Abstract
Introduction.
Studies of synaptic plasticity using the marine mollusk Aplysia californica as model system have been successfully used to identify proteins involved in learning and memory. The importance of molecular elements regulated by the learning- related neurotransmitter serotonin in Aplysia can then be explored in rodent models and finally tested for their relevance for human physiology and pathology.
Materials and methods.
Herein, 2-DE gel-based electrophoresis has been used to investigate protein level changes after treatment with serotonin in Aplysia abdominal ganglia.
Results.
Twenty-one proteins have been found to be regulated by serotonin, and protein level changes of actin depolymerizing factor (ADF), deleted in azoospermia associated protein (DAZAP-1), and Flotillin-1 have been verified by Western blotting.
Discussion.
Flotillin-1, a member of the flotillin/reggie family of scaffolding proteins, has been previously found to be involved in neuritic branching and synapse formation in hippocampal neurons in vitro. However, its importance for hippocampal- dependent learning and memory in the mouse has not been examined. Here, elevated levels of Flotillin-1 in hippocampal tissue of mice trained in the Morris water maze confirmed the relevance of Flotillin-1 for memory-related processes in a mammalian system. Thus, a translational approach—from invertebrates to rodents—led to the identification of Flotillin-1 as evolutionary-conserved memory-related protein.
Keywords: Aplysia, Flotillin-1, hippocampus, learning, mass spectrometry, serotonin
Key messages
Flotillin-1 is expressed in the invertebrate nervous system of Aplysia californica.
Protein levels of Flotillin-1 are up-regulated after learning-related paradigms in Aplysia abdominal ganglia and the mouse hippocampus.
The role of the evolutionary-conserved memory-related protein Flotillin-1 in memory formation and associated disorders in humans remains to be determined.
Introduction
The complexity of the neural circuitry organization of the mammalian brain have made organisms with a simpler nervous system an attractive tool for studying the neurobiological mechanisms mediating learning and memory. The gill- and siphon-withdrawal reflex of the marine invertebrate Aplysia californica is one of the most successfully used model systems for the investigation of the cellular and molecular basis underlying learning and memory-related synaptic plasticity. Behavioral sensitization of the gill-withdrawal reflex can be reconstituted in vitro by application of the modulatory neurotransmitter serotonin (5-HT), whereby repetitive stimulation induces protein synthesis-dependent long-term synaptic, strengthening or facilitation (1–5). Several of the molecular elements essential for modulating the gill-withdrawal reflex in Aplysia have been also identified to be required for other forms of learning and memory in the mammalian brain (see for review (6)). In mammals, learning-related synaptic plasticity has been most intensively studied in the hippocampus, a subcortical structure of the temporal lobe required for the formation of explicit memory in humans and other mammals (7–9). Investigations of the molecular mechanisms underlying memory-related synaptic plasticity in the hippocampus have been greatly advanced by studies in the rodent brain using well-established behavioral protocols for spatial learning, most prominently the Morris water maze (MWM) (10). However, significant differences in the expression of some of these molecular key players, even between strains of the same species, have been observed (11–13). Therefore, the parallel use of several animal model systems for the study of complex neural functions, such as learning and memory, may lead to the identification of homologous molecular principles required in different paradigms, providing stronger evidence for a causal involvement of these structures and inviting further translation studies testing their relevance for human physiology and pathology. Herein, such a translational approach—from invertebrates to rodents—has been used to search for novel proteins related to learning and memory. The first step was based upon 2-DE gel-based electrophoresis for the unbiased screening of protein level changes 24 and 48 hours after treatment with serotonin in Aplysia abdominal ganglia. Subsequently, proteins regulated by serotonin in Aplysia were tested for relevance for spatial learning and memory in rodents by probing hippocampal tissue of mice trained in the MWM.
Materials and methods
Aplysia and extraction of ganglia
Aplysia ganglia were identified in situ, and extraction of ganglia was performed essentially as previously reported (14). Aplysia californica were obtained from the Rosenstiel School of Marine and Atmospheric Science, National Resource for Aplysia, University of Miami/RSMAS, 4600 Rickenbacker Causeway, Miami, Florida 33149, USA. Dissection of Aplysia californica ganglia and handling were performed as previously described (14) with minor modifications. Briefly, adult Aplysia animals (body weights of about 120 g) were anesthetized by a direct injection of MgCl2 (0.35 M) into the internal body cavity. When body muscle contractions were not evident, indicating that animals were completely anesthetized, ganglia extraction was performed on a standard wax dissecting pan. Ventral-longitudinal skin opening cuts from the head to the tail were then carried out using small surgical scissors. Skin flaps were then smoothly separated and pinned down; the visceral mass was removed, and pairs of abdominal ganglia were quickly removed while in ice-cold artificial sea water (ASW). Individual ganglia were transferred to 35 mm Petri dishes containing 4 mL of L-15 media with 10 mg/mL of Dispase (Worthington, Catalogue Nr. LS02104, Lakewood, NJ, USA). Abdominal ganglia in separated Petri dishes were incubated for 1 h at 35°C. After partial photolytic digestion, ganglia were quickly desheathed, washed out several times with L-15/ASW (50%/50%), transferred to new Petri dishes containing L-15 medium (Leibovitz; L5520, Sigma–Aldrich Corp. St Louis, MO, USA) supplemented with NaCl 6.25 g; KCl 172 mg; CaCl2 744 mg; MgSO4 7H2O 3.12 g; MgCl2 6H2O 2.85 g; Dextrose 3.12 g, and NaHCO3 96 mg (per 500 mL of L-15) and allowed to recuperate overnight. Samples were then treated for about 2 h with 10 μM serotonin dissolved in supplemented L-15 medium as previously described (14). Treatment consisted of 5 min applications of serotonin and washing out (using supplemented L-15/ASW) at intervals of 15 min. Twenty-four and 48 hours after incubation in serotonin, time points reflecting the induction and the maintenance phase of long-term memory, respectively (15), ganglia were collected and samples were stored in cryotubes at –80°C until used for further analysis.
Mice and housing conditions
Adult C57BL/6J male mice (Janvier, France), 10–12 weeks old, were housed in pairs in standard transparent laboratory cages in a temperature-controlled colony room (21 ± 1°C) and were provided with food and water ad libitum. Mice were maintained on a 12 h light/dark cycle, and cages were cleaned once a week. All animal experiments are in line with the U.K. Animals (Scientific procedures) Act, 1996 and associated guidelines (86/609/EEC) and were approved by the local animal committee.
Morris water maze (MWM)
The MWM consisted of a circular pool (122 cm diameter, walls 76 cm depth) in which mice were trained to escape from water by swimming to a hidden platform (1.5 cm beneath water surface) whose location could be only identified using distal extra-maze cues attached to the room walls. Visual cues had different colors and dimensions and were kept constant during the whole experiment. Water temperature was maintained at 21 ± 1°C. The pool was divided into four quadrants by a computerized tracking/image analyzing system (Limelight Actimetrics, Elmwood, IL, USA). The platform was placed in the middle of the SW quadrant and remained at the same position during the acquisition phase (training). For yoked controls, no platform was placed in the tank during training sessions.
The acquisition phase consisted of 12 training trials: 3 training trials per day and 4 training days with an inter-trial interval of 60 min. Mice were released randomly with their heads facing the pool wall from the four compass locations (NE, NW, SW, and SE), and allowed to swim and search for the platform for 120 s. If mice did not locate the platform after 120 s, they were manually placed on the platform and allowed to remain on it for 15 s. On the first training day, mice were given an acclimatization training session in which they were placed on the hidden platform, were allowed to swim for 30 s, and were guided subsequently back to the platform. The day after the acquisition phase, mice underwent a probe trial in which the platform was removed. Animals were released from the longest distance to the platform and were allowed to swim freely for 60 s. The path the mouse swam was tracked and analyzed for the proportion of swimming time and/or path length spent in each quadrant of the pool. Animals were sacrificed by neck dislocation 4 h after termination of behavioral experiments. The hippocampus was rapidly dissected, snap-frozen in liquid nitrogen and the tissue was stored at –80°C until used.
Sample preparation
Preparation of samples followed the procedures previously described by our group (14).
Ganglia were first homogenized and then suspended in 1.2 mL of buffer (20 mM Tris, 7 M urea, 2 M thiourea, 4% (w/v) CHAPS, 10 mM 1.4-dithioerythritol, 1 mM EDTA, 1 mM PMSF, 1 tablet Complete from Roche Diagnostics (Mannheim, Germany), and 0.2% (v/v) phosphatase inhibitor cocktail from Calbiochem (Merck KGaA, Darmstadt, Germany)). Samples were then sonicated for approximately 30 s while in ice and then centrifuged at 15,000 g for 2 h at 12°C. Desalting procedure was performed at 12°C using an Ultrafree-4 centrifugal filter unit at 3,000 g with a cut-off molecular weight of 10 kDa (Millipore, Bedford, MA, USA) until getting an eluted volume of ˜4 mL. Bradford assay was used to determine the protein content of the supernatant.
Two-dimensional gel electrophoresis
Two-dimensional gel electrophoresis was carried out essentially as previously reported (14). Protein expression pattern of abdominal ganglia was analyzed by five independent gels (containing pooled samples of ten animals each) per group (no serotonin, 24 and 48 hours after serotonin treatment). Protein samples (700 μg) were immobilized by pH 3–10 non-linear gradient strips. Focusing voltage (V) started at 200 V and was progressively increased up to 8,000 V at a rate of 4 V/min. Conditions were kept constant for a further 3 h (approximately 150,000 Vh in total). The first incubation for 15 min was performed in DTT (1%) (w/v). For the second run, strips were equilibrated twice for 15 min in 10 mL of SDS equilibration buffer (50 mM pH 8.8 Tris-HCl, 6 M urea, 30% (v/v) glycerol, 2% (w/v) SDS, trace of bromophenol blue) while undergoing moderate shaking. The second step of incubation (15 min) was made in 4% iodoacetamide (w/v) for the second-dimensional run, and the separation was performed on 10%–16% gradient SDS-PAGE. Proteins were then fixated for 12 h in 50% methanol and 10% acetic acid. Gels underwent staining for 8 h with colloidal Coomassie blue (Novex, San Diego, CA, USA); standard 10–250 kDa markers (Bio-Rad Laboratories, Hercules, CA, USA) were used to estimate the peptide molecular masses. Standard immobilized pH gradient strips allowed the estimation of the isoelectric point values.
Quantification of protein levels
Protein spots from individual gels of the different ganglia groups were outlined (automatically and manually) and quantified using the Proteomweaver (Definiens, Munich, Germany) as previously described. The volume of each spot was determined as percentage of the sum of all spots reflecting the total protein content of the two-dimensional gel electrophoresis (14).
Western blotting
For Aplysia Western blots, aliquots of samples for 2-DE were used for Western blotting in order to verify observed expressional differences. Mouse hippocampal tissue (n = 6–8 per group) was ground under liquid nitrogen and homogenized in a protein lysis buffer containing 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.05% SDS, 0.5% Triton X100, 1 mM PMSF and protease inhibitor cocktail (1x, Roche Diagnostics, Mannheim, Germany).
Western blotting was carried out essentially as previously described (16). Membranes were blocked by incubating with 5% non-fat dry milk in 100 mM Tris pH 7.5, 150 mM NaCl, and 0.1% Tween 20 (TTBS). Membranes were then incubated with primary antibodies (DAZAP-1 [1:500], Santa Cruz Biotechnology Inc., CA, USA; ADF [1:500], Santa Cruz Biotechnology Inc.; Flotillin-1 [1:500], Abcam pIC, Cambridge, UK) overnight at 4°C, rinsed three times with TTBS, and incubated 1 h at 22°C with horseradish peroxidase-conjugated (horse anti-mouse IgG, goat anti-rabbit IgG [1:3000], Cell Signaling Technology Inc., MA, USA). Immunoreactivity was visualized by enhanced chemiluminescence (ECL, Amersham Biosciences, Piscataway, NJ, USA). Housekeeping protein β-tubulin (β-tubulin: Acris Antibodies; Hereford, Germany) was used as loading control to ensure equal amount of protein per sample on each gel. Quantification was performed by chemiluminescent imaging with a FluorChem HD2 (Alpha Innotech, San Leandro, CA, USA) using the respective software.
Statistical analysis
Student t tests were used for comparisons between two groups. Analysis of differences between three groups was performed by ANOVA. Bonferroni–Holm correction was applied to correct for multiple testing. An α-level of 0.05 was adopted in all instances. All analyses were carried out using BioStat 2009 professional software (AnalystSoft Inc., Alexandria, VA, USA).
Results
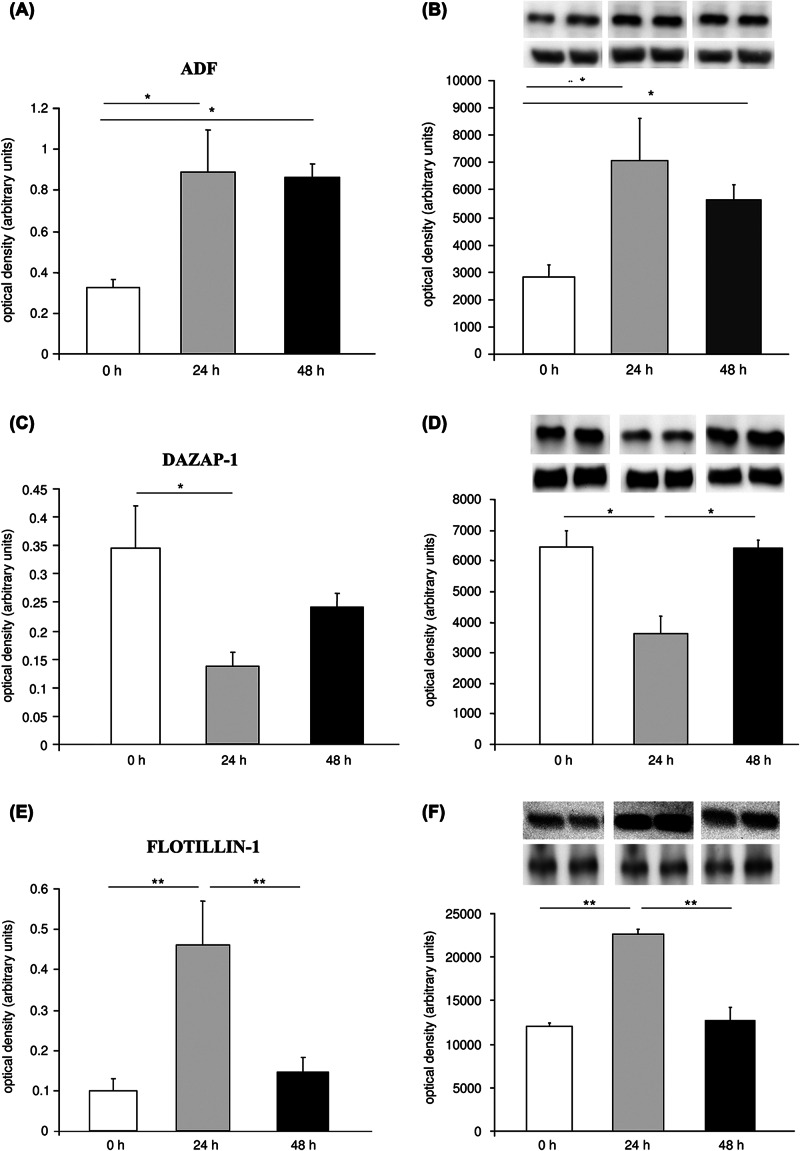
Five 2-DE gels per treatment group (untreated control, 24 h and 48 h after serotonin treatment) of Aplysia abdominal ganglia tissue pools of ten animals each were used for the software-assisted quantification of 275 spots, corresponding to 117 individual proteins recently identified by mass spectrometry from Aplysia ganglia (14). Optical density values of 24 spots representing 19 individual proteins were found to be significantly regulated by serotonin treatment (Figure 1). Information on protein levels and statistical results are summarized in Tables I and II. Subsequently, expressional changes identified by quantification of 2-DE gels were independently tested by Western blotting (WB). When commercially available antibodies were tested for their cross-reactivity with the respective Aplysia protein, antibodies for actin depolymerizing factor (ADF), deleted in azoospermia associated protein (DAZAP-1), and Flotillin-1 were found to recognize the corresponding Aplysia homologue (data not shown) and used for further WB experiments. Thus, protein level changes of ADF, DAZAP-1, and Flotillin-1 were verified in WB experiments using Aplysia abdominal ganglia tissue. As in 2-DE gels, ADF was up-regulated at 24 and 48 h after 5-HT treatment (both P < 0.05), while DAZAP-1 was down-regulated 24 h after 5-HT treatment in WB experiments (P < 0.05, Figure 2 A–D). WB further revealed significantly enhanced levels of Flotillin-1 24 h after 5-HT treatment (P < 0.05) in abdominal ganglia tissue (Figure 2 E and F).
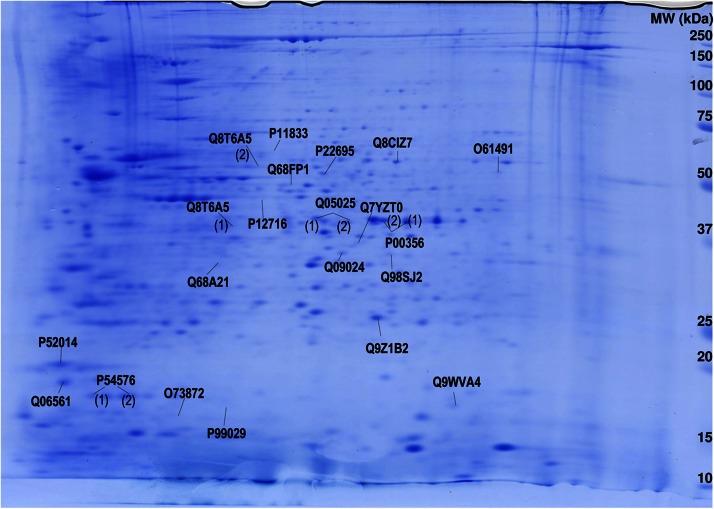
Figure 1.
Two-dimensional gel map of Aplysia abdominal ganglia proteins with statistically significant different levels after serotonin treatment. Aplysia abdominal ganglia proteins were extracted, and 700 μg were applied on an immobilized pH 3–10 non-linear gradient strip, followed by 9%–16% linear gradient polyacrylamide gel. Gels were stained with Coomassie Blue, spots were analyzed, and proteins were assigned using MASCOT software (14). UniProtKB accession numbers for protein identification are given.
Table I.
Protein levels (mean ± SD) of spots with significant differential expression at 0 (controls), 24, and 48 hours after serotonin treatment in Aplysia abdominal ganglia. Relative protein expression (arbitrary units) resulting from software-assisted quantification of 2-DE gels is given.
| Name | Accession nr. | 0 h | 24 h | 48 h |
|---|---|---|---|---|
| Actin | P12716 | 0.38 ± 0.06 | 0.39 ± 0.04 | 1.83 ± 0.36 |
| Actin-depolymerizing factor (ADF) | Q68FP1 | 0.32 ± 0.04 | 0.89 ± 0.21 | 0.86 ± 0.07 |
| Basement membrane proteoglycan | Q06561 | 1.22 ± 0.15 | 2.25 ± 0.30 | 1.65 ± 0.21 |
| Cytochrome b-c1 complex subunit 2 | P22695 | 0.22 ± 0.04 | 0.42 ± 0.05 | 0.30 ± 0.04 |
| DAZ-associated protein 1 (DAZAP-1) | Q98SJ2 | 0.35 ± 0.08 | 0.14 ± 0.02 | 0.24 ± 0.02 |
| Dihydrolipoyl dehydrogenase | Q8CIZ7 | 0.17 ± 0.02 | 0.35 ± 0.05 | 0.25 ± 0.04 |
| Flotillin-1 | O61491 | 0.10 ± 0.03 | 0.46 ± 0.11 | 0.15 ± 0.03 |
| Glutathione S-transferase Mu 5 | Q9Z1B2 | 1.68 ± 0.28 | 2.80 ± 0.34 | 3.13 ± 0.42 |
| Glyceraldehyde-3-phosphate dehydrogenase (1) | Q05025 | 0.43 ± 0.11 | 1.15 ± 0.17 | 0.77 ± 0.10 |
| Glyceraldehyde-3-phosphate dehydrogenase (2) | Q05025 | 0.37 ± 0.11 | 1.73 ± 0.29 | 0.21 ± 0.03 |
| Glyceraldehyde-3-phosphate dehydrogenase (1) | P00356 | 0.50 ± 0.07 | 1.32 ± 0.29 | 0.75 ± 0.14 |
| Glyceraldehyde-3-phosphate dehydrogenase (2) | P00356 | 0.19 ± 0.02 | 1.14 ± 0.16 | 0.22 ± 0.02 |
| Methyl-accepting chemotaxis protein (1) | P54576 | 0.82 ± 0.06 | 0.82 ± 0.06 | 0.50 ± 0.05 |
| Methyl-accepting chemotaxis protein (2) | P54576 | 0.60 ± 0.05 | 1.22 ± 0.18 | 0.84 ± 0.06 |
| Neural/ectodermal development factor IMP-L2 | Q09024 | 0.26 ± 0.02 | 0.46 ± 0.05 | 0.60 ± 0.09 |
| Peptidyl-prolyl cis-trans isomerase 6 | P52014 | 2.14 ± 0.23 | 1.55 ± 0.26 | 1.13 ± 0.11 |
| Peroxiredoxin-5 | P99029 | 2.29 ± 0.62 | 0.14 ± 0.03 | 0.20 ± 0.07 |
| Small heat shock protein p36 | Q7YZT0 | 0.26 ± 0.02 | 0.46 ± 0.04 | 0.60 ± 0.09 |
| Superoxide dismutase | O73872 | 0.32 ± 0.06 | 0.61 ± 0.06 | 0.55 ± 0.07 |
| Transcriptional activator protein Pur-beta | Q68A21 | 0.36 ± 0.09 | 0.72 ± 0.12 | 0.40 ± 0.06 |
| Transgelin-2 | Q9WVA4 | 0.37 ± 0.07 | 1.09 ± 0.18 | 0.80 ± 0.15 |
| Tubulin alpha-1 chain (1) | Q8T6A5 | 0.73 ± 0.13 | 0.43 ± 0.04 | 0.32 ± 0.04 |
| Tubulin alpha-1 chain (2) | Q8T6A5 | 0.31 ± 0.04 | 0.80 ± 0.12 | 0.53 ± 0.06 |
| Tubulin beta chain | P11833 | 0.27 ± 0.04 | 0.35 ± 0.06 | 0.49 ± 0.03 |
Table II.
Results of statistical evaluation of spots differentially expressed in Aplysia abdominal ganglia 0 (controls), 24, and 48 hours after serotonin treatment. Relative protein expression resulting from software-assisted quantification of 2-DE gels was subjected to statistical analysis (one-way ANOVA followed by Bonferroni post hoc tests).
| Name | Accession nr. | ANOVA | 0 versus 24 | 0 versus 48 | 24 versus 48 |
|---|---|---|---|---|---|
| Actin | P12716 | P < 0.001 | n.s. | P < 0.01 | P < 0.01 |
| Actin-depolymerizing factor (ADF) | Q68FP1 | P < 0.05 | P < 0.05 | P < 0.05 | n.s. |
| Basement membrane proteoglycan | Q06561 | P < 0.05 | P < 0.05 | n.s. | n.s. |
| Cytochrome b-c1 complex subunit 2 | P22695 | P < 0.05 | P < 0.05 | n.s. | n.s. |
| DAZ-associated protein 1 (DAZAP-1) | Q98SJ2 | P < 0.05 | P < 0.05 | n.s. | n.s. |
| Dihydrolipoyl dehydrogenase | Q8CIZ7 | P < 0.05 | P < 0.05 | n.s. | n.s. |
| Flotillin-1 | O61491 | P < 0.01 | P < 0.01 | n.s. | P < 0.05 |
| Glutathione S-transferase Mu 5 | Q9Z1B2 | P < 0.05 | n.s. | P < 0.05 | n.s. |
| Glyceraldehyde-3-phosphate dehydrogenase (1) | Q05025 | P < 0.01 | P < 0.01 | n.s. | n.s. |
| Glyceraldehyde-3-phosphate dehydrogenase (2) | Q05025 | P < 0.001 | P < 0.001 | n.s. | P < 0.001 |
| Glyceraldehyde-3-phosphate dehydrogenase (1) | P00356 | P < 0.05 | P < 0.05 | n.s. | n.s. |
| Glyceraldehyde-3-phosphate dehydrogenase (2) | P00356 | P < 0.001 | P < 0.001 | n.s. | P < 0.001 |
| Methyl-accepting chemotaxis protein (1) | P54576 | P < 0.01 | n.s. | n.s. | P < 0.01 |
| Methyl-accepting chemotaxis protein (2) | P54576 | P < 0.01 | P < 0.01 | n.s. | n.s. |
| Neural/ectodermal development factor IMP-L2 | Q09024 | P < 0.01 | n.s. | P < 0.01 | n.s. |
| Peptidyl-prolyl cis-trans isomerase 6 | P52014 | P < 0.05 | n.s. | P < 0.05 | n.s. |
| Peroxiredoxin-5 | P99029 | P < 0.01 | P < 0.01 | P < 0.01 | n.s. |
| Small heat shock protein p36 | Q7YZT0 | P < 0.01 | n.s. | P < 0.01 | n.s. |
| Superoxide dismutase | O73872 | P < 0.05 | n.s. | P < 0.05 | n.s. |
| Transcriptional activator protein Pur-beta | Q68A21 | P < 0.05 | P < 0.05 | n.s. | n.s. |
| Transgelin-2 | Q9WVA4 | P < 0.05 | P < 0.05 | n.s. | n.s. |
| Tubulin alpha-1 chain (1) | Q8T6A5 | P < 0.05 | n.s. | P < 0.05 | n.s. |
| Tubulin alpha-1 chain (2) | Q8T6A5 | P < 0.01 | P < 0.01 | n.s. | n.s. |
| Tubulin beta chain | P11833 | P < 0.05 | n.s. | P < 0.05 | n.s. |
n.s. = non-significant.
Figure 2.
Verification of quantitative results from 2-DE by Western blotting in abdominal ganglia. Protein level changes after serotonin treatment in abdominal ganglia of ADF detected by (A) 2-DE and (B) Western blotting; DAZAP-1 detected by (C) 2-DE and (D) Western blotting; Flotillin-1 detected by (E) 2-DE and (F) Western blotting. Inserts in B, D, and F depict representative images of original Western blots of target proteins and β-tubulin loading control, respectively. All data display optical densities as mean ± SEM. *P < 0.05; **P < 0.01.
To investigate the relevance of these three proteins for spatial learning and memory in the mammalian brain, their expression was analyzed in hippocampal homogenates of mice trained in the MWM and dissected 6 hours after the last training trial. Levels of Flotillin-1 were selectively augmented after MWM learning in mouse hippocampal tissue (P < 0.05), while no changes in levels of ADF and DAZAP-1 (P > 0.05) were observed (Figure 3).
Figure 3.
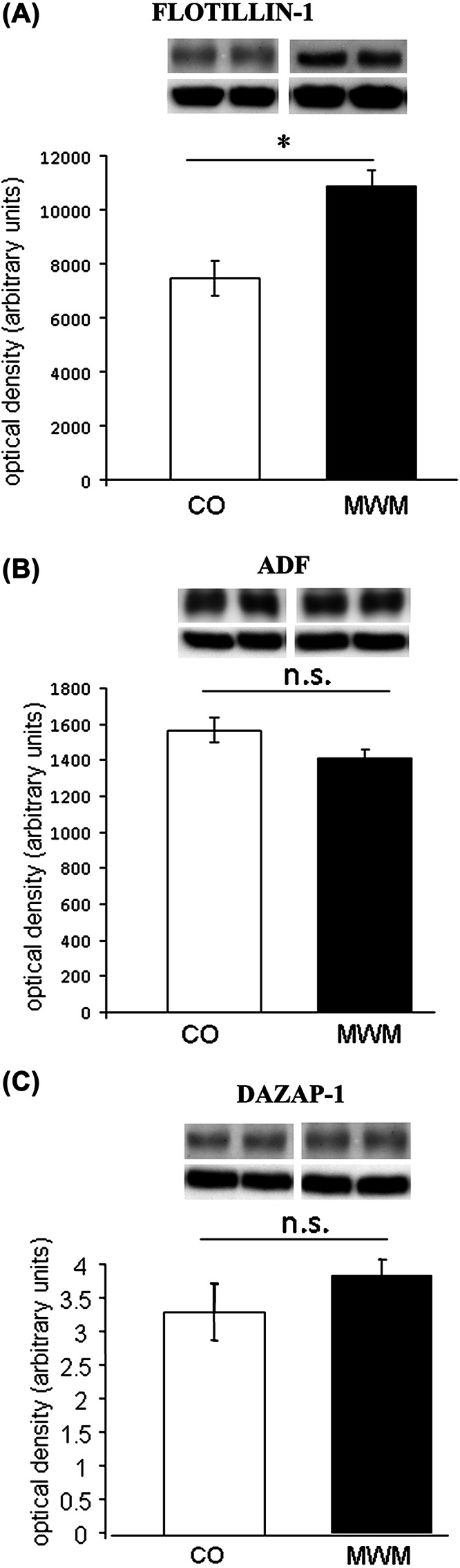
Protein levels of Flotillin-1 are increased after spatial learning in the mouse hippocampus. Levels of (A) Flotillin-1, (B) ADF, and (C) DAZAP-1 in hippocampal tissue of mice trained in the Morris water maze (MWM) and yoked controls (CO) (n = 6–8 per group). Inserts depict representative images of original Western blots of target proteins and β-tubulin loading control, respectively. All data display optical densities as mean ± SEM. *P < 0.05; n.s. = not significant.
Discussion
Herein, a translational approach—from invertebrates to rodents— was used to search for novel proteins linked to learning-related synaptic plasticity and memory function. The unbiased proteomic screening of proteins regulated by the learning-related neurotransmitter serotonin in the abdominal ganglia of Aplysia—which mediates the gill- and siphon-withdrawal reflex (17)—identified the modulation of expressional levels of 21 individual proteins. Of the three proteins—ADF, DAZAP-1, and Flotillin-1—verified to be regulated by 5-HT in Aplysia ganglia using WB. Only Flotillin-1 was found to be also related to spatial learning in the mouse hippocampus. Flotillin-1, belonging to a family of proteins characterized by an evolutionarily conserved stomatin/prohibitin/flotillin/HflK/C (SPFH)1 domain (18,19), is a lipid raft-associated protein which oligomerizes with Flotillin-2 to form homomeric and heteromeric tetramers (20). Although it is ubiquitously expressed (see for review: (21)), it has been shown to serve distinct functions in the nervous system. Flotillin-1 has been shown to be important for axonal regeneration (22,23), differentiation and outgrowth of hippocampal neurons (22,24), as well as formation of glutamatergic synapses in the hippocampus (24) and internalization of neurotransmitter transporters (25). However, its relevance for learning and memory processes in vivo has not been demonstrated so far. Although in the mouse hippocampus the effect of Flotillin-1 on synaptic activity has been mainly related to the activity of glutamate (24), we here show that modulation of the serotonergic system affects protein levels of Flotillin-1 in the Aplysia model system. Enhanced expression of Flotillin-1 may then in turn contribute to the neuronal remodeling accompanying 5-HT-induced long-term facilitation (15) since it promotes neurite outgrowth and neuritic branching (24). Up-regulation of Flotillin-1 after spatial learning in the mouse hippocampus is likely to reflect similar structural rearrangements including generation and maturation of dendritic spines, (24) necessary for long-term memory formation (see for review: (26)). Interestingly, when looking at the time-course of induction of protein expression in Aplysia, we found Flotillin-1 to be up-regulated at 24 hours but not 48 hours after stimulation with 5-HT. So far, although the cellular role of Flotillin-1 has been intensively investigated, little information on the regulation of its expression is available (27). Recently, it has been suggested that Flotillin-1 serves as scaffold at lipid rafts supporting the association of signaling molecules at the rafts, thus importantly modulating some of the major cellular signal transduction pathways, including those involved in learning and memory processes, such as the mitogen-activated protein kinase (MAPK) ERK1/2 cascade (27–29). Thus, it appears plausible that the protein levels of Flotillin-1 need to be tightly controlled in order to enable proper reactivity of cells to incoming stimuli. Additionally, the expression of Flotillin-1 has been found to be regulated by transcription factors which are also implicated in synaptic plasticity (such as Egr1 (30) and SRF (31)) and are selectively expressed at early time points after incoming stimulating events. Therefore, the selective up-regulation at 24 hours after 5-HT application in the Aplysia nervous system is in line with the available information on the regulation of Flotillin-1 expression. In the mouse hippocampus, only a single time point after Morris water maze learning (6 hours) has been evaluated in the present study, and future investigations using a time-course analysis may shed light on the temporal regulation of Flotillin-1 expression in learning-related events in the mammalian brain. Since this is the first demonstration of induction of Flotillin-1 in vivo in a behavioral learning paradigm, further experiments in experimental animals using gene knock-out or knock-down methodologies will be needed to determine whether expression of Flotillin-1 is not only related to but also necessary for long-term memory formation and storage. These approaches may then be suitable to address whether up-regulation of Flotillin-1 is cause or consequence of hippocampal-dependent learning in the intact animal and may provide further information for the targeted investigation of Flotillin-1 in human learning and memory and associated disorders. Unaltered levels of ADF and DAZAP-1 in hippocampal tissue of MWM-trained mice do not preclude a potential involvement of these proteins in learning and memory in the mammalian brain but suggest that they may not relate to the formation and retrieval of spatial information. Additionally, we cannot exclude the possibility that no changes in protein levels of ADF and DAZAP-1 were found because of the time point chosen for WB experiments. Future studies assessing a time-course of protein level changes in the mouse hippocampus after behavioral training may analyze this aspect in greater depth.
In summary, the most important result of this study is the identification of Flotillin-1 as an evolutionary-conserved memory-related protein in two distinct animal models systems involving implicit and explicit forms of learning.
Acknowledgments
Declaration of interest: Francisco J. Monje is supported by the ‘Hochschuljubiläums-Stiftung der Stadt Wien’. Daniela D. Pollak is supported by the Austrian Science Fund (FWF): stand-alone project P22424 and member of the research network SFB35. The authors declare no financial/commercial conflict of interest.
References
- 1.Carew TJ, Sahley CL. Invertebrate learning and memory: from behavior to molecules. Annu Rev Neurosci. 1986;9:435–87. doi: 10.1146/annurev.ne.09.030186.002251. [DOI] [PubMed] [Google Scholar]
- 2.Castellucci V, Pinsker H, Kupfermann I, Kandel ER. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167:1745–8. doi: 10.1126/science.167.3926.1745. [DOI] [PubMed] [Google Scholar]
- 3.Kupfermann I, Castellucci V, Pinsker H, Kandel E. Neuronal correlates of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167:1743–5. doi: 10.1126/science.167.3926.1743. [DOI] [PubMed] [Google Scholar]
- 4.Lukowiak K, Jacklet JW. Habituation and dishabituation: interactions between peripheral and central nervous systems in Aplysia. Science. 1972;178:1306–8. doi: 10.1126/science.178.4067.1306. [DOI] [PubMed] [Google Scholar]
- 5.Pinsker H, Kupfermann I, Castellucci V, Kandel E. Habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167:1740–2. doi: 10.1126/science.167.3926.1740. [DOI] [PubMed] [Google Scholar]
- 6.Mayford M, Kandel ER. Genetic approaches to memory storage. Trends Genet. 1999;15:463–70. doi: 10.1016/s0168-9525(99)01846-6. [DOI] [PubMed] [Google Scholar]
- 7.Morris RG, Schenk F, Tweedie F, Jarrard LE. Ibotenate lesions of hippocampus and/or subiculum: dissociating components of allocentric spatial learning. Eur J Neurosci. 1990;2:1016–28. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 8.Orr WB, Berger TW. Hippocampectomy disrupts the topography of conditioned nictitating membrane responses during reversal learning. Behav Neurosci. 1985;99:35–45. doi: 10.1037//0735-7044.99.1.35. [DOI] [PubMed] [Google Scholar]
- 9.Squire LR, Cohen NJ, Zouzounis JA. Preserved memory in retrograde amnesia: sparing of a recently acquired skill. Neuropsychologia. 1984;22:145–52. doi: 10.1016/0028-3932(84)90057-5. [DOI] [PubMed] [Google Scholar]
- 10.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 11.Pollak DD, John J, Scharl T, Leisch F, Schneider A, Hoeger H, et al. Strain-dependent regulation of neurotransmission and actin-remodelling proteins in the mouse hippocampus. Genes Brain Behav. 2006;5:200–4. doi: 10.1111/j.1601-183X.2006.00207.x. [DOI] [PubMed] [Google Scholar]
- 12.Pollak DD, John J, Schneider A, Hoeger H, Lubec G. Strain-dependent expression of signaling proteins in the mouse hippocampus. Neuroscience. 2006;138:149–58. doi: 10.1016/j.neuroscience.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Pollak DD, Scharl T, Leisch F, et al. Strain-dependent regulation of plasticity-related proteins in the mouse hippocampus. Behav Brain Res. 2005;165:240–6. doi: 10.1016/j.bbr.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Monje FJ, Pollak DD, Lubec G. A first partial Aplysia californica proteome. Amino Acids. 2011;41:955–68. doi: 10.1007/s00726-010-0795-9. [DOI] [PubMed] [Google Scholar]
- 15.Bailey CH, Kandel ER, Si K. The persistence of long-term memory: a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron. 2004;44:49–57. doi: 10.1016/j.neuron.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Monje FJ, Cabatic M, Divisch I, Kim EJ, Herkner KR, Binder BR, et al. Constant darkness induces IL-6-dependent depression-like behavior through the NF-kappaB signaling pathway. J Neurosci. 2011;31:9075–83. doi: 10.1523/JNEUROSCI.1537-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byrne JH, Baxter DA, Buonomano DV, Cleary LJ, Eskin A, Goldsmith JR, et al. Neural and molecular bases of nonassociative and associative learning in Aplysia. Ann N Y Acad Sci. 1991;627:124–49. doi: 10.1111/j.1749-6632.1991.tb25918.x. [DOI] [PubMed] [Google Scholar]
- 18.Rivera-Milla E, Stuermer CA, Malaga-Trillo E. Ancient origin of reggie (flotillin), reggie-like, and other lipid-raft proteins: convergent evolution of the SPFH domain. Cell Mol Life Sci. 2006;63:343–57. doi: 10.1007/s00018-005-5434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tavernarakis N, Driscoll M, Kyrpides NC. The SPFH domain: implicated in regulating targeted protein turnover in stomatins and other membrane-associated proteins. Trends Biochem Sci. 1999;24:425–7. doi: 10.1016/s0968-0004(99)01467-x. [DOI] [PubMed] [Google Scholar]
- 20.Solis GP, Hoegg M, Munderloh C, Schrock Y, Malaga-Trillo E, Rivera-Milla E, et al. Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem J. 2007;403:313–22. doi: 10.1042/BJ20061686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrow IC, Parton RG. Flotillins and the PHB domain protein family: rafts, worms and anaesthetics. Traffic. 2005;6:725–40. doi: 10.1111/j.1600-0854.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 22.Munderloh C, Solis GP, Bodrikov V, Jaeger FA, Wiechers M, Málaga-Trillo E, et al. Reggies/flotillins regulate retinal axon regeneration in the zebrafish optic nerve and differentiation of hippocampal and N2a neurons. J Neurosci. 2009;29:6607–15. doi: 10.1523/JNEUROSCI.0870-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulte T, Paschke KA, Laessing U, Lottspeich F, Stuermer CA. Reggie-1 and reggie-2, two cell surface proteins expressed by retinal ganglion cells during axon regeneration. Development. 1997;124:577–87. doi: 10.1242/dev.124.2.577. [DOI] [PubMed] [Google Scholar]
- 24.Swanwick CC, Shapiro ME, Vicini S, Wenthold RJ. Flotillin-1 promotes formation of glutamatergic synapses in hippocampal neurons. Dev Neurobiol. 2010;70:875–83. doi: 10.1002/dneu.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cremona ML, Matthies HJ, Pau K, Bowton E, Speed N, Lute BJ, et al. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat Neurosci. 2011;14:469–77. doi: 10.1038/nn.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuste R. Dendritic spines and distributed circuits. Neuron. 2011;71:772–81. doi: 10.1016/j.neuron.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banning A, Ockenga W, Finger F, Siebrasse P, Tikkanen R. Transcriptional regulation of flotillins by the extracellularly regulated kinases and retinoid X receptor complexes. PLoS One. 2012;7:45514. doi: 10.1371/journal.pone.0045514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amaddii M, Meister M, Banning A, Tomasovic A, Mooz J, Rajalingam K, et al. Flotillin-1/reggie-2 protein plays dual role in activation of receptor-tyrosine kinase/mitogen-activated protein kinase signaling. J Biol Chem. 2012;287:7265–78. doi: 10.1074/jbc.M111.287599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzucchelli C, Brambilla R. Ras-related and MAPK signalling in neuronal plasticity and memory formation. Cell Mol Life Sci. 2000;57:604–11. doi: 10.1007/PL00000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89:121–45. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knoll B, Nordheim A. Functional versatility of transcription factors in the nervous system: the SRF paradigm. Trends Neurosci. 2009;32:432–42. doi: 10.1016/j.tins.2009.05.004. [DOI] [PubMed] [Google Scholar]