity. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5131–5135. doi: 10.1073/pnas.83.14.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A., Nemeth S. P., Brugge J. S. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Feb;83(4):852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba H., Cross F. R., Garber E. A., Hanafusa H. Low level of cellular protein phosphorylation by nontransforming overproduced p60c-src. Mol Cell Biol. 1985 May;5(5):1058–1066. doi: 10.1128/mcb.5.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba H., Takeya T., Cross F. R., Hanafusa T., Hanafusa H. Rous sarcoma virus variants that carry the cellular src gene instead of the viral src gene cannot transform chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4424–4428. doi: 10.1073/pnas.81.14.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits E. B., Majors J. E., Varmus H. E. Hormonal regulation of the Rous sarcoma virus src gene via a heterologous promoter defines a threshold dose for cellular transformation. Cell. 1984 Oct;38(3):757–765. doi: 10.1016/0092-8674(84)90271-x. [DOI] [PubMed] [Google Scholar]
- Jernberg H., Björklund G., Nilsson K. Establishment of a new human myeloma cell line (U-2030) and selection of a hat-sensitive subline. Int J Cancer. 1987 Jun 15;39(6):745–751. doi: 10.1002/ijc.2910390615. [DOI] [PubMed] [Google Scholar]
- Johnson P. J., Coussens P. M., Danko A. V., Shalloway D. Overexpressed pp60c-src can induce focus formation without complete transformation of NIH 3T3 cells. Mol Cell Biol. 1985 May;5(5):1073–1083. doi: 10.1128/mcb.5.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S. A., Brugge J. S., Levine J. M. Induction of altered c-src product during neural differentiation of embryonal carcinoma cells. Science. 1986 Nov 14;234(4778):873–876. doi: 10.1126/science.3095923. [DOI] [PubMed] [Google Scholar]
- Marangos P. J., Campbell I. C., Schmechel D. E., Murphy D. L., Goodwin F. K. Blood platelets contain a neuron-specific enolase subunit. J Neurochem. 1980 May;34(5):1254–1258. doi: 10.1111/j.1471-4159.1980.tb09967.x. [DOI] [PubMed] [Google Scholar]
- Marangos P. J., Schmechel D., Parma A. M., Clark R. L., Goodwin F. K. Measurement of neuron-specific (NSE) and non-neuronal (NNE) isoenzymes of enolase in rat, monkey and human nervous tissue. J Neurochem. 1979 Jul;33(1):319–329. doi: 10.1111/j.1471-4159.1979.tb11735.x. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Bennich H., Johansson S. G., Pontén J. Established immunoglobulin producing myeloma (IgE) and lymphoblastoid (IgG) cell lines from an IgE myeloma patient. Clin Exp Immunol. 1970 Oct;7(4):477–489. [PMC free article] [PubMed] [Google Scholar]
- Odelstad L., Påhlman S., Nilsson K., Larsson E., Läckgren G., Johansson K. E., Hjertén S., Grotte G. Neuron-specific enolase in relation to differentiation in human neuroblastoma. Brain Res. 1981 Nov 9;224(1):69–82. doi: 10.1016/0006-8993(81)91117-3. [DOI] [PubMed] [Google Scholar]
- Parker R. C., Varmus H. E., Bishop J. M. Expression of v-src and chicken c-src in rat cells demonstrates qualitative differences between pp60v-src and pp60c-src. Cell. 1984 May;37(1):131–139. doi: 10.1016/0092-8674(84)90308-8. [DOI] [PubMed] [Google Scholar]
- Pontén J., Westermark B. Properties of human malignant glioma cells in vitro. Med Biol. 1978 Aug;56(4):184–193. [PubMed] [Google Scholar]
- Påhlman S., Esscher T., Bergvall P., Odelstad L. Purification and characterization of human neuron-specific enolase: radioimmunoassay development. Tumour Biol. 1984;5(2):127–139. [PubMed] [Google Scholar]
- Påhlman S., Esscher T., Nilsson K. Expression of gamma-subunit of enolase, neuron-specific enolase, in human non-neuroendocrine tumors and derived cell lines. Lab Invest. 1986 May;54(5):554–560. [PubMed] [Google Scholar]
- Påhlman S., Odelstad L., Larsson E., Grotte G., Nilsson K. Phenotypic changes of human neuroblastoma cells in culture induced by 12-O-tetradecanoyl-phorbol-13-acetate. Int J Cancer. 1981 Nov 15;28(5):583–589. doi: 10.1002/ijc.2910280509. [DOI] [PubMed] [Google Scholar]
- Pösö H., Karvonen E., Suomalainen H., Andersson L. C. A human neuroblastoma cell line with an altered ornithine decarboxylase. J Biol Chem. 1984 Oct 25;259(20):12307–12310. [PubMed] [Google Scholar]
- Rosen N., Bolen J. B., Schwartz A. M., Cohen P., DeSeau V., Israel M. A. Analysis of pp60c-src protein kinase activity in human tumor cell lines and tissues. J Biol Chem. 1986 Oct 15;261(29):13754–13759. [PubMed] [Google Scholar]
- Schmechel D. E., Brightman M. W., Marangos P. J. Neurons switch from non-neuronal enolase to neuron-specific enolase during differentiation. Brain Res. 1980 May 19;190(1):195–214. doi: 10.1016/0006-8993(80)91169-5. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Sejersen T., Björklund H., Sümegi J., Ringertz N. R. N-myc and c-src genes are differentially regulated in PCC7 embryonal carcinoma cells undergoing neuronal differentiation. J Cell Physiol. 1986 May;127(2):274–280. doi: 10.1002/jcp.1041270213. [DOI] [PubMed] [Google Scholar]
- Sidell N., Altman A., Haussler M. R., Seeger R. C. Effects of retinoic acid (RA) on the growth and phenotypic expression of several human neuroblastoma cell lines. Exp Cell Res. 1983 Oct;148(1):21–30. doi: 10.1016/0014-4827(83)90184-2. [DOI] [PubMed] [Google Scholar]
- Sorge J. P., Sorge L. K., Maness P. F. pp60c-src is expressed in human fetal and adult brain. Am J Pathol. 1985 Apr;119(1):151–157. [PMC free article] [PubMed] [Google Scholar]
- Sorge L. K., Levy B. T., Maness P. F. pp60c-src is developmentally regulated in the neural retina. Cell. 1984 Feb;36(2):249–257. doi: 10.1016/0092-8674(84)90218-6. [DOI] [PubMed] [Google Scholar]
- Tapia F. J., Polak J. M., Barbosa A. J., Bloom S. R., Marangos P. J., Dermody C., Pearse A. G. Neuron-specific enolase is produced by neuroendocrine tumours. Lancet. 1981 Apr 11;1(8224):808–811. doi: 10.1016/s0140-6736(81)92682-9. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]