Abstract
Objective.
The aim of this study was to evaluate the “real world” effects of the monoclonal antibody omalizumab (OMB) when used to treat severe persistent allergic asthma in UK clinical practice.
Methods.
A 10-center retrospective observational study was carried out to compare oral corticosteroid (OCS) use and exacerbation frequency in 12 months pre- versus post-OMB initiation in 136 patients aged ≥12 years with severe persistent allergic asthma. All patients received ≥1 dose of OMB. Patients who had received OMB in a clinical trial were excluded. Data were obtained from hospital and if necessary general practitioners’ (GPs’) records on OCS use, lung function, hospital resource use, and routinely used quality of life (QoL) measures at baseline (pre-OMB), 16 weeks, and up to 12 months post-OMB initiation.
Results.
Mean total quantity of OCS prescribed per year decreased by 34% between the 12 months pre- and post-OMB initiation. During the 12 months post-OMB initiation, 87 patients (64%) stopped/reduced OCS use by 20% or more and 66 (49%) stopped OCS completely. Mean percent predicted forced expiratory volume in one second (FEV1) increased from 66.0% at baseline to 75.2% at week 16 of OMB therapy. The number of asthma exacerbations decreased by 53% during the 12 months post-initiation. Accident and emergency visits reduced by 70% and hospitalizations by 61% in the 12 months post-OMB initiation.
Conclusion.
This retrospective analysis showed a reduction in exacerbations and improved QoL as per previous studies with OMB. However, the total reduction in annual steroid burden and improved lung function in this severely ill group of patients taking regular or frequent OCS is greater than that seen in previous trials.
Keywords: anti-asthmatic agents, observational study, hospital resource use, monoclonal antibodies, quality of life
Introduction
Omalizumab (OMB) is an anti-IgE recombinant humanized monoclonal antibody designed to treat IgE-mediated disease by reducing the plasma concentration of free IgE antibody. The efficacy and safety profile of OMB in severe persistent allergic asthma was described in international clinical trials (1–4) in which OMB as an add-on therapy reduced the number of asthma exacerbations, reduced the concomitant medication burden, improved symptom severity, and improved quality of life (QoL) compared to standard therapy alone.
OMB became available for prescription in the United Kingdom (UK) in October 2005. It was accepted for use in the National Health Service (NHS) in Scotland by the Scottish Medicines Consortium (SMC) in October 2007 for patients aged ≥12 years (5) (extended to cover patients aged 6 to <12 years (6) in March 2010), where it is restricted to initiation and monitoring by hospital physicians experienced in the diagnosis and treatment of severe persistent asthma and to patients prescribed systemic steroids and in whom all other treatments have failed. The National Institute for Health and Clinical Excellence (NICE) recommended its use in England and Wales in November 2007 (7), within the licensed indication, for patients aged ≥12 years, with severe unstable disease requiring hospital treatment in the previous year.
Patients with unstable severe allergic asthma have a high unmet medical need and are at increased risk of hospitalization for exacerbations or asthma death. Despite efforts to minimize chronic oral corticosteroid (OCS) use due to the well-documented long-term side effects (8), a significant number of patients with severe asthma need OCS. In addition to the personal burden on patients, the direct cost of asthma to the NHS was estimated at £889 million in 2001 (9).
Although randomised controlled trial (RCT) evidence has demonstrated the efficacy of OMB, clinical trial results do not always translate into routine practice, where the drug is used in a less controlled manner in a broader range of patients. To date, there are international real-life experience data (10–16) but only limited UK studies (17–20), describing the outcomes achieved in routine clinical practice where access to OMB has been restricted by several criteria including severity of illness and previous treatment and to specialist prescribers. The primary objective of this study was to investigate the steroid-sparing effect of OMB in the UK context, by comparing the total quantity of OCS prescribed in the 12 months pre- and post-OMB initiation. Secondary objectives were to compare exacerbation rate, hospital resource use, lung function, patient-reported asthma control and QoL.
Methods
Ten UK centers with a special interest in severe and difficult asthma where OMB had been in use for ≥12 months, with ≥8 patients (per center) having received OMB treatment, were purposefully selected to participate in this retrospective observational study. All treated, consenting patients were included.
The study was approved by the Moorfields and Whittington Research Ethics Committee on 15 December 2009 (reference 09/H0721/74). Local management (R&D) approval was obtained in each center.
Patients who had received ≥1 dose of OMB, were aged ≥12 at initiation, and in whom OMB was initiated ≥12 months before data collection were identified by the Principal Investigator from a clinic database or diary. As this was a retrospective observational study, the criteria for selection of patients to receive OMB were not set as part of this study; the aim was to study all patients who received the drug as part of routine clinical practice in the UK NHS, incorporating any slight local variations in patient selection, to give a “real world” perspective on the outcomes achievable with OMB. However, patients in the participating centers are routinely assessed to exclude alternative diagnoses which may mimic severe allergic asthma, such as acute bronchopulmonary aspergillosis and vasculitides. Similarly, the need for allergen or salicylate avoidance is assessed, strategies advised where appropriate and their effect monitored before OMB prescribing is considered. Patient informed consent was sought for research access to the medical record and researcher contact with the general practitioner (GP) if needed to complete the study dataset. Patients were excluded if they declined to consent, had received OMB in a clinical trial, or if their hospital medical records were unavailable.
Anonymized-coded data were collected from paper and electronic hospital medical records between February 2010 and January 2011 by two researchers from pH Associates (an independent research company), according to detailed data collection rules. Date of OMB initiation was between 10 April 2006 and 7 January 2010. Data collected included patient demographics, OMB dosing, OCS prescriptions, documented exacerbations (defined as an increase in symptoms requiring treatment with systemic corticosteroids), and hospital visits and admissions for the 12 months pre- and post-OMB initiation; lung function test results, FEV1 (L and % predicted), were available in hospital records, but they had not been standardized as pre- or post-bronchodilator values and patient-reported outcomes from the Asthma Control Test (ACT) (21) and Asthma Quality of Life Questionnaire (AQLQ) (22) at baseline (immediately pre-OMB initiation), 16 weeks, and most recently up to 12 months post-OMB initiation (exact time points for assessments could not be stipulated for the study as this was a retrospective observational study of routine clinical practice). Details of any adverse events occurring during OMB treatment were also recorded, to comply with European pharmacovigilance regulations. For patients whose initial secondary care referral was <12 months before OMB initiation, the patient’s secondary care consultant requested data (using a standard letter) from the patient’s GP on OCS prescribed and hospital admissions for asthma within the study period prior to referral.
Data were entered into a study database and analyzed according to a pre-specified analysis plan by the sponsor, Novartis, UK. The data of all patients who had received at least one dose of OMB were analyzed.
Patient demographics and other baseline characteristics were summarized using descriptive statistics—contingency tables for qualitative variables (gender, race, and age group), and mean, standard deviation, median, minimum, and maximum for quantitative variables (age, weight, and duration of severe asthma). Age of subjects, duration of disease, and so on were calculated with respect to the date of OMB initiation.
Analysis of The Primary Objective
The primary objective to quantify the steroid-sparing effect of OMB was evaluated by calculating the difference in the mean total quantity of OCS prescribed per patient during the 12 months pre- versus post-initiation of OMB (post-OMB minus pre-OMB) and its 95% confidence interval (CI). The null hypothesis that there was no difference in mean total quantity of OCS during the 12 months pre- versus post-initiation of OMB was tested by paired t-test. OCS prescribed doses were converted to prednisolone-equivalent doses according to the equivalence table in the British National Formulary (23).
Analysis of Secondary Objectives
Mean daily dose per patient was compared over the same 12 months periods (pre- and post-OMB initiation), but averaged only for the days on which each patient was receiving OCS. Other variables, such as lung function tests, hospital resource use, and so on, were analyzed similarly (by calculating the mean difference (post-OMB minus pre-OMB), its 95% CI, and applying a paired t-test). Sub-group analyses included outcomes in patients who were and were not taking continuous OCS (defined as daily OCS for the 6 months preceding OMB without break) at OMB initiation.
Results
Demographics
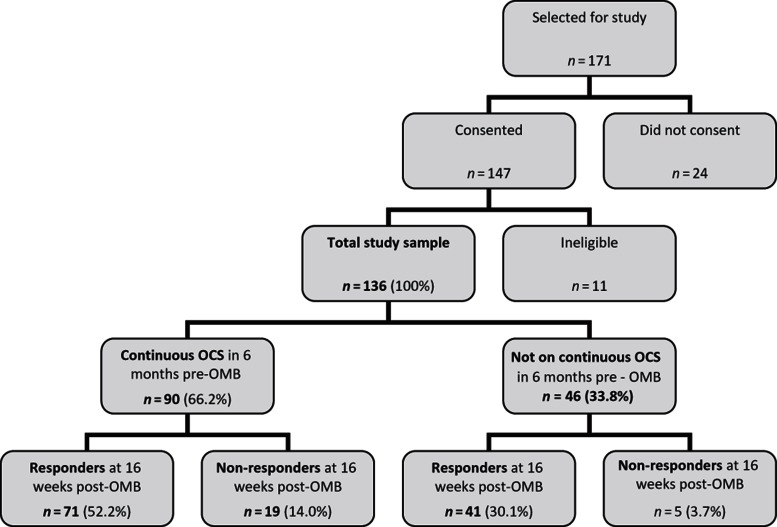
A total of 171 patients were invited to allow their records to be used for the study and 147 (86%) consented. Of these, 11 were ineligible (clinical trial patients, n = 8; data collected twice in error, n = 1; and medical records unavailable, n = 2) and 136 were included in the study (range: 7–36 patients per center), an inclusion rate of 80% (Figure 1). The study sample was composed of 68% females. Median age at diagnosis of asthma was 8.0 years (range: birth–72 years); median age at data collection was 42.8 years (range: 12.1–83.6 years) (Table 1).
Figure 1.
—Study flow of patient numbers.
Table 1.
—Patient characteristics.
| Age (years) (n = 136) | Mean (SD) | 41.26 (14.522) |
| Median (range) | 42.82 (12.1–83.6) | |
| Age distribution (n = 136) | 12–18 years | 9 (6.6%) |
| 18–45 years | 69 (50.7%) | |
| >45 years | 58 (42.6%) | |
| Sex (n = 136) | Male | 43 (31.6%) |
| Female | 93 (68.4%) | |
| Smoking history (n = 136) | Current smoker | 3 (2.2%) |
| Ex-smoker | 24 (17.6%) | |
| Never smoked | 89 (65.4%) | |
| Not recorded | 20 (14.7%) | |
| Pack-years (n = 16) | Mean (SD) | 10.76 (14.378) |
| Median (range) | 7.50 (0.2-60.0) | |
| Last recorded weight pre-OMB (kg) (n = 132) | Mean (SD) | 81.16 (20.632) |
| Median (range) | 81.25 (40.3–133.0) | |
| Age at asthma diagnosis (years) (n = 120) | Mean (SD) | 14.59 (15.657) |
| Median (range) | 8.00 (0–72.0) | |
| Duration of severe persistent allergic asthma (years) (n = 120) | Mean (SD) | 26.44 (14.266) |
| Median (range) | 25.80 (2.9–57.5) | |
| Allergies pre-dating OMB initiation (n = 136) | Allergen | No. patients (%) |
| Animal fur (inc. cats, dogs) | 79 (58.1%) | |
| Pollen | 64 (47.1%) | |
| House dust (mite) | 62 (45.6%) | |
| Plant material | 32 (23.5%) | |
| Fruit (inc. strawberries) | 14 (10.3%) | |
| Antibiotic | 13 (9.6%) | |
| Other drug | 11 (8.1%) | |
| Nuts (inc. peanut) | 11 (8.1%) | |
| Moulds | 9 (6.6%) | |
| Milk | 6 (4.4%) | |
| Fish/shellfish | 6 (4.4%) | |
| Egg | 5 (3.7%) | |
| Feathers | 2 (1.5%) | |
| Other | 36 (26.5%) | |
| None | 8 (5.9%) |
Most patients (65%) had never smoked, 2% were current smokers at OMB initiation and 18% were ex-smokers (median: 7.5 pack-years); smoking status was not recorded in 15%. Prior to OMB initiation, 94% of patients had at least one documented allergy, with the most common being to animal fur (58%), pollen (47%), and house dust (46%). Eighty-two percent of patients suffered from one or more respiratory or allergic comorbidity, most commonly seasonal (46%) and perennial (28%) rhinitis (Table 1). Systemic asthma medication (excluding OCS) prescribed in the year before OMB initiation included theophyllines (n = 99 patients, 73%), leukotriene receptor antagonists (n = 82, 60%), immunosuppressants (methotrexate, ciclosporin, azathioprine, or tacrolimus) (n = 14, 10%) and oral long-acting β-antagonist (bambuterol) (n = 4, 3%). For 17 patients, no systemic asthma medications were documented in the hospital records in the year pre-OMB.
OMB Dosing
Patients received a mean (SD) of 18.3 (8.19) doses of OMB over 315.6 (103.64) days during the study period. This includes 24 patients (17.6%) classified as “non-responders” according to the product licence criteria at 16 weeks (which stipulate a clinician assessment taking into account peak expiratory flow (PEF), day- and nighttime symptoms, rescue medication use, spirometry, and exacerbations (24)), in whom treatment was stopped for this reason and 112 (82.4%) patients classified as “responders” at 16 weeks (Figure 1). Most patients (n = 85, 62.5%) received doses of between 225 and 375 mg every 2 weeks; the remainder (n = 51, 37.5%) received 150–300 mg every 4 weeks. No patient received more than 375 mg every 2 weeks.
OCS Use
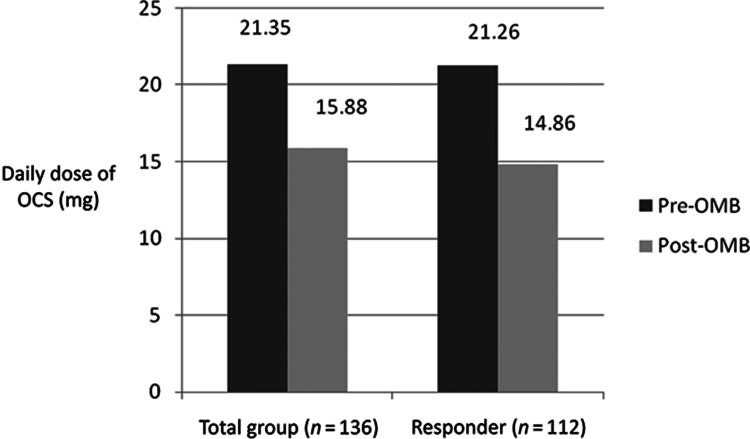
The mean total quantity of OCS prescribed in the whole study sample (n = 136) decreased from 5.5 g in the 12 months period pre-OMB to 3.6 g in the 12 months post-OMB (p < .001); a 34% decrease in total annual OCS burden. Mean daily OCS dose (on OCS-treated days) decreased by 5.5 mg (25.6%), from 21.4 mg pre-OMB to 15.9 mg post-OMB (p < .001) (Figure 2). Sixty-six patients (48.5%) stopped OCS within 1 year of OMB initiation and 87 (64%) stopped or reduced OCS dose by ≥20%. Of those patients on continuous OCS for the 6 months pre-OMB (n = 90), 35 (38.9%) stopped OCS and 59 (65.6%) stopped or reduced OCS dose by ≥20%.
Figure 2.
—Daily dose of OCS (mg) in the 1 year pre- and post-OMB.
Concomitant Medications and Exacerbations
There was little difference in the concomitant asthma medications between the pre- and post-OMB study periods; only five patients started a new systemic asthma medication (excluding OCS) in the year post-OMB initiation (theophylline, n = 3; montelukast, n = 1; and methotrexate, n = 1) while 56 systemic medications (excluding OCS) were stopped at or before OMB initiation (theophyllines, n = 24; montelukast, n = 19; immunosuppressants, n = 11; and long-acting β-agonists, n = 2). The number of patients stopping systemic medications during OMB treatment was not recorded for the study. The same 17 patients with no systemic asthma medications documented in the hospital records in the year pre-OMB also had none documented in the year post-OMB initiation.
The mean number of asthma exacerbations decreased from 3.67 events/year pre-OMB to 1.7 events/year post-OMB (p < .001).
Lung Function Tests
Lung function test results, where available from clinical records (numbers available for the analysis are shown following each result), showed a 12.8% increase in mean FEV1 (% predicted) from 62.9% at baseline to 71.0% at 16 weeks (p < .001) (n = 111) and a 12.4% increase from 69.9% to 78.6% at the most recent assessment (approximately 12 months post-OMB initiation) (p = .002) (n = 32) (Table 2). Mean FEV1 (L) also increased from 1.99 L at baseline to 2.10 L at 16 weeks and 2.22 L at the most recent assessment (p < .001). Mean PEF (L/min) increased from 296.9 at baseline to 348.5 at 16 weeks (p < .001) (n = 91) and from 299.2 at baseline to 326.8 in the 58 patients with a most recent assessment (p = .004) (Table 2).
Table 2.
—Lung function tests.
| N = 136 | Baseline | Post-Omalizumab | Difference | % Difference | P- value |
|---|---|---|---|---|---|
| Mean FEV1(% predicted) | |||||
| 16 weeks (n = 111) | 62.94 | 70.98 | 8.05 | 12.8% | <.001 |
| Most recent (approx. 12 months) (n = 32) | 69.90 | 78.60 | 8.70 | 12.4% | .0020 |
| Mean FEV1(L) | |||||
| 16 weeks (n = 88) | 1.99 | 2.10 | 0.10 | 5.5% | .2157 |
| Most recent (approx. 12 months) (n = 70) | 1.99 | 2.22 | 0.24 | 12.1% | <.001 |
| Mean PEF(L/min) | |||||
| 16 weeks (n = 91) | 296.89 | 348.51 | 51.62 | 17.4% | <.001 |
| Most recent (approx. 12 months) (n = 58) | 299.24 | 326.79 | 27.55 | 9.2% | .0040 |
QoL and Patient-Reported Asthma Control
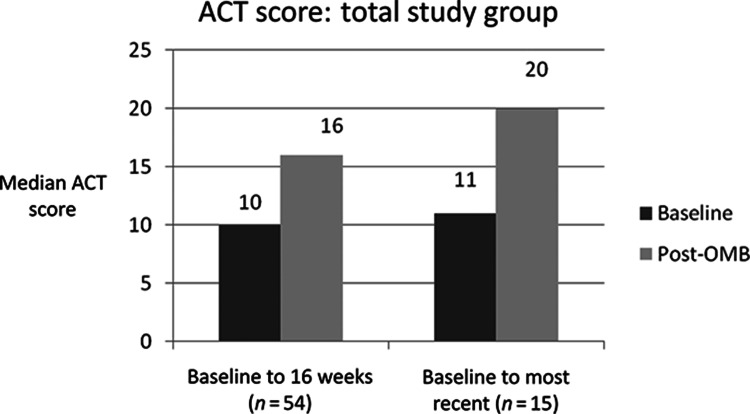
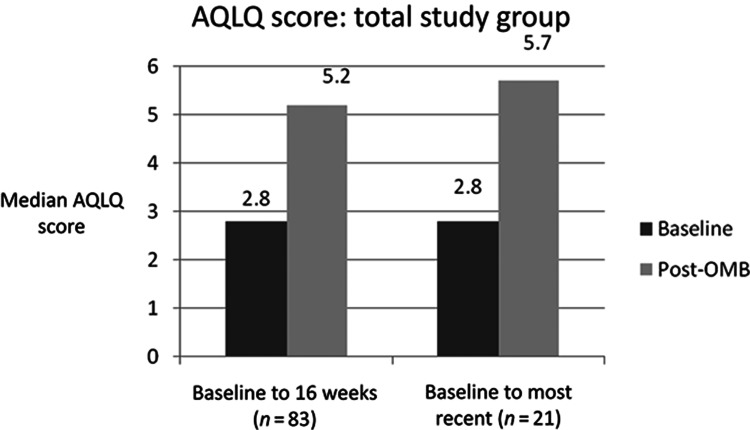
QoL, measured by median AQLQ scores and asthma control, measured by median ACT scores, improved significantly from baseline, both at 16 weeks and at the most recent measurement up to 12 months post-OMB initiation (Figures 3 and 4).
Figure 3.
—Median ACT score. An ACT score below 19/25 indicates poorly controlled asthma.
Figure 4.
—Median AQLQ score. The AQLQ works on a maximum possible score 7, where lower scores indicate a poorer quality of life.
Resource Use
Resource use (mean (SD)) decreased in the 12 months post-OMB initiation, compared with the 12 months pre-OMB initiation in terms of A&E visits (0.46 (1.42) vs. 1.52 (2.19) (p < .001)), inpatient hospitalizations (0.51 (1.10) vs. 1.30 (1.73) (p < .001)) and bed days (2.97 (6.34) vs. 9.10 (14.44) (p < .001)) (Table 3). There were also statistically significant reductions in consultant (3.82 (3.25) vs. 4.54 (3.28) (p = .017)) and telephone consultations (0.11 (0.42) vs. 0.23 (0.88) (p = .038)). There was no significant difference in the use of other secondary care services such as outpatient appointments, nurse, and pharmacist consultations but it should be noted that visits made only for OMB dosing were not recorded (Table 3).
Table 3.
—Hospital resource use (excluding visits for OMALIZUMAB administration).
| Mean (SD) | Mean (SD) | |||
|---|---|---|---|---|
| Resource | pre-OMALIZUMAB | post-OMALIZUMAB | Difference | Paired t-test |
| A&E visits | 1.52 (2.194) | 0.46 (1.419) | −1.07 (2.383) | p < .001 |
| Inpatient hospitalizations | 1.30 (1.731) | 0.51 (1.102) | −0.79 (1.830) | p < .001 |
| Bed days (all subjects) | 9.10 (14.438) | 2.97 (6.343) | −6.13 (14.243) | p < .001 |
| Inpatient hospitalizations (hospitalized subset a , n = 81) | 2.19 (1.761) | 0.65 (1.247) | −1.53 (1.963) | p < .001 |
| Bed days (hospitalized subset, n = 81) | 14.86 (16.341) | 3.83 (6.939) | −11.04 (16.176) | p < .001 |
| Respiratory outpatient visits | 6.00 (3.432) | 5.71 (4.360) | −0.29 (4.968) | p = .49 |
| Telephone consultations | 0.23 (0.877) | 0.11 (0.416) | −0.12 (0.656) | p= .0384 |
| Nurse consultations | 1.24 (2.209) | 1.69 (3.954) | 0.46 (3.559) | p = .1375 |
| Doctor consultations | 4.54 (3.277) | 3.82 (3.250) | −0.71 (3.434) | p = .0168 |
| MDT consultations | 0.06 (0.266) | 0.00 (0.000) | −0.06 (0.266) | p = .0109 |
| Pharmacist consultations | 0.05 (0.372) | 0.06 (0.360) | 0.01 (0.333) | p = .7973 |
Note: aPatients hospitalized in the 12 months pre-OMALIZUMAB initiation.
Discussion
Study Limitations
As with any retrospective study, the data quality relied heavily on the accuracy and completeness of available clinical records. Incomplete data may have been obtained on OCS prescribing (due to many patients self-managing dosing and/or obtaining further supplies from their GP), concomitant primary care prescribing and admissions to other hospitals. Where data were incomplete, assumptions were made regarding the typical course of steroids for exacerbations; these were applied identically in the pre- and post-OMB periods.
Although overall OCS usage may be underestimated, it is likely that the underestimate is greater in the pre-OMB period, as patients are more closely monitored following OMB initiation. Hence, the reduction in OCS usage and exacerbation rate shown is likely to be an underestimate of the true effect size. Nevertheless, it is possible that the more regular contact with asthma nurses for OMB administration in the post-OMB year may have been a contributing factor in the reduction in hospital admissions and exacerbations seen.
This is a limited sample size, although it is estimated that this covers approximately 1/8 of the UK population receiving OMB for severe persistent allergic asthma at the time of the study. The study sites were all large specialist asthma centers and it is possible that the outcomes achieved in these centers may not be representative of all UK centers.
Unlike studies comparing parallel groups, the comparison of the 12 months pre- and post-OMB initiation periods does not take account of any spontaneous improvement in asthma morbidity. However, this is unlikely to be a significant confounding factor in this severely ill group of patients.
Finally, the lung function data have to interpreted with caution as there was no standardization of lung function done pre- or post-bronchodilator, as this was a retrospective data collection.
Discussion Of Main Results
Despite the significant limitations of the study, there are some important individual findings. First, the 34% (1.87 g) reduction in total quantity of OCS prescribed in the 12 months pre- versus post-OMB initiation also expressed as a 5.47 mg (26%) reduction in mean daily OCS dose per patient is clinically important. Approximately 50% of patients stopped OCS and 64% reduced OCS dose by at least 20%; results were similar even in the sub-group on continuous OCS pre-OMB (i.e., most severe). Reducing OCS use is an important goal of therapy for patients with severe asthma, in view of the well-documented adverse effects of long-term exposure to systemic corticosteroids (25, 26).
These results, achieved in routine clinical practice in the UK NHS, are in line with those reported in a previous observational study conducted in France (27) in which 64% of patients reduced or discontinued OCS and mean daily OCS dose reduced by 8.7 mg, and also with the PERSIST study (10), conducted in Belgium, where mean daily dose of methylprednisolone reduced by 39%. In a study in Irish patients (28), although the number of OCS courses reduced from 3.08 to 1.16 in the 6 months pre- and post-OMB, respectively, the median OCS dose remained constant at 10 mg. However, in that study there were fewer patients on continuous OCS than in our study (13% vs. 66%).
The demographic profile of the study sample represents a typical severe persistent asthma group. The higher ratio of females to males in the study is consistent with a higher prevalence of asthma in adult females vs. males reported in the literature (29).
There was a reduction in the number of asthma exacerbations requiring OCS following OMB therapy with a similar effect size to RCT data (2) and previous international observational studies (10, 28, 30, 31). As all exacerbations were treated with OCS, the reduction in exacerbations may be a contributing factor in the reduction also seen in the number of patients requiring OCS and the reduction in total quantity and mean daily OCS dose. As very few patients (n = 5) started other systemic asthma medications during OMB treatment, this and other outcomes seen in the study cannot be attributed to the effect of medication taken concurrently with OMB.
Improvements in lung function were also seen and the difference from baseline was statistically significant for all measures (FEV1 (L and % predicted); peak expiratory flow rate (PEFR)) at one or more time points post-OMB initiation. This effect has not been demonstrated consistently in previous RCTs, but a similar effect was seen in one other observational study (28). However, the mean change did not consistently reach 10% (a magnitude of change deemed potentially clinically significant in this population), for all measures at all time points. Furthermore, the recording of lung function measurements was patchy, resulting in analysis based on small sample sizes for some measures and/or time points and was not consistently recorded pre- or post-bronchodilator in routine practice at all centers, this should be addressed in future observational studies.
There was a low rate of admissions to A&E and general hospital wards in the study cohort, suggesting this is a group of patients who are predominantly self-managing at home. Nevertheless, the results suggest that OMB use is associated with a significant reduction in hospitalization rates and length of stay, with a saving of over a week of hospital stay per patient per year; a valuable improvement from both the patient’s and the NHS’ perspectives. While a health economic analysis of this entire dataset would be of interest, until the full long-term cost of the deleterious effect of treating side effects of OCSs is calculated, such an analysis would be futile.
Both the ACT and AQLQ improved by clinically significant amounts and it is noteworthy that overall the ACT moved out of the poorly controlled range. From the patient’s perspective, this is clearly important. The positive effect of OMB on patients’ lives is further supported by the improvement in QoL, seen in the increase in median AQLQ score in the 12 months post-OMB initiation. The improvement in AQLQ scores was better than in the PERSIST study (10) at both 16 and 52 weeks—but PERSIST patients had a slightly higher mean baseline score (3.24 PERSIST vs. 2.8 this study).
In this study, patients were classified as “responders” if they had continued with OMB treatment beyond 16 weeks (n = 112, 82.4%), as response to treatment was a condition for continued funding of OMB by NHS payers. Although this method of classification is not directly comparable with those used in other studies of OMB, it is notable that the proportion of “responders” in this study was considerably higher than the 60.5% of OMB-treated patients judged to have an “excellent” response in the Investigation of Omalizumab in Severe Asthma Treatment study (INNOVATE) trial (2). This is particularly so because patients in this study had, on an average, more severe asthma than the INNOVATE patients (2) (mean 3.67 asthma exacerbations in 12 months pre-OMB vs. 2.64 in 14 months pre-OMB in INNOVATE). The “response” rate was the same (82%) as those with “good” or “excellent” Global Effectiveness ratings in a comparable European observational study “Real-life” effectiveness of omalizumab in patients with severe persistent allergic asthma study (PERSIST) (10), suggesting that patients are being carefully selected for OMB treatment and it is not being used inappropriately. Although compliance with NICE/SMC guidance was not formally assessed in the study, local payers require these patients have a detailed work-up prior to the use of OMB. All of the centers involved in this study have an interest in severe and difficult asthma and patients are extensively investigated to exclude comorbid and associated conditions known to present to severe and difficult asthma clinics. These conditions include reflux-induced cough, hyperventilation, poor compliance, and bronchiectasis. The rigorous exclusion of these patients may have led to treatment of a group who had a greater likelihood of responding to treatment with OMB. It is also possible that some of the large QoL responses may in part be due to concomitant management of the coexistent comorbidities and cannot all be assumed to be directly related to the OMB treatment.
Further Study
Prospectively planned observational data collection would improve the consistency of data available across the various lung function and QoL measures employed in clinical practice. Patient-held steroid diaries may also elicit more complete data on OCS usage. It would be interesting to understand the criteria by which patients are being selected and by which successful management is occurring. It appears that the strict criteria set down by NICE may not take account of the self-management of this severely ill patient group.
Conclusion
This retrospective analysis showed a reduction in exacerbations and improved QoL in this UK patient population as per previous studies with OMB. However, the total reduction in annual steroid burden and improved lung function in this severely ill group of patients taking regular or frequent OCS was greater than that seen in previous trials. OMB use was also associated with a significant positive impact on unplanned hospital resource use in the year after its initiation. These are important quality improvements in the management of asthma for these previously difficult-to-treat patients.
Declaration of Interests
The study was sponsored by Novartis Pharmaceuticals UK Ltd. The sponsor was involved in the design of the study and conducted the analysis according to a detailed analysis plan agreed by the investigators. The interpretation of the results is that of the authors of this paper. Neil Barnes. The work under consideration: Grant paid to institution (Novartis). Financial activities outside the submitted work: Board membership (Glaxo). Consultancy (GlaxoSmithKline, Novartis, TEVA, Nycomed). Payment for lectures including service on speakers bureaus (GlaxoSmithKline, Novartis, Nycomed, Chiesi). Travel/accommodation/meeting expenses. Grants/grants pending paid to institution (GlaxoSmithKline, Boehringer, Novartis, Astrazeneca, Merck, Sharp & Dohme). Andrew Menzies-Gow. Financial activities outside the submitted work: Board membership (Novartis/Genentech/Roche). Payment for lectures including service on speakers’ bureaus (GlaxoSmithKline, Astra Zeneca, Novartis, Chiesi). Payment for development of educational presentations (GlaxoSmithKline). Travel/accommodation/meeting expenses (GlaxoSmithKline, Novartis, Boeringer Ingelheim). Adel Mansur. The work under consideration: Consulting fee/honorarium (lecturing advisory board meeting). Fees paid to institution to cover admin time. Financial activities outside the submitted work: SAB meeting fees, payment for lecturing sponsored by company, sponsorship to attend ERS. Educational grant paid to institution. Money paid to institution for sponsorship of local meetings. David Spencer. Previous member of advisory board for Novartis. Frances Percival. Frances Percival is an employee of pH Associates, a professional consultancy specializing in the design and implementation of real world data projects. pH Associates received payment from Novartis to undertake this study. pH Associates has also been paid to design and conduct studies by other pharmaceutical companies. Amr Radwan. Amr Radwan is employed as a Medical Therapy Area Head at Novartis Pharmaceuticals UK Ltd. Rob Niven. The work under consideration: Expenses paid for travel to investigator meeting. Financial activities outside the submitted work: Fees for lectures sponsored by Novartis. Sponsored by Novartis for support to attend international conferences twice in last 5 years.
References
- 1.Holgate ST, Chuchalin AG, Hébert J, Lötvall J, Persson GB, Chung KF, Bousquet J, Kerstjens HA, Fox H, Thirlwell J, Cioppa GD. Omalizumab 011 International Study Group. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy. 2004;34:632–638. doi: 10.1111/j.1365-2222.2004.1916.x. [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, Beeh K-M, Ramos S, Canonica G W, Hedgecock S, Fox H, Blogg M, Surrey K. Benefits of omalizumab as add on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–316. doi: 10.1111/j.1398-9995.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 3.Fritscher L, Chapman KR. Omalizumab for asthma: Pharmacology and clinical profile. Expert Rev Resp Med. 2009;3((2)):119–127. doi: 10.1586/ers.09.7. [DOI] [PubMed] [Google Scholar]
- 4.Bousquet J, Cabrera P, Berkman N, Buhl R, Holgate S, Wenzel S, Fox H, Hedgecock S, Blogg M, Cioppa GD. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy. 2005;60:302–308. doi: 10.1111/j.1398-9995.2004.00770.x. [DOI] [PubMed] [Google Scholar]
- 5.http://www.scottishmedicines.org.uk/SMC_Advice/Advice/Omalizumab__Xolair_/omalizumab_150mg_powder_and_solvent_for_injection__Xolair_1 Scottish Medicines Consortium. Omalizumab 150mg powder and solvent for injection (Xolair®) (No. 259/06). 2007. Available at.
- 6.http://www.scottishmedicines.org.uk/files/OmalizumabXolair_Abbreviated_Mar2010.pdf Scottish Medicines Consortium. Omalizumab 150 mg powder and solvent for solution for injection (Xolair®) (No: 611/10). 2010 Product Update. Available at.
- 7.http://guidance.nice.org.uk/TA133 National Institute for Health and Clinical Excellence. Omalizumab for severe persistent allergic asthma. NICE technology appraisal TA133. Available at.
- 8.Lewis LD, Cochrane GM. Systemic steroids in chronic severe asthma. Br Med J (Clin Res Ed) 1986;292:1289–1290. doi: 10.1136/bmj.292.6531.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuen P, Office of Health Economics. Compendium of Health Statistics. 2003–2004. Office of Health Economics, London 2003.
- 10.Brusselle G, Michils A, Louis R, Dupont L, Van de Maele B, Delobbe A, Pilette C, Lee CS, Gurdain S, Vancayzeele S, Lecomte P, Hermans C, MacDonald K, Song M, Abraham I. “Real-life” effectiveness of omalizumab in patients with severe persistent allergic asthma: The PERSIST study. Respir Med. 2009;103:1633–1642. doi: 10.1016/j.rmed.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Molimard M, de Blay F, Didier A, Le Gros V. Effectiveness of omalizumab (Xolair®) in the first patients treated in real-life practice in France. Respir Med. 2008;102:71–76. doi: 10.1016/j.rmed.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Zureik M, Molimard M, Aubier M, Levy J, Humbert M, Grimaldi-Bensouda L, Abenhaim L. European Respiratory Society (ERS) Congress. Barcelona; Spain: 18-Sep. 2010. Pharmacoepidemiology of Asthma and Xolair (PAX) Study Group Effect of omalizumab on the risk of hospitalisation in patients with uncontrolled severe asthma in real-life. The PAX-LASER cohort. [Google Scholar]
- 13.Korn S, Thielen A, Seyfried S, Taube C, Kornmann O, Buhl R. Omalizumab in patients with severe persistent allergic asthma in a real-life setting in Germany. Respir Med. 2009;103:1725–1731. doi: 10.1016/j.rmed.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Vennera MDC, De Llano LP, Bardagi S, Ausin P, Sanjuas C, González H, Gullón JA, Martínez-Moragón E, Carretero JA, Vera E, Medina JF, Álvarez FJ, Entrenas LM, Padilla A, Irigaray R, Picado C, on behalf of the Spanish Registry. Omalizumab therapy in severe asthma: Experience from the Spanish Registry—Some new approaches. J Asthma. 2012;49:416–422. doi: 10.3109/02770903.2012.668255. [DOI] [PubMed] [Google Scholar]
- 15.Eisner MD, Zazzali JL, Miller MK, Bradley MS, Schatz M. Longitudinal changes in asthma control with Omalizumab: 2 year interim data from the EXCELS study. J Asthma. 2012;49:642–648. doi: 10.3109/02770903.2012.690477. [DOI] [PubMed] [Google Scholar]
- 16.Dal Negro RW, Tognella S, Pradelli LA. 36 month study on the cost/utility of add-on omalizumab in persistent difficult-to-treat atopic7asthma in Italy. J Asthma. 2012;49:843–848. doi: 10.3109/02770903.2012.717659. [DOI] [PubMed] [Google Scholar]
- 17.Niven R, McBryan D. A UK survey of oral corticosteroid use in patients treated with omalizumab . Thorax. 2007;62((3)):A98. Abstract P91) [Google Scholar]
- 18.Simons S, Regan K, Aziz A, Saralaya D. Real-life effectiveness of omalizumab in patients with severe persistent allergic (IgE-mediated) asthma at a single UK hospital. Am J Respir Crit Care Med. 2010;181:A1336. [Google Scholar]
- 19.Knowles V, Nordstrom M, Britton M. Real-life effectiveness of omalizumab in patients with severe persistent allergic (IgE-mediated) asthma at a single UK hospital. Eur Respir J. 2009;34((suppl. 53)) 315s, E1875 (Abstract). [Google Scholar]
- 20.Gibeon D, Campbell DA, Regan SE, Menzies-Gow A. The effectiveness of omalizumab in severe allergic asthma is comparable in patients selected by NICE or SMC criteria. Am J Respir Crit Care Med. 2009;179:A2812. (Abstract) [Google Scholar]
- 21.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: A survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health-related quality of life in asthma: Development of a questionnaire for use in clinical trials. Thorax. 1992;47:76–83. doi: 10.1136/thx.47.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joint Formulary Committee. British National Formulary. London: BMJ Group and Pharmaceutical Press; 2012. [Google Scholar]
- 24.http://www.medicines.org.uk/emc/medicine/17029#POSOLOGY European Medicines Agency. Xolair 150 mg powder and solvent for solution for injection summary of product characteristics [online]. Available at.
- 25.Walsh L, Wong C, Oborne J, Cooper S, Lewis SA, Pringle M, Hubbard R, Tattersfield AE. Adverse effects of oral corticosteroids in relation to dose in patients with lung disease. Thorax. 2001;56:279–284. doi: 10.1136/thorax.56.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Covar RA, Leung DYM, McCormick D Steelman J, Zeitler P, Spahn JD. Risk factors associated with glucocorticoid-induced adverse effects in children with severe asthma. The Journal of Allergy and Clinical Immunology. 2000;106:651–9. doi: 10.1067/mai.2000.109830. [DOI] [PubMed] [Google Scholar]
- 27.Molimard M, Chanez P, Pison C, Tétu L, Le Gros V. Long term follow-up of patients under omalizumab initiated during the pre-approval period in France. European Respiratory Society Annual Congress. 2010. (Abstract #250607)
- 28.Costello RW, Long DAA, Gaine S, Mc Donnell T, Gilmartin JJ, Lane SJ. Therapy with omalizumab for patients with severe allergic asthma improves asthma control and reduces overall healthcare costs. Ir J Med Sci. 2011;180:637–641. doi: 10.1007/s11845-011-0716-2. [DOI] [PubMed] [Google Scholar]
- 29.Schatz M, Camargo CA., Jr. The relationship of sex to asthma prevalence, health care utilization and medications in a large managed care organization. Ann Allergy Asthma Immunol. 2003;91:553–558. doi: 10.1016/S1081-1206(10)61533-5. [DOI] [PubMed] [Google Scholar]
- 30.Cazzola M, Camiciottoli G, Bonavia M, et al. Italian real-life experience of omalizumab. Respir Med. 2010;104:1410–1416. doi: 10.1016/j.rmed.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Braunstahl GJ, Leo J, Chen CW, Maykut R, Georgiou P, Peachey G. The eXpeRience registry: Monitoring the ‘real-world’ effectiveness of omalizumab in allergic asthma. Poster presentation (P3953) at the European Respiratory Society Annual Congress. Amsterdam; NL: 2011. [Google Scholar]