Abstract
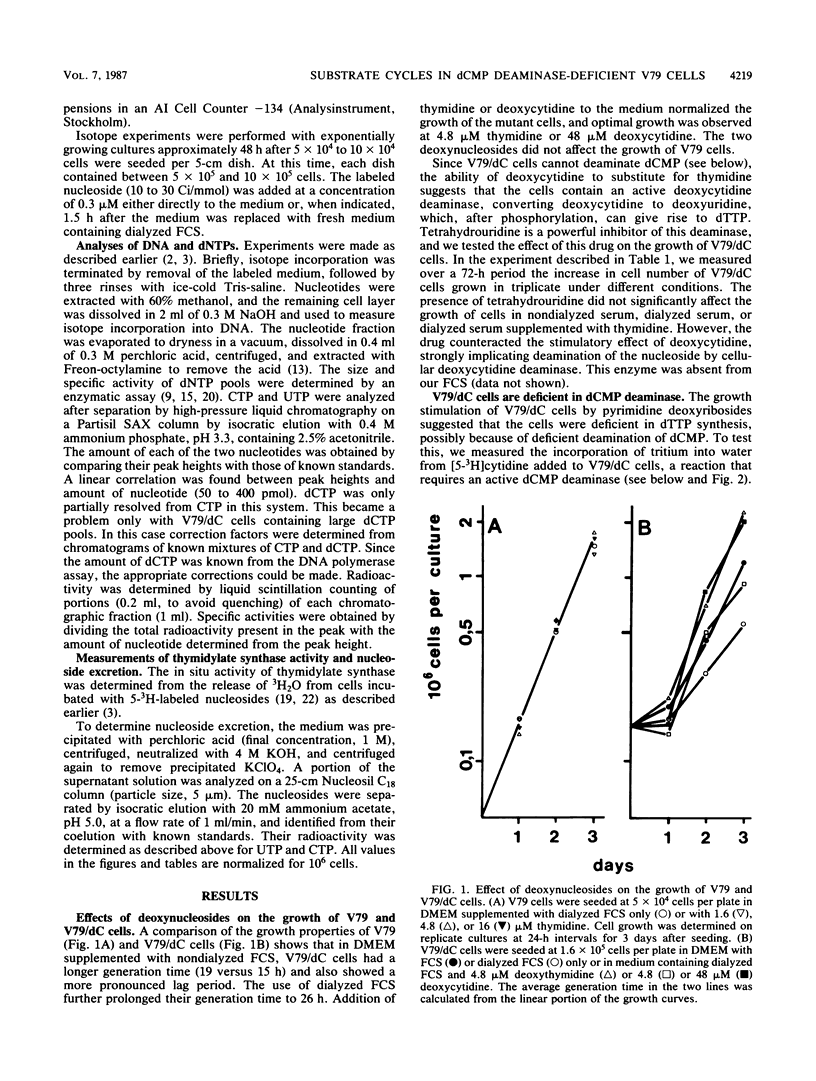
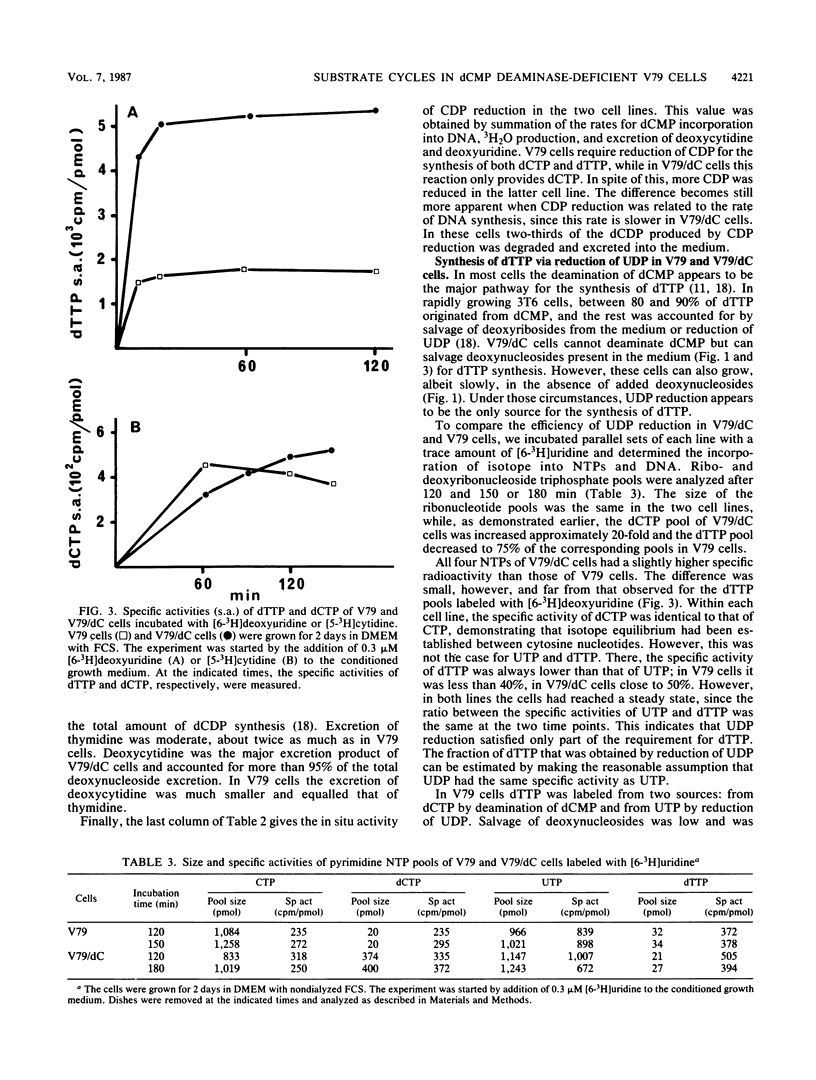
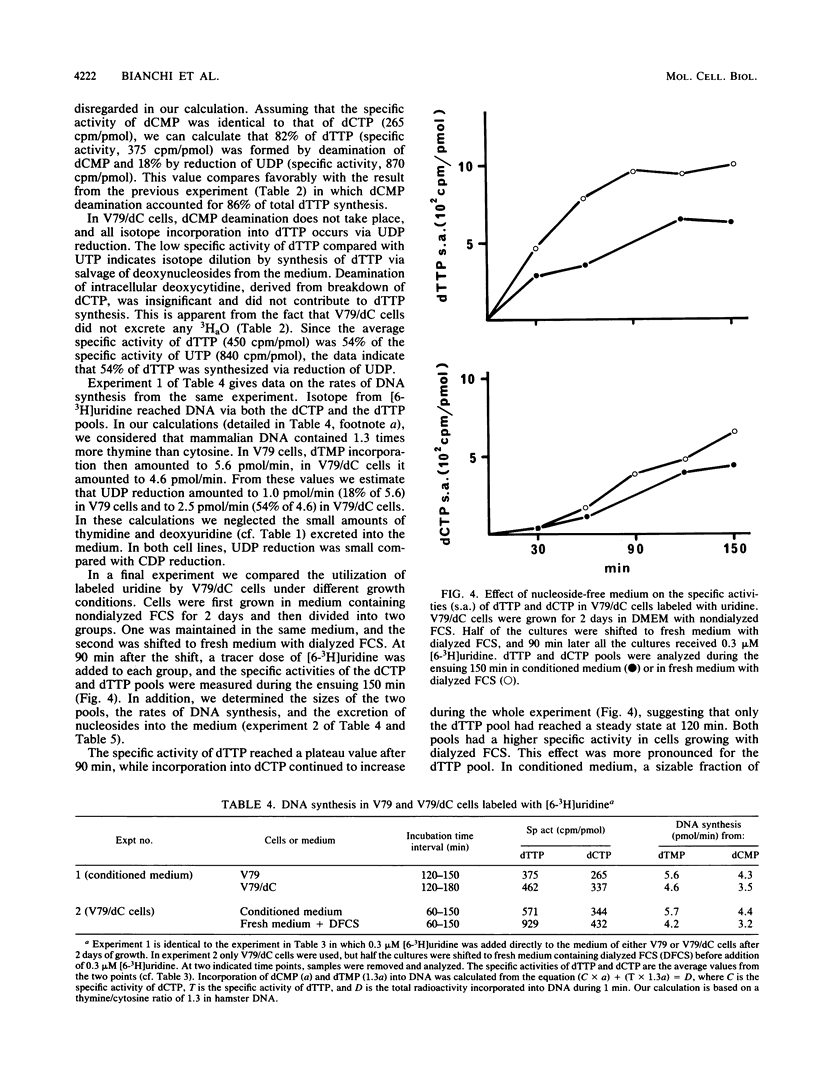
A mutant V79 hamster fibroblast cell line lacking the enzyme dCMP deaminase was used to study the regulation of deoxynucleoside triphosphate pools by substrate cycles between pyrimidine deoxyribosides and their 5'-phosphates. Such cycles were suggested earlier to set the rates of cellular import and export of deoxyribosides, thereby influencing pool sizes (V. Bianchi, E. Pontis, and P. Reichard, Proc. Natl. Acad. Sci. USA 83:986-990, 1986). While normal V79 cells derived more than 80% of their dTTP from CDP reduction via deamination of dCMP, the mutant cells had to rely completely on UDP reduction for de novo synthesis of dTTP, which became limiting for DNA synthesis. Because of the allosteric properties of ribonucleotide reductase, CDP reduction was not diminished, leading to a large expansion of the dCTP pool. The increase of this pool was kept in check by a shift in the balance of the deoxycytidine/dCMP cycle towards the deoxynucleoside, leading to massive excretion of deoxycytidine. In contrast, the balance of the deoxyuridine/dUMP cycle was shifted towards the nucleotide, facilitating import of extracellular deoxynucleosides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronow B., Watts T., Lassetter J., Washtien W., Ullman B. Biochemical phenotype of 5-fluorouracil-resistant murine T-lymphoblasts with genetically altered CTP synthetase activity. J Biol Chem. 1984 Jul 25;259(14):9035–9043. [PubMed] [Google Scholar]
- Bianchi V., Pontis E., Reichard P. Changes of deoxyribonucleoside triphosphate pools induced by hydroxyurea and their relation to DNA synthesis. J Biol Chem. 1986 Dec 5;261(34):16037–16042. [PubMed] [Google Scholar]
- Bianchi V., Pontis E., Reichard P. Interrelations between substrate cycles and de novo synthesis of pyrimidine deoxyribonucleoside triphosphates in 3T6 cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):986–990. doi: 10.1073/pnas.83.4.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E. H., McLaren J. D., Li I. C., Lamb B. Pleiotropic mutants of Chinese hamster cells with altered cytidine 5'-triphosphate synthetase. Biochem Genet. 1984 Aug;22(7-8):701–715. doi: 10.1007/BF00485854. [DOI] [PubMed] [Google Scholar]
- Eriksson S., Skog S., Tribukait B., Jäderberg K. Deoxyribonucleoside triphosphate metabolism and the mammalian cell cycle. Effects of thymidine on wild-type and dCMP deaminase-deficient mouse S49 T-lymphoma cells. Exp Cell Res. 1984 Nov;155(1):129–140. doi: 10.1016/0014-4827(84)90774-2. [DOI] [PubMed] [Google Scholar]
- Eriksson S., Thelander L., Akerman M. Allosteric regulation of calf thymus ribonucleoside diphosphate reductase. Biochemistry. 1979 Jul 10;18(14):2948–2952. doi: 10.1021/bi00581a005. [DOI] [PubMed] [Google Scholar]
- Hellgren D., Nilsson S., Reichard P. Effects of arabinosyl-cytosine on thymidine triphosphate pools and polyoma DNA replication. Biochem Biophys Res Commun. 1979 May 14;88(1):16–22. doi: 10.1016/0006-291x(79)91690-5. [DOI] [PubMed] [Google Scholar]
- Ives D. H., Durham J. P. Deoxycytidine kinase. 3. Kinetics and allosteric regulation of the calf thymus enzyme. J Biol Chem. 1970 May 10;245(9):2285–2294. [PubMed] [Google Scholar]
- Jackson R. C. The regulation of thymidylate biosynthesis in Novikoff hepatoma cells and the effects of amethopterin, 5-fluorodeoxyuridine, and 3-deazauridine. J Biol Chem. 1978 Oct 25;253(20):7440–7446. [PubMed] [Google Scholar]
- Kaufman E. R. Resistance to 5-fluorouracil associated with increased cytidine triphosphate levels in V79 Chinese hamster cells. Cancer Res. 1984 Aug;44(8):3371–3376. [PubMed] [Google Scholar]
- Khym J. X. An analytical system for rapid separation of tissue nucleotides at low pressures on conventional anion exchangers. Clin Chem. 1975 Aug;21(9):1245–1252. [PubMed] [Google Scholar]
- Lindberg U., Skoog L. A method for the determination of dATP and dTTP in picomole amounts. Anal Biochem. 1970 Mar;34:152–160. doi: 10.1016/0003-2697(70)90096-5. [DOI] [PubMed] [Google Scholar]
- Martin D. W., Jr, Gelfand E. W. Biochemistry of diseases of immunodevelopment. Annu Rev Biochem. 1981;50:845–877. doi: 10.1146/annurev.bi.50.070181.004213. [DOI] [PubMed] [Google Scholar]
- Meuth M., L'Heureux-Huard N., Trudel M. Characterization of a mutator gene in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6505–6509. doi: 10.1073/pnas.76.12.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicander B., Reichard P. Evidence for the involvement of substrate cycles in the regulation of deoxyribonucleoside triphosphate pools in 3T6 cells. J Biol Chem. 1985 Aug 5;260(16):9216–9222. [PubMed] [Google Scholar]
- Reichard P. Regulation of deoxyribotide synthesis. Biochemistry. 1987 Jun 16;26(12):3245–3248. doi: 10.1021/bi00386a001. [DOI] [PubMed] [Google Scholar]
- Rode W., Scanlon K. J., Moroson B. A., Bertino J. R. Regulation of thymidylate synthetase in mouse leukemia cells (L1210). J Biol Chem. 1980 Feb 25;255(4):1305–1311. [PubMed] [Google Scholar]
- Skoog L. An enzymatic method for the determination of dCTP and dGTP in picomole amounts. Eur J Biochem. 1970 Dec;17(2):202–208. doi: 10.1111/j.1432-1033.1970.tb01154.x. [DOI] [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Tomich P. K., Chiu C. S., Wovcha M. G., Greenberg G. R. Evidence for a complex regulating the in vivo activities of early enzymes induced by bacteriophage T4. J Biol Chem. 1974 Dec 10;249(23):7613–7622. [PubMed] [Google Scholar]
- Trudel M., Van Genechten T., Meuth M. Biochemical characterization of the hamster thy mutator gene and its revertants. J Biol Chem. 1984 Feb 25;259(4):2355–2359. [PubMed] [Google Scholar]
- Weinberg G., Ullman B., Martin D. W., Jr Mutator phenotypes in mammalian cell mutants with distinct biochemical defects and abnormal deoxyribonucleoside triphosphate pools. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2447–2451. doi: 10.1073/pnas.78.4.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Saint Vincent B. R., Déchamps M., Buttin G. The modulation of the thymidine triphosphate pool of Chinese hamster cells by dCMP deaminase and UDP reductase. Thymidine auxotrophy induced by CTP in dCMP deaminase-deficient lines. J Biol Chem. 1980 Jan 10;255(1):162–167. [PubMed] [Google Scholar]


