Abstract
Background:
A healthy lifestyle following a cancer diagnosis may improve long-term outcomes. No studies have examined health behaviour change among UK cancer survivors, or tracked behaviours over time in survivors and controls. We assessed smoking, alcohol and physical activity at three times (0–2 years before a cancer diagnosis, 0–2 years post-diagnosis and 2–4 years post-diagnosis) and at matched times in a comparison group.
Methods:
Data were from waves 1–5 of the English Longitudinal Study of Ageing; a cohort of older adults in England. Behavioural measures were taken at each wave. Generalised estimating equations were used to examine differences by group and time, and group-by-time interactions.
Results:
Of the 5146 adults included in the analyses, 433 (8.4%) were diagnosed with cancer. Those with a cancer diagnosis were less likely to be physically active (P<0.01) and more likely to be sedentary (P<0.001). There were no group differences in alcohol or smoking. Smoking, alcohol and activity reduced over time in the whole group. Group-by-time interactions were not significant for smoking (P=0.17), alcohol (P=0.20), activity (P=0.17) or sedentary behaviour (P=0.86), although there were trends towards a transient improvement from pre-diagnosis to immediately post-diagnosis.
Conclusion:
We found little evidence that a cancer diagnosis motivates health-protective changes. Given the importance of healthy lifestyles, strategies for effective support for behaviour change in cancer survivors need to be identified.
Keywords: cancer survivors, smoking, alcohol, physical activity, longitudinal, teachable moment
Advances in early detection and treatment of cancer have led to steady increases in survival (Jemal et al, 2008). However, there is growing evidence that cancer survivors are at greater risk than the general population of a range of conditions including cardiovascular disease, diabetes and osteoporosis as well as second primary cancers (Brown et al, 1993; Travis et al, 2006). These conditions are all linked with behaviours such as smoking, diet and physical activity (Hu et al, 2001; Mokdad and Ford, 2003; US Department of Health and Human Services, 2004; Warburton et al, 2006); stimulating interest in promoting healthier lifestyles to improve long-term outcomes. Trials have shown that interventions are effective in achieving behaviour change in cancer survivors (Demark-Wahnefried et al, 2007; Morey et al, 2009).
Evidence from some early surveys and qualitative studies has indicated that some cancer survivors make positive lifestyle changes following diagnosis, including increasing physical activity (Hounshell et al, 2001; Blanchard et al, 2003; Patterson et al, 2003) and eating more healthily (Hounshell et al, 2001; Maskarinec et al, 2001; Maunsell et al, 2002; Salminen et al, 2002; Patterson et al, 2003), although interpretation of these results is limited by small sample sizes, the possibility of retrospective bias, and the absence of a control group. However, the observation of health behaviour changes has led to the suggestion that a cancer diagnosis could be a ‘teachable moment', in which individuals are motivated to adopt risk-reducing health behaviours' (e.g., McBride et al, 2003).
In contrast to the qualitative studies, larger scale prevalence studies typically find that levels of smoking, alcohol consumption and physical activity in cancer survivors are similar to the general population (Bellizzi et al, 2005; Coups and Ostroff, 2005; Eakin et al, 2007; Blanchard et al, 2008; Courneya et al, 2008; Grimmett et al, 2009; Smith et al, 2011). However, it is possible that pre-diagnostic lifestyles are less healthy in the cancer samples, and therefore parity with the general population post-diagnosis constitutes improvement.
There have now been a number of longitudinal studies. Two studies have used data from the Health and Retirement Study (HRS), a population-based cohort of older adults in the United States. One analysed data only on smokers and found significantly higher odds of quitting in the 2 years after a cancer diagnosis compared with smokers without any serious diagnosis (Keenan, 2009). The other examined the full HRS sample and found a significantly greater reduction in smoking rates among those who had received a diagnosis of cancer within the previous 2 years (from 23.7% to 16.1%) than those without any new serious diagnosis (22.8 to 20.8%), but there was no significant group difference in alcohol intake, and a greater reduction in physical activity in the cancer group (Newsom et al, 2012a). However, because the comparison groups in these analyses were not only free of cancer, but also free of heart disease, diabetes, stroke or lung disease, it is difficult to determine the specific influence of a diagnosis of cancer.
In men from the Diet, Cancer and Health Study in Denmark (Karlsen et al, 2012), a cancer diagnosis was associated with significantly reduced tobacco consumption from pre- to post-cancer diagnosis (an average of 6 years), compared with the rest of the sample, although there were no differences in alcohol use or weight. Results from the same study for women with breast cancer showed no evidence of differential change in body mass index, tobacco or alcohol (Bidstrup et al, 2013). In a Canadian sample (Newsom et al, 2012b), a cancer diagnosis was associated with a greater reduction in smoking rates (from 17.2% to 13.5%) 2 years after a cancer diagnosis than in the healthy comparison group (23 to 21%), but no significant group differences in diet, alcohol or physical activity.
All previous studies have been limited to two time points. The primary aim of the present study was therefore to examine the effect of a cancer diagnosis on changes in health behaviours across three times (0–2 years before a cancer diagnosis, 0–2 years post-diagnosis and 2–4 years post-diagnosis) using data from a population-based sample in the United Kingdom.
Materials and methods
Design and participants
Data were from waves 1–5 of the English Longitudinal Study of Ageing (ELSA), which were collected biennially between 2002 and 2010. ELSA is a population-based cohort of adults aged ⩾50 years drawn from participants in the Health Survey for England (HSE) in 1998, 1999 or 2001. It is a ‘sister' study to the HRS and has a partly harmonised data collection protocol (Steptoe et al, 2012). The initial core sample size at wave 1 was 11 391, of whom an average of 47% have taken part in all biennial examinations.
The cancer survivor group comprised respondents who reported a new cancer diagnosis in waves 2, 3 or 4. A cancer diagnosis was defined as answering ‘yes' to the question: ‘Have you ever been told by a doctor or other health professional that you had cancer or any other kind of malignancy'. The first wave in which they responded ‘yes' became their ‘peri-diagnosis' point (T1), the previous wave was their pre-diagnosis point (T0), and the subsequent wave was their post-diagnosis point (T2). Individuals reporting a cancer diagnosis at wave 1 or a new diagnosis at wave 5 were excluded from the analysis because of the absence of pre- or post-diagnosis data, respectively. Data from waves 2, 3 and 4 were used as T0, T1 and T2, respectively, for the comparison group, which comprised all individuals who had not received a cancer diagnosis in any wave. This was favoured over a completely healthy control group as it enabled us to determine the specific influence of a cancer diagnosis independent of other chronic diseases. For both samples, we only included individuals with data available from three consecutive waves for at least one variable of interest.
Measures
Age and gender were included as control variables, with household non-pension wealth used as an indicator of socioeconomic status (SES), because it has been identified as particularly appropriate to this age group (Banks et al, 2003).
Smoking status was based on the answer to the question ‘Do you smoke cigarettes at all nowadays' (yes/no).
In wave 1, alcohol consumption was assessed with the question ‘In the past 12 months have you taken an alcoholic drink (twice a day or more/daily or almost daily/once or twice a week/once or twice a month/special occasions only/not at all)'. Those who responded ‘twice a day or more' or ‘daily or almost daily' were classified as daily alcohol drinkers. In waves 2 to 5, alcohol consumption was assessed with the question ‘On how many days out of the last seven did you have an alcoholic drink' with response options of one to seven. Those who responded ‘five', ‘six' or ‘seven' were classified as daily alcohol drinkers. For the present analyses, we compared daily alcohol drinkers with all other categories combined.
Physical activity and sedentary behaviour were assessed with three questions adapted from the Whitehall II study: ‘Do you take part in any sports that are (vigorous/moderately energetic/mildly energetic) with response options of (more than once a week/once a week/one to three times a month/hardly ever or never)' (Marmot et al, 1991). For the present analyses, participants were divided into those who did moderate or vigorous activity at least once a week (active) vs less than this (non-active), and as sedentary if they hardly ever or never participated in even mildly energetic activity.
Statistical analyses
Demographic characteristics of the two groups were compared using t-tests for continuous variables and χ2 for categorical variables. Generalised estimating equation (GEE) models were used to examine main effects of group (overall group differences in prevalence of smoking, daily alcohol consumption, physical activity and sedentary behaviour independent of time), main effects of time (changes in behaviours over time independent of group), and group-by-time interactions (differences in behaviour change over time between groups). Age, sex and wealth were entered as covariates for all analyses.
Results
The analysed sample (n=5146) comprising individuals with a new cancer diagnosis in waves 2–4 (n=433) and individuals with no cancer diagnosis in any wave (n=4173) and data on at least one health behaviour for three consecutive waves are shown in Table 1. The cancer diagnosis group was older (69.9 vs 66.5 years; P<0.001), and had a more equal gender balance than the comparison group, but the groups did not differ in wealth (P=0.935). The analysed sample was slightly younger, wealthier and had slightly better health behaviours (were less likely to smoke and were more active), than the full ELSA sample (data not shown).
Table 1. Demographic characteristics of sample – percentage (n), mean (s.d.).
| Characteristics | Cancer group (n=433) | Comparison group (n=4713) | P |
|---|---|---|---|
|
Age |
69.92 (9.67) |
66.52 (8.97) |
0.000 |
|
Sex | |||
| Male | 49.6% (207) | 43.7% (2061) | |
| Female |
50.4% (210) |
56.3% (2652) |
0.020 |
|
Wealth quintiles | |||
| 1 (lowest) | 17.9% (77) | 18.7% (862) | |
| 2 | 18.6% (80) | 18.6% (861) | |
| 3 | 22.6% (97) | 21.0% (969) | |
| 4 | 20.3% (87) | 20.1% (927) | |
| 5 (highest) | 20.5% (88) | 21.6% (998) | 0.935 |
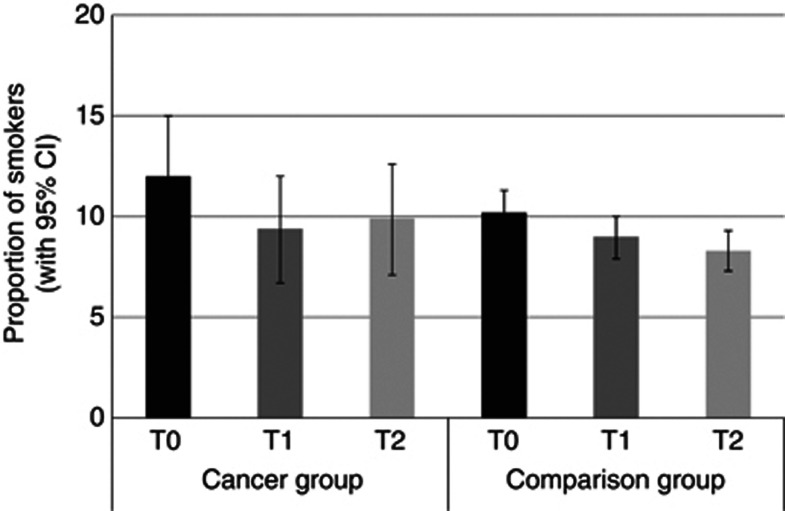
Smoking
Figure 1 shows the proportion of smokers in each group at each time. There was no significant group effect, indicating that the proportion of smokers was not significantly different overall between groups (P=0.34). There was a significant effect of time, with the proportion of smokers decreasing over time independent of group (P<0.001). Between T0 and T1, smoking rates dropped from 12.0% to 9.4% in the cancer group and 10.2% to 9.0% in the comparison group. Between T1 and T2, smoking rates increased from 9.4% to 9.9% in the cancer group and dropped from 9.0% to 8.3% in the comparison group. The group-by-time interaction was not statistically significant (P=0.17).
Figure 1.
The proportion of each group who smoked at each time point (adjusted for age, sex and wealth).
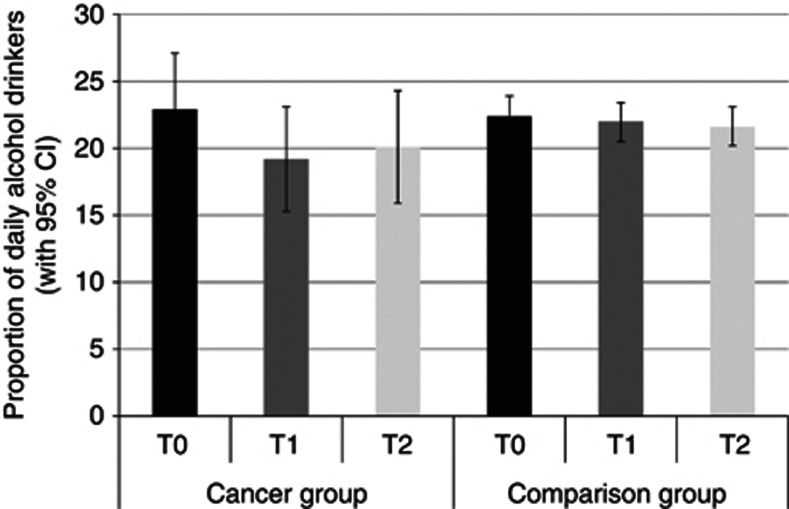
Daily alcohol consumption
Figure 2 shows the proportion of daily alcohol drinkers in each group at each time. The group difference was not statistically significant (P=0.49). There was a borderline significant effect of time, with the proportion of daily drinkers decreasing over time independent of group (P=0.06). Between T0 and T1, daily alcohol consumption dropped from 22.9% to 19.2% in the cancer group and from 22.4% to 22.0% in the comparison group. Between T1 and T2, daily alcohol consumption rose from 19.2% to 20.1% in the cancer group and dropped from 22.0% to 21.6% in the comparison group. However, the group-by-time interaction was not significant (P=0.20).
Figure 2.
The proportion of each group who drank alcohol daily at each time point (adjusted for age, sex and wealth).
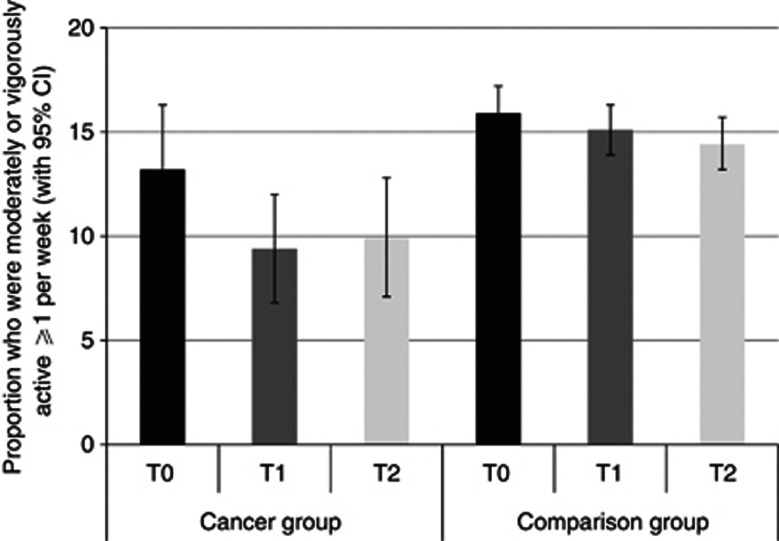
Physical activity
Figure 3 shows the results for being moderately or vigorously active at least once a week. The cancer group was less physically active than the comparison group overall (P<0.01). The proportion of respondents who were physically active reduced over time independent of group (P<0.05). The pattern of results suggested a greater change between T0 and T1 in the group getting a cancer diagnosis (13.2 to 9.4% vs 15.9 to 15.1% in the comparison group) while between T1 and T2, physical activity increased from 9.4% to 9.9% in the cancer group and dropped from 15.1% to 14.4% in the comparison group. However, the group-by-time interaction was not statistically significant (P=0.17).
Figure 3.
The proportion of each group who were moderately or vigorously active ≥1 per week at each time point (adjusted for age, sex and wealth).
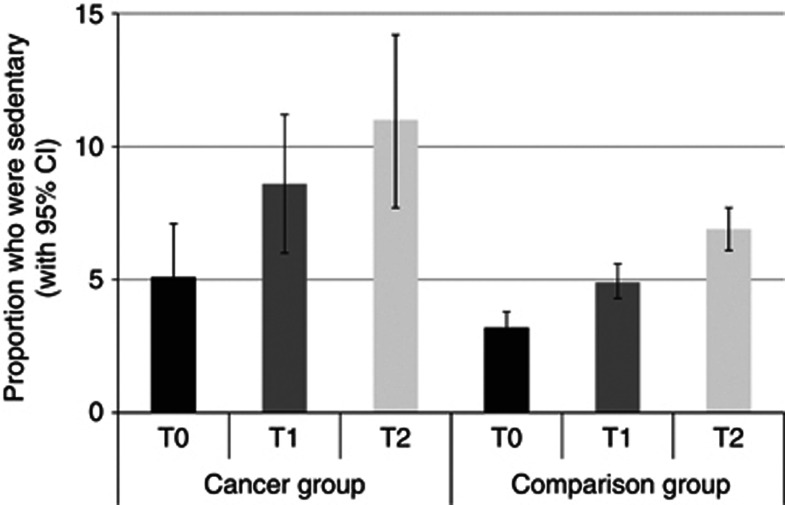
Sedentary behaviour
Figure 4 shows the results for being sedentary (no mild, moderate or vigorous activity). The cancer group was more sedentary than the comparison group independent of time (P<0.001), and the proportion of participants who were completely sedentary increased over time independent of group (P<0.001). Between T0 and T1, sedentary behaviour increased from 5.1% to 8.6% in the cancer group and 3.2% to 4.9% in the comparison group. Between T1 and T2, sedentary behaviour increased from 8.6% to 11.0% in cancer group and 4.9% to 6.9% in the comparison group. The group-by-time interaction was not statistically significant (P=0.86).
Figure 4.
The proportion of each group who were sedentary at each time point (adjusted for age, sex and wealth).
Pattern of changes in health behaviours
Although the planned analysis tested group-by-time interactions across the three waves and were all non-significant, the pattern of results suggested there may be non-linearity in the cancer group for smoking, alcohol and activity, with a trend towards slightly greater changes from T0 to T1 in the cancer group, followed by a small rebound from T1 to T2. We tested for this using a linear-by-linear association test and failed to confirm linearity for smoking, alcohol and activity in the cancer group. Because this could have affected the analyses over the three time points, we repeated the analyses to examine group-by-time interactions specifically for the T0 to T1 transition. These results showed consistent, near-significant, trends towards the cancer group making greater changes than the comparison group from T0 to T1: smoking (P=0.11), alcohol (P=0.07) and physical activity (P=0.07). Interactions over the T1 to T2 transition were not significant for smoking (P=0.14), alcohol (P=0.55) or activity (P=0.56).
Discussion
This longitudinal study investigated the impact of a cancer diagnosis on health behaviour change in a population-based sample of older adults living in England. In comparison with participants not receiving a cancer diagnosis, we saw no evidence that a cancer diagnosis was associated with sustained improvements in lifestyle from before diagnosis to at least 2 years after diagnosis.
In both groups, the overall pattern of health behaviour change over the 4-year period indicated beneficial trends for smoking and alcohol consumption (declining rates), and adverse trends on sedentary behaviour (increasing) and vigorous physical activity (declining). Across the three time points, there were no overall significant group differences in alcohol or smoking, but individuals who received a cancer diagnosis were less active and more sedentary overall.
Although a cancer diagnosis was not associated with significant differential change across the three time points in any of the behaviours, there was a difference in the pattern of the results, with approximately linear time trends in the comparison group, but non-linear time trends in the cancer group. A slightly larger proportion of participants receiving a cancer diagnosis had quit smoking and reduced their alcohol intake by the first assessment after diagnosis, but this was followed with a slight rebound by the next follow-up. The activity effect – albeit indicating adverse changes – showed a similar pattern. We therefore tested the effects separately for each time transition. From T0 to T1, the group-by-time interactions approached significance for all three health behaviours (P=0.07, 0.07, 0.11). The T1 to T2 difference did not approach statistical significance, but nonetheless diminished the health behaviour differences that had emerged immediately post-diagnosis. The consistency of this pattern across the three behaviours suggests that with a larger sample size, we might have seen significant ups and downs in health behaviours associated with a cancer diagnosis compared with steadier time trends in the comparison group.
Previous research has found evidence for higher rates of smoking cessation following a cancer diagnosis (Falba, 2005; Keenan, 2009; Karlsen et al, 2012; Newsom et al, 2012a). For two of the studies, this could be because the comparison group was not only free of a cancer diagnosis, but also free from heart disease, diabetes, stroke and lung disease, and these conditions could also contribute to the motivation to change (Keenan, 2009; Newsom et al, 2012a). All three of the other samples (from the United States, Canada and Denmark) had higher smoking rates, especially the Danish men, than were observed in ELSA. This may be partly because UK adults get excellent quitting advice, but it could also mean any remaining smokers among UK adults are a relatively ‘hard core' group (Jarvis, 2003). Either way, the observation that 75% of smokers with a cancer diagnosis failed to quit suggests that a cancer diagnosis is an underused opportunity for intervention. The need for assistance to quit smoking has been reported by patients following heart disease and may apply equally following a cancer diagnosis (Wilkes and Evans, 1999).
None of the previous studies, either longitudinal (Karlsen et al, 2012) or cross-sectional (Bellizzi et al, 2005; Coups and Ostroff, 2005; Eakin et al, 2007), have found evidence that a cancer diagnosis is associated with significant change in alcohol intake, and the overall effect in this study was also non-significant. However, the possibility of a transient acute effect was indicated by the post hoc analyses, and may have been missed in studies assessing alcohol consumption longer after the diagnosis.
The sample as a whole had low levels of activity, and those who received a cancer diagnosis were even less active; consistent with findings from cross-sectional studies (Grimmett et al, 2009; Smith et al, 2011). We did not see evidence of differential change in physical activity except for the non-significant trend for an acute worsening immediately post-diagnosis. This result gives no support to the idea that individuals who get a cancer diagnosis make positive activity changes either immediately after diagnosis or in the subsequent 2 years. A similar pattern was found for sedentary behaviour, with a larger proportion of the cancer diagnosis group being sedentary from before they received their diagnosis and no improvement after diagnosis. The finding that the whole sample became progressively more sedentary over time highlights the need for interventions to increase activity and reduce sedentary time among older adults. Specific advice on being more active in the context of a cancer diagnosis could also contribute to improved long-term outcomes.
Overall, our findings provide little evidence that a cancer diagnosis is associated with spontaneous positive lifestyle changes over and above lifestyle trends in the older adult population. There were encouraging downward trends over time in smoking and alcohol regardless of diagnosis. The slightly higher smoking rates among those who got a cancer diagnosis dropped to match the comparison group over time, but the differential change was not statistically significant in this sample. Participants in both groups became steadily more sedentary, and there was no sign that cancer survivors became more active either immediately post-diagnosis or 2 years after that.
Given that life events such as a cancer diagnosis have been hypothesised to motivate positive lifestyle change (Rabin, 2009), it is surprising that our results provided little evidence of such change. One explanation may be that cancer survivors make some immediate, but short-lived, changes. However, it is also possible that cancer patients in the United Kingdom are not receiving adequate support and advice on lifestyle change. This would be consistent with findings from UK surveys indicating that fewer than half of cancer specialists routinely discuss exercise with their patients (Daley et al, 2008; Macmillan Cancer Support/ICM, 2011). Cancer survivorship has been on the agenda in the United States since the 1990s, whereas it really only rose to prominence in the United Kingdom following the Cancer Reform Strategy in 2007 (Department of Health, 2007), so there are likely to be improvements in the UK statistics. We have shown that the friends and relatives of patients with a cancer diagnosis are supportive of them receiving lifestyle advice (Williams et al, 2013) and it would be timely to establish patient preferences for format and timing.
This study had a number of limitations. Cancer data were self-reported and diagnoses of non-melanoma skin cancer were not excluded. We did not have the exact date of diagnosis, which could have been any time from just after T0 or just before T1 (a range of 2 years). It is therefore possible that more participants made short-lived changes in smoking and alcohol consumption that were not picked up. However, even if this were the case, the lack of longer term maintenance highlights an opportunity for intervention. The questions assessing frequency of alcohol consumption were changed after wave 1, but the prevalence of drinking alcohol at all was very similar across waves, so it is unlikely that the measurement issues biased this finding. The ELSA data set did not include a direct measure of sedentary behaviour so we used lack of physical activity. The activity measure itself did not map directly onto the current physical activity guidelines, but we used the closest available cut points; and overall levels were extremely low. To examine changes over multiple time points, our analyses were restricted to cases with three consecutive waves of data; excluding those who died, dropped out or did not answer the cancer diagnosis question. This reduced the sample size to ∼45% of the initial 11 391 sample in wave 1. Compared with the total ELSA sample, the analysed sample was slightly younger, wealthier and had slightly better health behaviours; consistent with retention in other longitudinal studies (Mendes de Leon, 2007). The results may therefore give a conservative estimate of health behaviour change. The sample size was not large enough to analyse by cancer site and there may be differences between sites. Finally, with a larger sample, the small group differences in smoking immediately post-diagnosis (T0–T1) might have been significant, but it seems unlikely that without more intervention, cancer survivors will achieve lower smoking rates than the general population.
In conclusion, our results provide little evidence that a cancer diagnosis is a stimulus for health-protective changes, with only modest reductions in smoking rates and an increase in sedentary behaviour, both broadly similar to age-related changes in the general population. The growing evidence that healthier lifestyles are important for long-term cancer outcomes highlights the need to identify effective support for health behaviour change in cancer survivors.
Acknowledgments
Kate Williams and Jane Wardle are supported by funding from Cancer Research UK. Andrew Steptoe is supported by funding from the British Heart Foundation. The English Longitudinal Study of Ageing was developed by a team of researchers based at University College London, the Institute of Fiscal Studies and the National Centre for Social Research, with funding from the National Institute on Aging (grants 2RO1AG7644-01A1 and 2RO1AG017644) and a consortium of UK government departments coordinated by the Office for National Statistics. A grant to Jane Wardle from Cancer Research UK C1418/A14134 supported these analyses. The data are lodged with the UK Data Archive.
References
- Banks J, Karlsen S, Oldfield Z.2003Socio-economic positionIn Health, Wealth and Lifestyles of the Older Population in England Marmot M, Banks J, Blundell R, Lessof C, Nazroo J, (eds) pp71–125.Institute for Fiscal Studies: London [Google Scholar]
- Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23:8884–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- Bidstrup PE, Dalton SO, Christensen J, Tjonneland A, Larsen SB, Karlsen R, Brewster A, Bondy M, Johansen C. Changes in body mass index and alcohol and tobacco consumption among breast cancer survivors and cancer-free women: a prospective study in the Danish Diet, Cancer and Health Cohort. Acta Oncol. 2013;52:327–335. doi: 10.3109/0284186X.2012.746466. [DOI] [PubMed] [Google Scholar]
- Blanchard CM, Courneya KS, Stein K. Cancer survivors' adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society's SCS-II. J Clin Oncol. 2008;26:2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- Blanchard CM, Denniston MM, Baker F, Ainsworth SR, Courneya KS, Hann DM, Gesme DH, Reding D, Flynn T, Kennedy JS. Do adults change their lifestyle behaviors after a cancer diagnosis. Am J Health Behav. 2003;27:246–256. doi: 10.5993/ajhb.27.3.6. [DOI] [PubMed] [Google Scholar]
- Brown BW, Brauner C, Minnotte MC. Noncancer deaths in white adult cancer patients. J Natl Cancer Inst. 1993;85:979–987. doi: 10.1093/jnci/85.12.979. [DOI] [PubMed] [Google Scholar]
- Coups EJ, Ostroff JS. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Prev Med. 2005;40:702–711. doi: 10.1016/j.ypmed.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Katzmarzyk PT, Bacon E. Physical activity and obesity in Canadian cancer survivors. Cancer. 2008;112:2475–2482. doi: 10.1002/cncr.23455. [DOI] [PubMed] [Google Scholar]
- Daley AJ, Bowden SJ, Rea DW, Billingham L, Carmicheal AR. What advice are oncologists and surgeons in the United Kingdom giving to breast cancer patients about physical activity. Int J Behav Nutr Phys Act. 2008;5:46. doi: 10.1186/1479-5868-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Clipp EC, Lipkus IM, Lobach D, Snyder DC, Sloane R, Peterson B, Macri JM, Rock CL, McBride CM, Kraus WE. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;25:2709–2718. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- Department of Health 2007Cancer Reform Strategy. Retrieved from http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_081006
- Eakin EG, Youlden DR, Baade PD, Lawler SP, Reeves MM, Heyworth JS, Fritschi L. Health behaviors of cancer survivors: data from an Australian population-based survey. Cancer Causes Control. 2007;18:881–894. doi: 10.1007/s10552-007-9033-5. [DOI] [PubMed] [Google Scholar]
- Falba T. Health events and the smoking cessation of middle aged Americans. J Behav Med. 2005;28:21–33. doi: 10.1007/s10865-005-2560-1. [DOI] [PubMed] [Google Scholar]
- Grimmett C, Wardle J, Steptoe A. Health behaviours in older cancer survivors in the English Longitudinal Study of Ageing. Eur J Cancer. 2009;45:2180–2186. doi: 10.1016/j.ejca.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Hounshell J, Tomori C, Newlin R, Knox K, Rundhaugen L, Tallman M, Bennett C. Changes in finances, insurance, employment, and lifestyle among persons diagnosed with hairy cell leukemia. Oncologist. 2001;6:435–440. doi: 10.1634/theoncologist.6-5-435. [DOI] [PubMed] [Google Scholar]
- Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- Jarvis MJ. Prevalence of hardcore smoking in England, and associated attitudes and beliefs: cross sectional study. BMJ. 2003;326:1061. doi: 10.1136/bmj.326.7398.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siege R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer Statistics 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Karlsen RV, Bidstrup PE, Christensen J, Larsen SB, Tjønneland A, Dalton SO, Johansen C. Men with cancer change their health behaviour: a prospective study from the Danish Diet, Cancer and Health Study. Br J Cancer. 2012;107:201–206. doi: 10.1038/bjc.2012.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan PS. Smoking and weight change after new health diagnoses in older adults. Arch Intern Med. 2009;169:237–242. doi: 10.1001/archinternmed.2008.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan Cancer Support/ICM 2011. Online survey of 400 health professionals who deal with cancer patients (100 GPs, 100 practice nurses, 100 oncologists, and 100 oncology nurses, of whom 52 were oncology clinical nurse specialists). Fieldwork conducted 23 May–12 June 2011. Survey results are unweighted
- Marmot MG, Stansfeld S, Patel C, North F, Head J, White I, Brunner E, Feeney A, Marmot MG, Smith GD. Health inequalities among British civil servants: the Whitehall II study. Lancet. 1991;337:1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- Maskarinec G, Murphy S, Shumay DM, Kakai H. Dietary changes among cancer survivors. Eur J Cancer Care. 2001;10:12–20. doi: 10.1046/j.1365-2354.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- Maunsell E, Drolet M, Brisson J, Robert J, Deschênes L. Dietary change after breast cancer: extent, predictors, and relation with psychological distress. J Clin Oncol. 2002;20:1017–1025. doi: 10.1200/JCO.2002.20.4.1017. [DOI] [PubMed] [Google Scholar]
- McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18:156–170. doi: 10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- Mendes de Leon CF. Aging and the elapse of time: a comment on the analysis of change. J Gerontol B Psychol Sci Soc Sci. 2007;62:S198–S202. doi: 10.1093/geronb/62.3.s198. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Ford ES. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, Miller P, Mitchell DC, Demark-Wahnefried W. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsom J, Huguet N, McCarthy MJ, Ramage-Morin P, Kaplan MS, Bernier J, McFarland BH, Oderkirk J. Health behavior change following chronic illness in middle and later life. J Gerontol B Psychol Sci Soc Sci. 2012a;67:279–288. doi: 10.1093/geronb/gbr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsom J, Huguet N, Ramage-Morin P, McCarthy MJ, Bernier J, Kaplan MS, McFarland BH. Health behaviour changes after diagnosis of chronic illness among Canadians aged 50 or older. Health Rep (Statistics Canada, Catelogue no. 82-003-XPE) 2012b;23:3–7. [PMC free article] [PubMed] [Google Scholar]
- Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J Am Diet Assoc. 2003;103:323–328. doi: 10.1053/jada.2003.50045. [DOI] [PubMed] [Google Scholar]
- Rabin C. Promoting lifestyle change among cancer survivors: when is the teachable moment. Am J Lifestyle Med. 2009;3:369–378. [Google Scholar]
- Salminen E, Heikkilä S, Poussa T, Lagström H, Saario R, Salminen S. Female patients tend to alter their diet following the diagnosis of rheumatoid arthritis and breast cancer. Prev Med. 2002;34:529–535. doi: 10.1006/pmed.2002.1015. [DOI] [PubMed] [Google Scholar]
- Smith WA, Nolan VG, Robison LL, Hudson MM, Ness KK. Physical activity among cancer survivors and those with no history of cancer – a report from the National Health and Nutrition Examination Survey 2003-2006. Am J Transl Res. 2011;3:342–350. [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Breeze E, Banks J, Nazroo J.2012Cohort profile: the English longitudinal study of ageing Int J Epidemiole-pub ahead of print;doi: 10.1093/ije/dys168 [DOI] [PMC free article] [PubMed]
- Travis LB, Rabkin CS, Brown LM, Allan JM, Alter BP, Ambrosone CB, Begg CB, Caporaso N, Chanock S, DeMichele A, Figg WD, Gospodarowicz MK, Hall EJ, Hisada M, Inskip P, Kleinerman R, Little JB, Malkin D, Ng AK, Offit K, Pui CH, Robison LL, Rothman N, Shields PG, Strong L, Taniguchi T, Tucker MA, Greene MH. Cancer survivorship--genetic susceptibility and second primary cancers: research strategies and recommendations. J Natl Cancer Inst. 2006;98:15–25. doi: 10.1093/jnci/djj001. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services 2004The Health Consequences of Smoking: A Report of the Surgeon General US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA; Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK44704/ [Google Scholar]
- Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. Can Med Assoc J. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes S, Evans A. A cross-sectional study comparing the motivation for smoking cessation in apparently healthy patients who smoke to those who smoke and have ischaemic heart disease, hypertension or diabetes. Fam Pract. 1999;16:608–610. doi: 10.1093/fampra/16.6.608. [DOI] [PubMed] [Google Scholar]
- Williams K, Beeken RJ, Wardle J. Health behaviour advice to cancer patients: the perspective of social network members. Br J Cancer. 2013;108:831–835. doi: 10.1038/bjc.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]