Abstract
The interactions between bioactive rich food components within a complex human diet for the inhibition of prostate carcinogenesis (PCa) are largely unknown and difficult to quantify in humans. Tomato and soy products have each shown anti-PCa activity in laboratory studies. The objective of this study was to determine the efficacy of dietary tomato and soy germ, alone and in combination, for the inhibition of prostate carcinogenesis in the transgenic adenocarcinoma of the mouse prostate (TRAMP) model. At 4 weeks of age, male C57BL/6 × FVB TRAMP mice (n=119) were randomized to consume: AIN-93G control, 10% whole tomato powder (TP), 2% soy germ powder (SG) or 10% tomato powder with 2% soy germ powder (TP+SG) for 14 weeks. 100% of mice fed the control diet had PCa, while PCa incidence was significantly lower in mice consuming TP (61%, p<0.001), SG (66%, p<0.001) and TP+SG (45%, p<0.001). Although the protection offered by the combination of TP and SG was not synergistic, it was the most effective intervention. TP, SG and TP+SG increased apoptotic index (AI) and modestly reduced the proliferative index (PI) in the prostate epithelium of TRAMP mice exhibiting primarily prostatic intraepithelial neoplasia. The dramatic reduction in the PI/AI ratio by the dietary interventions suggests that the control mice experience a stronger stimulus for malignant progression in the prostate microenvironment. Maximally effective and safe strategies for PCa prevention may result from optimizing combinations of nutrients and bioactives through an orchestration of dietary patterns.
Keywords: Tomato, lycopene, prostate cancer, soy, equol
INTRODUCTION
The combination of pharmacologic agents based upon selection of different mechanisms of action and non-overlapping toxicity has led to dramatic success in the chemotherapy for certain cancers (1, 2). Yet, these principles have not been effectively applied to translational human cancer prevention studies. Numerous epidemiological studies suggest that consumption of soy foods or tomato products is associated with a lower risk of prostate cancer (PCa) (3, 4) and this manuscript explores the potential benefit of combining them for PCa prevention. Soy products are a rich source of bioactive components, such as isoflavones, saponins, and lignans. Tomatoes also provide various polyphenols, but are best known as a source of carotenoids such as lycopene and precursors, phytoene and phytofluene (5). Anti-PCa activity of soy or tomato components tested in cell culture or rodent models include protection against oxidative stress, inhibition of proliferation, enhanced sensitivity to apoptotic death signals, inhibition of inflammation and angiogenesis, and disruption of hormonal and growth factor signaling, among others(5–8). Experimental models of prostate tumorigenesis and carcinogenesis suggest that both soy and tomato products and several of their components have significant anti-cancer bioactivity (8–18).
Our research team is particularly interested in examining the hypothesis that whole foods or novel food products containing a diverse array of bioactive phytochemicals, each individually at modest concentrations, may have greater activity for cancer prevention than any individual component developed as a pharmaceutical chemopreventive agent. For example, we found that tomato powder is more effective than lycopene in reducing testosterone and prostate carcinogenesis in rodents (9, 19). Recent in vitro evidence suggests that whole soy extract is more effective than individual isoflavones and sera from men consuming tomato paste was more effective than sera from men consuming lycopene alone in inducing cell cycle arrest and apoptosis in PCa cells (20, 21). People consume complex diets, yet there is limited research on the potential additive or interactive anti-cancer effects for consumption of multiple foods in combination. The combination of tomato and broccoli was more effective at reducing PCa progression in rats than when these vegetables are consumed individually (9), and the combination of dietary soy and tea, but not soy or tea alone, was effective in reducing inflammation and the development of prostate neoplasms in rats (10). The reductionist approach of testing single bioactive components derived from foods is useful in identifying potential anticarcinogenic drugs or to provide insight into mechanisms of action for a food; however, it is increasingly important to investigate whole foods and combinations of foods, which may be more efficacious due to the presence of multiple bioactive compounds with complementary or synergistic activity.
Epidemiological and laboratory studies support the hypotheses that diets rich in soy or tomato products may reduce the risk of PCa, and the limited, but positive research on the added benefits of whole foods and mixtures of foods suggest that a combination of tomato and soy may be more protective. High compliance for a dietary intervention with the combination of tomato and soy products in a short phase II study in men with active PCa has previously been completed (22), supporting feasibility and justifying future investigations regarding the efficacy of the combination of these foods for biological activity in the prostate. In vivo models provide an opportunity to examine the efficacy of interventions for future translation into clinical prevention studies. The TRAMP (transgenic adenocarcinoma of the mouse prostate) model, which develops a prostate epithelium-specific cancer, is well-characterized and has been used extensively for investigation of dietary and pharmacologic chemoprevention strategies (23). The objective of this study was to test the hypotheses that dietary interventions with tomato or soy germ, alone and in combination, would reduce the incidence of PCa in TRAMP mice.
MATERIALS AND METHODS
Animals and Experimental Design
The University of Illinois Laboratory Animal Care Advisory Committee approved all animal procedures. Male C57BL/6 × TRAMP mice, heterozygous for the probasin-Tag transgene, female C57BL/6 and male and female FVB/NJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Female and male heterozygous TRAMP (C57BL/6) mice from our colony were bred with FVB/NJ mice to obtain [TRAMPxFVB/NJ] F1 offspring used for the study. Mouse tail DNA from male offspring was isolated with an Extract-N-Amp™ Tissue PCR kit (Sigma Aldrich, St. Louis, MO), and mice were genotyped via PCR-based DNA screening. Offspring were weaned at three weeks of age, individually housed in shoe-box cages under controlled conditions (12 h light-dark cycle, 22°C, 60% humidity) and acclimated to a powdered, modified AIN-93G diet for one week. At 4 weeks of age, mice were randomized to consume experimental diets: AIN-93G control (n=29), 10% whole tomato powder (n=31) (FutureCeuticals, Momence, IL), 2% soy germ powder (n=32) (Frutarom, North Bergen, NJ), and 10% tomato powder with 2% soy germ powder (n=27). Diets were balanced for protein, fat, energy and fiber (Table 1), and stored at 4°C in the dark. Non-transgenic litter mates (n=5 per a dietary group) were included in the study to confirm the effects of the transgene. Mice were weighed weekly, and individual feed intake was measured three times a week when fresh diet was provided.
Table 1.
Composition of Experimental Diets
| g/100g total diet | ||||
|---|---|---|---|---|
| Control | 10% Tomato Powder a |
2% Soy Germ b |
10% Tomato Powder + 2% Soy Germ ab |
|
| Cornstarch | 39.75 | 36.5 | 39.75 | 36.5 |
| Casein | 20.0 | 18.7 | 19.12 | 17.82 |
| Maltodextrin | 13.2 | 9.95 | 13.2 | 9.95 |
| Sucrose | 10.0 | 10.0 | 10.0 | 10.0 |
| Fiber c | 5.0 | 3.3 | 4.36 | 2.66 |
| Mineral Mix d | 3.5 | 3.5 | 3.5 | 3.5 |
| Vitamin Mix e | 1.0 | 1.0 | 1.0 | 1.0 |
| L-Cystine | 0.3 | 0.3 | 0.3 | 0.3 |
| Choline Bitrate | 0.25 | 0.25 | 0.25 | 0.25 |
| Cottonseed Oil | 7.0 | 6.5 | 6.52 | 6.02 |
| Tomato Powder f | 0.0 | 10.0 | 0.0 | 10.0 |
| Soy Germ g | 0.0 | 0.0 | 2.0 | 2.0 |
Contains 268 µg total lycopene per gram of diet
Contains 200 µg total daidzein, 156 µg total glycitein, and 45 µg total genistein per gram of diet. For all three isoflavones, glucosides accounted for 58%, acetyl glucosides 32–39%, and aglycones 4–10% of total isoflavones.
Non-nutritive cellulose,
AIN93-MX formulation,
AIN93-VX formulation
FutureCeuticals Tomato Powder 20 N8
Frutarom SoyLife® Complex Micro
At 18 weeks of age, mice were asphyxiated by CO2 and blood was collected by cardiac puncture. When possible, the prostate was microdissected into individual lobes (anterior, dorsal, lateral and ventral). Liver, testes and half of each prostate lobe were flash frozen in liquid nitrogen and stored at −80°C. All animals were thoroughly examined for gross metastases. Lungs, liver sections, enlarged peri-aortic lymph nodes and half of each prostate lobe were fixed in 10% phosphate-buffered formalin overnight and transferred to 70% ethanol.
Histopathology
Tissues were paraffin embedded and 4µm sections were stained with hematoxylin/eosin for pathologic grading. A blinded examiner (SKC), with extensive experience with transgenic mouse models of PCa, evaluated all prostate lobes for incidence and severity of pathology according to a grading scheme which has been previously described (24).
Immunohistochemical analysis
Paraffin-embedded tissue sections (4 µm) were prepared, deparaffinized and rehydrated. Slides were placed in a decloaking chamber and treated in a citrate buffer (pH 6.0) for 30 seconds at 125°C and 10 seconds at 90°C for antigen retrieval. The subsequent steps were completed in a BioGenex i6000 Automated Staining System (BioGenex, San Ramon, CA). Endogenous peroxidase was quenched with a 3% H202 solution for 15 minutes. Slides were blocked with Power Block™ (BioGenex) for 10 min, avidin blocked for 15 min and biotin blocked for 15 min. Slides were incubated with rabbit anti-proliferating cell nuclear antigen (PCNA) antibody (Abcam, Cambridge, MA) or rabbit anti-cleaved caspase-3 (Cell Signaling, Danvers, MA) for 30 minutes and visualized using a SuperSensitive™ Link-Label IHC Detection System (Biogenex). Slides were stained with DAB and counterstained with hematoxylin. Tissue from mouse small intestine was used as a positive control for PCNA, and mouse thymus was used as a positive control for cleaved caspase-3. Negative control slides were obtained by omitting the primary antibody. Stained slides were scanned with a NanoZoomer 2.0-HT digital slide scanner (Hamamtsu, Bridgewater, NJ) with Olympus Uplansapo 20× objective at 40× digital zoom, giving 0.23µm resolution. Images were captured with NDP view software (Hamatsu). A representative image of the dorsal lobe and a representative image of the lateral lobe of the prostate were captured for each mouse. Proliferation and apoptotic index were calculated as previously described in our laboratory (9, 25). Apoptotic index (AI) from cleaved-caspase-3 stained prostate sections was calculated as: AI = (cleaved caspase-3 positive cell count / total epithelial cells counted) × 100. Proliferation index (PI) from PCNA stained prostate sections was calculated as: PI = (PCNA positive epithelial cell count / total epithelial cells counted) × 100. These indices were established by counting at least 1,000 cells from each image.
Carotenoid Analysis
Carotenoid extraction and analysis of diet, serum and tissues was performed as previously described (26). Serum and testes were pooled within groups to facilitate HPLC detection of carotenoids, and liver was extracted in duplicate.
Isoflavone Analysis
Serum, prostate and diet samples were analyzed for genistein, glycitein and daidzein and their conjugates and metabolites at The Ohio State University Comprehensive Cancer Center Nutrient and Phytochemical Analytic Shared Resource. Briefly, 100–200µL of serum was extracted with two volumes of acetonitrile. Extracts were resuspended in 2:1 acetonitrile/water, probe sonicated, centrifuged, and the supernatant was collected. This was repeated, and the supernatants were pooled, dried under nitrogen, and resuspended in 0.5 mL 2M acetate buffer (pH 5.5). Isoflavones were deconjugated with β-glucuronidase and sulfatase in 2 M acetate buffer (pH 5.5) for 1 h at 37 °C. Digests were extracted twice with three volumes of ether and dried under nitrogen. Extracts were redissolved in 150µL methanol with bath sonication and filtered through 0.2µm nylon before injection. Approximately 40 mg of prostate tissue was transferred to a 1.5mL microcentrifuge tube, suspended in 400µL of water and probe sonicated with a Fisher dismembrator at 35% amplitude setting for 5 seconds. 800µL of acetonitrile was added to induce protein precipitation and extract isoflavone metabolites. Samples were centrifuged for 5 min at 21,500 rcf to pellet solids, and supernatants were transferred to a 4mL glass vial. Pellets were resuspended in 1.2 mL of 2:1 acetonitrile/water, probe sonicated and centrifuged. Supernatants were pooled with first supernatants in a speedvac (45°C, 0.1 vacuum) until dry (1.5 hrs). Residues were resuspended in 1mL of 2M acetate (pH 5.5) and isoflavones were deconjugated with β-glucuronidase and sulfatase in 0.2% NaCl for 3hrs at 37°C. Extracts were digested twice with three volumes ether (3mL). Ether extracts were pooled, dried under nitrogen, redissolved in 150µL methanol with bath sonication, and filtered through 0.2 µm nylon before injection. Samples were analyzed by UPLC/MS with a Phenomenex Synergi Fusion RP Column (2×50mm, 2.5µm) and quadrupole mass spectrometer (Quattro Ultima, Micromass, Manchester, UK) via an electrospray probe operated at negative polarity. Authentic standards of daidzein, dihydrodaidzein, o-desmethylangolensin, equol, glycitein, genistein, and dihydrogenistein were used as external calibrants.
Serum VEGF
Serum VEGF was analyzed by ELISA according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
Statistical analysis
SAS (version 9.3; SAS Institute, Cary, NC) was used for all statistical analysis, and a p < 0.05 was considered significant. Serum lycopene and isoflavones were analyzed by a Student’s t- test. Weight gain, feed intake, gain:feed ratio, serum VEGF, proliferation index and apoptotic index were compared by two-tailed analysis of variance using the mixed model procedure in SAS with a Dunnett’s post-hoc test. Fisher’s exact test was used to compare incidence of pathology between an experimental group and the controls.
RESULTS
All experimental diets readily consumed by TRAMP mice
There were no significant differences in weight gain or gain:feed ratio between a dietary intervention group and the control. Feed intake in the TP and SG groups was not significantly different from the controls. The average daily feed intake in the TP+SG group (5.57 ± 0.03 grams) was significantly lower than the control group (5.71 ± 0.03 grams, p=0.003); however, this minimal difference in feed intake is likely not biologically significant.
Tomato powder feeding increases serum and tissue lycopene accumulation
Lycopene, the primary carotenoid in tomatoes, was detected in serum and tissues of mice consuming TP or TP+SG (Table 2). Mean serum lycopene levels were significantly higher in TP fed mice than mice fed TP+SG (p = 0.03). Testes lycopene was nearly three times higher in mice that consumed TP compared to TP+SG (p = 0.006). Although not statistically significant, lycopene accumulation in the prostate tumors (p = 0.2) and liver (p = 0.3) was also lower in TP+SG fed mice.
Table 2.
Serum and Tissue Lycopene and Isoflavones
| Control | 10% Tomato Powder |
2% Soy Germ | 10% Tomato Powder + 2% Soy Germ |
|
|---|---|---|---|---|
| Serum (µM) and Tissue (nmol/g) Lycopene | ||||
| Serum | ND1 | 0.67 ± 0.07 | ND1 | 0.44 ± 0.04* |
| Liver | ND1 | 4.56 ± 0.91 | ND1 | 3.14 ± 0.56 |
| Prostate Tumor | ND1 | 0.25 ± 0.05 | ND1 | 0.18 ± 0.04 |
| Testes | ND1 | 1.42 ± 0.23 | ND1 | 0.47 ± 0.10* |
| Serum Isoflavones (µM) | ||||
| Total Isoflavones | NA2 | NA2 | 3.93 ± 0.60 | 4.02 ± 0.26 |
| Total Parent | NA2 | NA2 | 0.61 ± 0.22 | 0.74 ± 0.14 |
| Daidzein | NA2 | NA2 | 0.29 ± 0.85 | 0.48 ± 0.09 |
| Glycitein | NA2 | NA2 | 0.14 ± 0.39 | 0.24 ± 0.05 |
| Genisteina | NA2 | NA2 | 0.18 ± 0.10 | 0.01 ± 0.01 |
| Total Metabolites | NA2 | NA2 | 3.32 ± 0.49 | 3.28 ± 0.17 |
| Dihydrodaidzein | NA2 | NA2 | 0.02 ± 0.01 | 0.08 ± 0.03 |
| Dihydrogenistein | NA2 | NA2 | 0.01 ± 0.01 | 0.03 ± 0.01 |
| O-desmethylangolensin | NA2 | NA2 | 0.03 ± 0.01 | 0.07 ± 0.03 |
| Equol | NA2 | NA2 | 3.26 ± 0.49 | 3.10 ± 0.18 |
| Prostatic Isoflavones (nmol/g) | ||||
| Total Isoflavones | NA2 | NA2 | 0.201 ± 0.083 | 0.224 ± 0.068 |
| Total Parent | NA2 | NA2 | 0.048 ± 0.022 | 0.041 ± 0.005 |
| Daidzein | NA2 | NA2 | 0.035 ± 0.017 | 0.029 ± 0.003 |
| Glycitein | NA2 | NA2 | 0.010 ± 0.004 | 0.008 ± 0.002 |
| Genistein | NA2 | NA2 | 0.003 ± 0.001 | 0.003 ± 0.001 |
| Total Metabolites | NA2 | NA2 | 0.152 ± 0.062 | 0.183 ± 0.063 |
| Dihydrodaidzein | NA2 | NA2 | 0.032 ± 0.014 | 0.034 ± 0.011 |
| Dihydrogenistein | NA2 | NA2 | 0.003 ± 0.001 | 0.003 ± 0.001 |
| O-desmethylangolensin | NA2 | NA2 | 0.001 ± 0.001 | 0.002 ± 0.001 |
| Equol | NA2 | NA2 | 0.117 ± 0.047 | 0.142 ± 0.050 |
Not detectable
Isoflavones were not detected in the analysis of AIN-93G or 10% tomato powder diets; therefore serum from mice consuming these diets was not analyzed for isoflavones.
genistein was not detected in 3 mice in the 2% soy germ group and 6 mice in the 10% tomato group + 2% soy germ group.
Values are means ± SEM. Liver n = 7, serum and testes lycopene n = 5, tumor n = 5 – 8, serum isoflavones n = 7–8, prostate isoflavones n=4.
within rows indicate significant difference between treatments (P < 0.05).
Isoflavones and isoflavone metabolites are detected in serum and prostate of TRAMP mice consuming soy germ
Parent isoflavones and isoflavone metabolites were detected in the serum and prostate of SG and TP+SG fed mice (Table 2). Individual and total isoflavone concentrations in the serum and prostate were not significantly different between SG or TP+SG fed mice. Equol, a metabolite of daidzein, was the primary isoflavone detected in the serum and prostate, and equol concentrations were higher than all other metabolites and parent isoflavones combined. Serum genistein was below detection limits in 3 out of 7 SG fed mice and 6 out of 8 TP+SG mice (limit of detection = 5×10−9 mol/L).
Range of pathology identified in TRAMP mice
Non-transgenic mice in all of the dietary groups had histologically normal prostates. At the time of necropsy (age 18 weeks), all TRAMP mice had evidence of prostatic intraepithelial neoplasia (PIN) or PCa. Representative images of the range of pathological progression in mice observed are shown in Figure 1. Cancerous lesions categorized as well-differentiated (WD), moderately-differentiated (MD) or poorly-differentiated (PD) carcinoma were identified in 68% of the TRAMP mice (Table 3). As previously described in the TRAMP model, cancer lesions were more frequent in the dorsal, lateral and ventral lobes (27), and the most common pathology was PD carcinoma.
Figure 1.
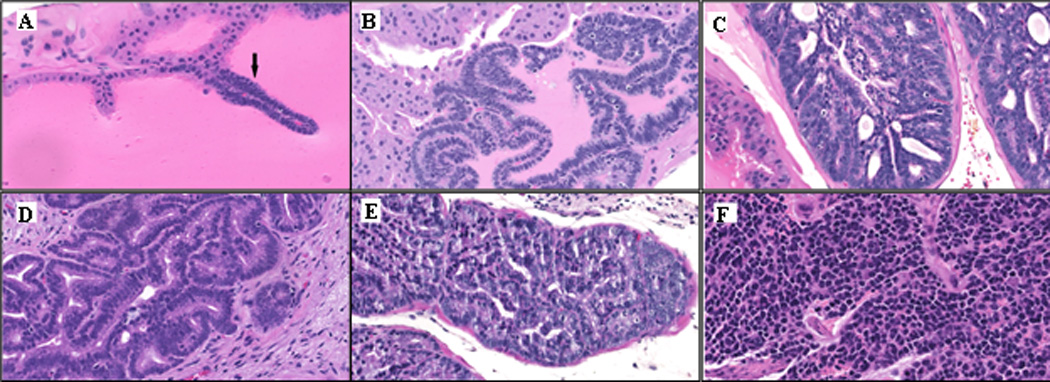
Prostate pathology in 18-week old TRAMP mice. (A) Low-grade PIN, (B) Moderate-grade PIN, (C) High-grade PIN, (D) Well-differentiated adenocarcinoma, (E) Moderately-differentiated adenocarcinoma, (F) Poorly-differentiated carcinoma. Images were captured from Nanozoomer scanned slides with NDP view software at 40× digital zoom.
Table 3.
Distribution of pathology (most severe lesion) by prostatic lobe in 18-week old TRAMP mice
| PIN | Adenocarcinoma | |||||
|---|---|---|---|---|---|---|
| LG | MG | HG | WD | MD | PD | |
| Anterior | 3% | 39% | 53% | 0% | 3% | 2% |
| Ventral | 5% | 24% | 17% | 3% | 3% | 48% |
| Dorsal | 2% | 21% | 27% | 4% | 2% | 44% |
| Lateral | 1% | 21% | 18% | 8% | 3% | 49% |
PIN = prostatic intraepithelial neoplasia, LG = Low-Grade, MG = Moderate-Grade, HG = High-Grade, WD = Well-Differentiated, MD = Moderately-Differentiated, PD = Poorly-Differentiated.
Consumption of tomato powder, soy germ, and the combination significantly reduced prostate cancer incidence
Cancer incidence was defined as the presence of an adenocarcinoma (WD, MD, PD) lesion in at least one prostatic lobe. 100% of mice fed control diets showed evidence of PCa in at least one lobe, and compared to the controls, overall incidence was significantly lower in TRAMP mice consuming TP (61%, p<0.001), SG (66%, p<0.001) and TP+SG (45%, p<0.001) (Table 4). In parallel to the low cancer incidence, TRAMP mice in the dietary intervention groups had significantly higher incidence of non-cancerous PIN lesions compared to the controls.
Table 4.
Analysis of histopathologicaly data based upon the most severe lesion found in the entire prostate for TRAMP mice fed tomato powder or soy germ, alone or in combination.
| PIN | Adenocarcinoma | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | n | LG | MG | HG | WD | MD | PD | Prostate Cancer (WD – PD) |
| Control | 29 | 0% | 0% | 0% | 10% | 7% | 83% | 100% |
| 10% Tomato | 31 | 0% | 7% | 32%*** | 6% | 0% | 55%* | 61%*** |
| 2% Soy Germ | 32 | 0% | 6% | 28%** | 6% | 7% | 53%* | 66%*** |
| 10% Tomato + 2% Soy Germ | 27 | 0% | 7% | 48%*** | 0% | 4% | 41%** | 45%*** |
LG = Low-Grade, MG = Moderate-Grade, HG = High-Grade, PIN = prostatic intraepithelial neoplasia, WD = Well-Differentiated, MD = Moderately-Differentiated, PD = Poorly-Differentiated.
Results are the incidence of each stage of pathology and overall prostate cancer incidence (sum of WD-PD) within dietary groups.
P < 0.05,
P < 0.01,
P < 0.001 compared to control group by Fisher’s exact test.
We also employed a histopathological scoring system to assign a numerical score to each lobe based on the severity and overall extent of pathology within the prostate (24). Reassuringly, the quantitative numerical scoring system also identified a significant impact of diet on prostate carcinogenesis. The most severe lesion score in the prostate was significantly lower in the TP (14.7 ± 1.1, p < 0.01), SG (15.6 ± 1.1, p = 0.03) and TP+SG (13.5 ± 1.2, p < 0.001) groups compared to the control (19.3 ± 0.4).
Dietary interventions impacted the severity of carcinogenesis in prostate lobes
Compared to the controls, PCa incidence in the SG group was significantly lower in the lateral and anterior lobes, and TP and TP+SG groups had significantly lower PCa incidence in the dorsal, lateral and ventral lobes (Table 5). The incidence of MG PIN in the anterior lobe was significantly higher in TP, SG and TP+SG groups compared to the controls due to the higher incidence of more severe pathology in the controls. Although not significantly different from TP or SG, cancer incidence in the dorsal, lateral and ventral lobes was lowest in the TP+SG group.
Table 5.
Histopathological analysis (most severe lesion) of individual prostate lobes in TRAMP mice fed tomato powder or soy germ, alone or in combination
| n | PIN | Adenocarcinoma | Prostate Cancer (WD – PD) |
|||||
|---|---|---|---|---|---|---|---|---|
| LG | MG | HG | WD | MD | PD | |||
| Dorsal Prostate | ||||||||
| Control | 29 | 0% | 4% | 17% | 10% | 7% | 62% | 79% |
| 10% Tomato | 31 | 0% | 26%* | 35% | 7% | 0% | 32%* | 39%** |
| 2% Soy Germ | 32 | 6% | 16% | 28% | 0% | 0% | 50% | 50% |
| 10% Tomato + 2% Soy Germ | 27 | 3% | 41%*** | 26% | 0% | 0% | 30%* | 30%*** |
| Lateral Prostate | ||||||||
| Control | 29 | 0% | 0% | 14% | 7% | 10% | 69% | 86% |
| 10% Tomato | 31 | 0% | 26%** | 22% | 10% | 0% | 42%* | 52%** |
| 2% Soy Germ | 31 | 3% | 32%*** | 13% | 0% | 0% | 52% | 52%** |
| 10% Tomato + 2% Soy Germ | 27 | 0% | 41%*** | 18% | 8% | 0% | 33%* | 41%*** |
| Ventral Prostate | ||||||||
| Control | 29 | 0% | 14% | 7% | 7% | 7% | 65% | 79% |
| 10% Tomato | 31 | 7% | 32% | 22% | 3% | 0% | 36%* | 39%** |
| 2% Soy Germ | 32 | 6% | 28% | 7% | 3% | 3% | 53% | 59% |
| 10% Tomato + 2% Soy Germ | 27 | 8% | 22% | 33%* | 0% | 0% | 37% | 37%** |
| Anterior Prostate | ||||||||
| Control | 27 | 0% | 11% | 74% | 0% | 8% | 7% | 15% |
| 10% Tomato | 28 | 4% | 57%*** | 39%* | 0% | 0% | 0% | 0% |
| 2% Soy Germ | 30 | 3% | 43%** | 54% | 0% | 0% | 0% | 0%* |
| 10% Tomato + 2% Soy Germ | 22 | 5% | 45%** | 45% | 0% | 5% | 0% | 5% |
LG = Low-Grade, MG = Moderate-Grade, HG = High-Grade, PIN = prostatic intraepithelial neoplasia, WD = Well-Differentiated, MD = Moderately-Differentiated, PD = Poorly-Differentiated.
Results are the incidence for each histopathologic grade and overall prostate cancer incidence (sum of WD-PD) within dietary groups.
P < 0.05,
P < 0.01,
P < 0.001 compared to control group by Fisher s exact test.
Dietary impacts on regional lymph node metastases
Due to our desire to examine early phases of carcinogenesis and the young age (18 weeks) at which mice were necropsied, the overall metastasis rate was low and the study lacked statistical power to fully assess local/regional or distant metastatic rates. However, the descriptive data reinforces the conclusions regarding dietary impact on prostate disease. The incidence of gross lymph node metastasis was lower in TP (9.4%), SG (12.9%), and TP+SG fed mice (11.1%) compared to the controls (17.2 %). In mice with advanced, diffuse, PD carcinoma (n = 25), we observed that 6 mice had distant micrometastases, defined as the presence of microscopic T-antigen positive prostate cancer cells in the liver and/or lungs (data not shown), and half of those animals (n=3) were control fed mice.
Dietary interventions with TP, SG, and TP+SG altered apoptosis
We chose a histological subset of prostate samples to examine proliferation and apoptotic index. Sections from the dorsal and lateral prostate lobes in which the predominant pathology was PIN were stained and quantitated. The apoptotic index in the prostate of control fed mice (0.7 ± 0.2%) was nearly half that of mice consuming TP (1.2 ± 0.2%, p = 0.007), SG (1.4 ± 0.2%, p = 0.002) and TP+SG (1.3± 0.2%, p = 0.004). Proliferation in TP (77 ± 4%), SG (74 ± 3%) and TP+SG (76 ± 4%) groups was quantitatively lower but not significantly different than the controls (81 ± 3%). The ratio of the proliferation index to the apoptotic index in control fed mice (154 ± 17) was over two times higher than mice consuming TP (71 ± 18, p = 0.007), SG (63 ± 17, p = 0.002) and TP+SG (70 ± 18, p = 0.006).
Serum VEGF is increased in mice with poorly differentiated and metastatic adenocarcinoma
Serum VEGF was not significantly different between groups (Control = 97±11 pg/mL, TP = 97±11 pg/mL, SG = 97±11 pg/mL, TP+SG = 86±11 pg/mL, diet main effect p=0.88). In this study of early carcinogenesis, we observed no major overall elevation in serum VEGF in TRAMP compared to normal, wild-type mice, or an overall impact of diet on baseline VEGF concentrations. However, serum VEGF levels were significantly elevated in TRAMP mice that had PD carcinoma with metastasis (Wild-Type = 68 ± 19 pg/mL; TRAMP without metastasis = 89 ± 5 pg/mL, TRAMP mice with metastasis = 139 ± 15 pg/mL, p = 0.01). Since too few mice had metastatic disease, the study is underpowered to assess an impact of diet on cancer associated elevations in serum VEGF.
DISCUSSION
There are a number of in vivo and in vitro studies that have investigated the anti-carcinogenic properties of pure phytochemicals derived from foods using the principles of pharmacognosy and pharmacology in hopes of developing novel drugs for cancer chemoprevention. However, there are far fewer studies focusing upon interventions with whole foods or novel processed food products. In this study we examine the combination of soy and tomato products for their anticancer activity in the TRAMP model in order to guide future development of food products for human PCa prevention strategies.
Preclinical rodent studies of prostate carcinogenesis with tomato products alone have been promising. The current study shows that consumption of 10% tomato powder reduced the overall incidence of early PCa in the TRAMP model by nearly 40% (p < 0.001). Tomato powder at 10% of the diet has also been shown to increase survival and reduce the incidence of advanced, poorly differentiated PCa in the TRAMP model (11), and interventions in other rodent models also support the protective effects of tomato products on prostate carcinogenesis (9, 12, 19). Lycopene concentrations in the serum (0.36 – 0.84 µM) and prostate (0.11 – 0.48 nmol/g tissue) of TRAMP mice in this study are comparable to levels observed in men consuming 25–30 mg lycopene/day from tomato products (22, 28, 29), which can be achieved by intake of approximately one cup of tomato sauce, ½ cup tomato paste or 6 cups of raw tomatoes. Therefore, the tissue and serum concentrations of lycopene achieved in this study can be obtained by intake of whole foods, along with an array of bioactives, in humans without intake of pure lycopene supplements.
The majority of interventions with soy protein isolate or soy phytochemical extracts in rodent models of PCa have shown anti-cancer activity, suggesting that soy consumption may inhibit carcinogenesis, reduce tumorigenesis, and reduce expression of biomarkers related to proliferation or angiogenesis (13, 30). In this study, consumption of soy germ was selected as it is a phytochemical rich fraction of the soybean that can be utilized by food scientists to produce novel functional foods for future cancer prevention studies or commercial products (31, 32). To our knowledge, this is the first study to evaluate the potential anti-carcinogenic properties of soy germ. Soy germ, the hypocotelydon of the soybean, is separated during milling of whole soybeans and is a concentrated source of bioactives including isoflavones, saponins and phytosterols. In contrast to soy protein isolates, concentrations of daidzein and glycitein in soy germ are considerably higher than genistein (as quantified by LC/MS). The anti-carcinogenic properties of genistein have been extensively studied and may be one contributor to the anti-cancer activity associated with consumption of soy products (33). However, in our study, diets containing soy germ provided a modest 45mg genistein/ kg diet, which is substantially lower than doses previously suggested to be protective with in vivo studies (16, 17, 34–36). In fact, serum genistein was below detection limits from several of the mice that consumed diets containing soy germ and was only 4–5% of total isoflavones in the prostate. Rather, equol, produced from the metabolism of daidzein by intestinal microflora, was the predominant isoflavone in the serum and prostate. Equol has a longer half-life and is a more potent antioxidant than parent isoflavones (37). Equol has a greater affinity to estrogen receptors than daidzein and can concentrate in the prostate and the prostatic fluid at higher concentrations than genistein (37, 38). However, it is estimated that only 30–40% of the Western population can metabolize daidzein to equol, and it is of increasing interest to determine if health benefits from soy consumption are related to equol synthesis or other metabolites either produced by the host or the intestinal microflora. Thus, our findings suggest that other soy phytochemicals, in addition to genistein may contribute to the anticancer activity of soy germ. Prostatic isoflavone concentrations in this study are comparable to what have been identified in men consuming soy foods or isoflavone supplements (39–41). Asian populations, which historically have demonstrated a substantially lower incidence of PCa compared to Western countries, consume an average of 25–50 mg isoflavones/ day (42), which can be achieved in the diet by consumption of 1–2 servings of soy foods (One serving = 4oz tofu, 1oz soy nuts or 8oz soymilk), including a recently developed tomato-soy juice (31).
Soy isoflavones and tomato carotenoids have distinctly different mechanisms of absorption, yet, long-term combined consumption of TP+SG resulted in slightly reduced serum and testes lycopene than when TP was consumed alone. Results from this study support our previous findings where SG consumption significantly reduced carotenoid accumulation in the liver, testes, seminal vesicles and prostate of male rats (26). In our previous study, reduced carotenoid bioaccumulation was not explained by mRNA expression of scavenger receptor class B type I (a protein involved in carotenoid absorption) or carotenoid metabolizing enzymes in the prostate, liver or duodenal mucosa (26). Future studies should measure parent lycopene and their metabolites (cleavage products that may be more bioactive) to identify potential interactions between SG and TP that may impact biodistribution and activity (43).
It is a challenge to identify specific mechanisms of action when investigating whole food products containing an array of bioactives with diverse activities. Indeed, many pathways may be modulated. We assessed the dynamic changes in the epithelial cell proliferation/apoptotic relationship. In order to accurately examine this relationship we chose a similar area of the prostate (dorsal and lateral lobes) exhibiting the same histopathologic grade of cancer progression (PIN) from mice on each diet. We clearly observed that TP, SG and TP+SG increased apoptotic index (AI) and modestly reduced the proliferative index (PI), resulting in a dramatic reduction in the PI/AI ratio, a finding that would favor the accumulation of malignant epithelial cells in the control group. These findings from an in vivo and very aggressive model support the in vitro findings suggesting that many polyphenols in soy and tomato can inhibit growth factor signaling transduction pathways and enhance sensitivity to activation of apoptotic cascades (15, 44, 45). Tomato sauce consumption has previously been reported to increase apoptosis in both PCa and benign prostatic hyperplasia (46). In vitro, isoflavone and isoflavone metabolites induced apoptosis in benign prostatic epithelial cells at concentrations within ranges identified in the prostatic fluid of men consuming a soy product (47). Isoflavones and lycopene have both been suggested to reduce proliferation by modulating IGF-1 signaling (20, 35, 44). In vitro evidence is useful in identifying potential bioactive compounds and mechanisms of action and provides preliminary evidence and support for preclinical trials investigating the efficacy of a whole food containing multiple bioactive compounds. Our findings of reduced cancer incidence by TP, SG and TP+SG are supported by a large body of in vitro evidence, including hundreds of published studies on soy or tomato polyphenols, that bioactive compounds in these foods have anti-carcinogenic properties.
We have previously observed a decline in serum VEGF in men with metastatic PCa fed tomato and soy components (22). We did examine serum VEGF in this study, but the early age of termination at 18 weeks, when most mice have microscopic cancer or PIN, was too early for the clear assessment of diet on serum VEGF or other markers of angiogenesis. Indeed, when evaluating our data, only mice with advanced, poorly differentiated cancers with regional or distant metastases showed elevations in serum VEGF. Thus, our study, terminated at an early point in the carcinogenesis process, prevented an assessment of the impact of diet on serum VEGF as seen in our human study.
Interestingly, we observed a 30% reduction of PCa by TP and SG, a level of protection similar to what has been calculated from meta-analyses of epidemiological studies measuring soy or tomato product consumption (3, 4). This is the first study to investigate the efficacy of a combination of tomato and soy for inhibition of prostate carcinogenesis in an animal model. Over half of the TRAMP mice that consumed the combination of TP+SG were without a single cancerous lesion in the prostate compared to 100% cancer incidence in the control group. The combination of TP and SG was quantitatively the most effective intervention and suggests a positive interaction between tomato and soy foods for prevention of PCa. Interestingly, in PCa patients, lycopene supplementation was more effective at reducing serum prostate specific antigen (PSA) progression than the combined supplementation of lycopene and soy isoflavones (48), suggesting a potential negative interaction between soy isoflavones and lycopene. The small clinical trial had no control group and did not measure the efficacy of soy isoflavones alone; therefore, it is difficult to know if the soy isoflavones had any impact on cancer outcomes and if there was truly a negative interaction between these bioactive components.
This study reinforces the public health recommendations of many organizations such as the American Institute for Cancer Research and the United States government through the 2010 Dietary Guidelines for Americans which recommends that a variety of fruits and vegetables should serve as the foundation of a healthy diet (49, 50). In addition to supporting national public health goals, this work suggests that continued efforts to develop novel functional foods containing an array of bioactives are a reasonable strategy for PCa prevention or as an adjunct to therapy. The demonstrated benefit from the combination of dietary components that have been selected based upon evidence derived from laboratory and epidemiologic studies provides a stimulus for food scientists to develop novel tomato-soy food products with defined and consistent composition (22, 31) for future clinical trials. Maximally effective and safe strategies for PCa prevention may result from optimizing combinations of nutrients and bioactives through an orchestration of dietary patterns or the development of novel food products that can be tested in prospective trials.
Acknowledgements
The authors would like to thank FutureCeuticals for donating the tomato powder and Frutarom for donation of the soy germ powder. The authors are grateful to Dr. Steven J. Schwartz and Dr. Ken Riedl, of The Ohio State University Comprehensive Cancer Center Nutrient and Phytochemical Analytic Shared Resource for their advice regarding soy isoflavone analysis and interpretation.
Grant Support
K. Zuniga is supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award (1 F31 CA153804-01A1). Research supported in part by NIH grant PHS-1-R01 CA125384 to Drs. Erdman and Clinton, and the University of Illinois Division of Nutritional Sciences Margin of Excellence Research Program. Supported by the Ohio State University Comprehensive Cancer Center’s Nutrient and Phytochemical Analytic Shared Resource
Footnotes
CONFLICTS OF INTEREST: None
References
- 1.Kummar S, Chen HX, Wright J, Holbeck S, Millin MD, Tomaszewski J, et al. Utilizing targeted cancer therapeutic agents in combination: Novel approaches and urgent requirements. Nat Rev Drug Discov. 2010;9(11):843–856. doi: 10.1038/nrd3216. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey RW, Brockway-Lunardi LM, Bonk DT, Dohoney KM, Doroshow JH, Meech SJ, et al. Opportunities and challenges in the development of experimental drug combinations for cancer. J Natl Cancer Inst. 2011;103(16):1222–1226. doi: 10.1093/jnci/djr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: A revisit of a meta-analysis. Am J Clin Nutr. 2009;89(4):1155–1163. doi: 10.3945/ajcn.2008.27029. [DOI] [PubMed] [Google Scholar]
- 4.Etminan M, Takkouche B, Caamano-Isorna F. The role of tomato products and lycopene in the prevention of prostate cancer: A meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev. 2004;13(3):340–345. [PubMed] [Google Scholar]
- 5.Engelmann N, Clinton S, Erdman J. Nutritional aspects of phytoene and phytofluene, carotenoid precursors to lycopene. Adv Nutr. 2011;2(1):51–61. doi: 10.3945/an.110.000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goetzl MA, Van Veldhuizen PJ, Thrasher JB. Effects of soy phytoestrogens on the prostate. Prostate Cancer Prostatic Dis. 2007;10(3):216–223. doi: 10.1038/sj.pcan.4500953. [DOI] [PubMed] [Google Scholar]
- 7.Ford N, Moran N, Smith J, Clinton S, Erdman J. An interaction between carotene-15,15'-monooxygenase expression and consumption of a tomato or lycopene-containing diet impacts serum and testicular testosterone. Int J Cancer. 2012;131(2):E143–E148. doi: 10.1002/ijc.26446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan H, Thomas Ahner J, Grainger E, Wan L, Francis D, Schwartz S, et al. Tomato-based food products for prostate cancer prevention: What have we learned? Cancer Metastasis Rev. 2010;29(3):553–568. doi: 10.1007/s10555-010-9246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canene-Adams K, Lindshield BL, Wang S, Jeffery EH, Clinton SK, Erdman JW., Jr Combinations of tomato and broccoli enhance antitumor activity in dunning r3327-h prostate adenocarcinomas. Cancer Res. 2007;67(2):836–843. doi: 10.1158/0008-5472.CAN-06-3462. [DOI] [PubMed] [Google Scholar]
- 10.Hsu A, Bruno R, Lhr C, Taylor A, Dashwood R, Bray T, et al. Dietary soy and tea mitigate chronic inflammation and prostate cancer via NFkB pathway in the noble rat model. J Nutr Biochem. 2011;22(5):502–510. doi: 10.1016/j.jnutbio.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pannellini T, Iezzi M, Liberatore M, Sabatini F, Iacobelli S, Rossi C, et al. A dietary tomato supplement prevents prostate cancer in TRAMP mice. Cancer Prev Res. 2010;3(10):1284–1291. doi: 10.1158/1940-6207.CAPR-09-0237. [DOI] [PubMed] [Google Scholar]
- 12.Mossine V, Chopra P, Mawhinney T. Interaction of tomato lycopene and ketosamine against rat prostate tumorigenesis. Cancer Res. 2008;68(11):4384–4391. doi: 10.1158/0008-5472.CAN-08-0108. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Yu L, Zhong Y, Blackburn G. Soy phytochemicals and tea bioactive components synergistically inhibit androgen-sensitive human prostate tumors in mice. J Nutr. 2003;133(2):516–521. doi: 10.1093/jn/133.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou JR, Yu L, Zhong Y, Nassr RL, Franke AA, Gaston SM, et al. Inhibition of orthotopic growth and metastasis of androgen-sensitive human prostate tumors in mice by bioactive soybean components. Prostate. 2002;53(2):143–153. doi: 10.1002/pros.10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou JR, Gugger ET, Tanaka T, Guo Y, Blackburn GL, Clinton SK. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J Nutr. 1999;129(9):1628–1635. doi: 10.1093/jn/129.9.1628. [DOI] [PubMed] [Google Scholar]
- 16.Mentor-Marcel R, Lamartiniere C, Eltoum I, Greenberg N, Elgavish A. Dietary genistein improves survival and reduces expression of osteopontin in the prostate of transgenic mice with prostatic adenocarcinoma (TRAMP) J Nutr. 2005;135(5):989–995. doi: 10.1093/jn/135.5.989. [DOI] [PubMed] [Google Scholar]
- 17.Mentor-Marcel R, Lamartiniere CA, Eltoum IE, Greenberg NM, Elgavish A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP) Cancer Res. 2001;61(18):6777–6782. [PubMed] [Google Scholar]
- 18.El Touny L, Banerjee P. Akt GSK-3 pathway as a target in genistein-induced inhibition of TRAMP prostate cancer progression toward a poorly differentiated phenotype. Carcinogenesis. 2007;28(8):1710–1717. doi: 10.1093/carcin/bgm103. [DOI] [PubMed] [Google Scholar]
- 19.Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW, Jr, Clinton SK. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst. 2003;95(21):1578–1586. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- 20.Talvas J, Caris-Veyrat C, Guy L, Rambeau M, Lyan B, Minet-Quinard R, et al. Differential effects of lycopene consumed in tomato paste and lycopene in the form of a purified extract on target genes of cancer prostatic cells. Am J Clin Nutr. 2010;91(6):1716–1724. doi: 10.3945/ajcn.2009.28666. [DOI] [PubMed] [Google Scholar]
- 21.Hsu A, Bray TM, Helferich WG, Doerge DR, Ho E. Differential effects of whole soy extract and soy isoflavones on apoptosis in prostate cancer cells. Exp Biol Med. 2010;235(1):90–97. doi: 10.1258/ebm.2009.009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grainger EM, Schwartz SJ, Wang S, Unlu NZ, Boileau TW, Ferketich AK, et al. A combination of tomato and soy products for men with recurring prostate cancer and rising prostate specific antigen. Nutr Cancer. 2008;60(2):145–154. doi: 10.1080/01635580701621338. [DOI] [PubMed] [Google Scholar]
- 23.Klein RD. The use of genetically engineered mouse models of prostate cancer for nutrition and cancer chemoprevention research. Mutat Res. 2005;576(1–2):111–119. doi: 10.1016/j.mrfmmm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Berman Booty L, Sargeant A, Rosol T, Rengel R, Clinton S, Chen C, et al. A review of the existing grading schemes and a proposal for a modified grading scheme for prostatic lesions in TRAMP mice. Toxicol Pathol. 2012;40(1):5–17. doi: 10.1177/0192623311425062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao Z, Boileau TW, Erdman JW, Jr, Clinton SK. Interrelationships among angiogenesis, proliferation, and apoptosis in the tumor microenvironment during N-methyl-N-nitrosourea androgen-induced prostate carcinogenesis in rats. Carcinogenesis. 2002;23(10):1701–1711. doi: 10.1093/carcin/23.10.1701. [DOI] [PubMed] [Google Scholar]
- 26.Zuniga K, Erdman J. Combined consumption of soy germ and tomato powders results in altered isoflavone and carotenoid bioavailability in rats. J Agric Food Chem. 2011;59(10):5335–5341. doi: 10.1021/jf2004157. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, et al. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55(3):219–237. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- 28.Bowen P, Chen L, Stacewicz-Sapuntzakis M, Duncan C, Sharifi R, Ghosh L, et al. Tomato sauce supplementation and prostate cancer: Lycopene accumulation and modulation of biomarkers of carcinogenesis. Exp Biol Med. 2002;227(10):886–893. doi: 10.1177/153537020222701008. [DOI] [PubMed] [Google Scholar]
- 29.Van Breemen RB, van Breemen Liquid chromatography - mass spectrometry of cis- and all-trans-lycopene in human serum and prostate tissue after dietary supplementation with tomato sauce. J Agric Food Chem. 2002;50(8):2214–2219. doi: 10.1021/jf0110351. [DOI] [PubMed] [Google Scholar]
- 30.Messina MJ. Emerging evidence on the role of soy in reducing prostate cancer risk. Nutr Rev. 2003;61(4):117–131. doi: 10.1301/nr.2003.apr.117-131. [DOI] [PubMed] [Google Scholar]
- 31.Bohn T, Blackwood M, Francis D, Tian Q, Schwartz SJ, Clinton SK. Bioavailability of phytochemical constituents from a novel soy fortified lycopene rich tomato juice developed for targeted cancer prevention trials. Nutr Cancer. doi: 10.1080/01635581.2011.630156. 2011 11/18; 2013/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Consumer products [Internet] Available from: http://www.soylife.com/consumer-products-p3741-en.html.
- 33.Perabo FGE, Von-Lw EC, Ellinger J, von-Rcker A, Mller SC, Bastian PJ. Soy isoflavone genistein in prevention and treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2008;11(1):6–12. doi: 10.1038/sj.pcan.4501000. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Eltoum I, Lamartiniere C. Genistein chemoprevention of prostate cancer in TRAMP mice. J Carcinog. 2007;6:3. doi: 10.1186/1477-3163-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Eltoum I, Lamartiniere C. Genistein alters growth factor signaling in transgenic prostate model (TRAMP) Mol Cell Endocrinol. 2004;219(1–2):171–180. doi: 10.1016/j.mce.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 36.El Touny L, Banerjee P. Identification of a biphasic role for genistein in the regulation of prostate cancer growth and metastasis. Cancer Res. 2009;69(8):3695–3703. doi: 10.1158/0008-5472.CAN-08-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Setchell KDR, Brown N, Lydeking Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132(12):3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 38.Hedlund T, Maroni P, Ferucci P, Dayton R, Barnes S, Jones K, et al. Long-term dietary habits affect soy isoflavone metabolism and accumulation in prostatic fluid in caucasian men. J Nutr. 2005;135(6):1400–1406. doi: 10.1093/jn/135.6.1400. [DOI] [PubMed] [Google Scholar]
- 39.Brössner C, Petritsch K, Fink K, Auprich M, Madersbacher S, Adlercreutz H, et al. Phytoestrogen tissue levels in benign prostatic hyperplasia and prostate cancer and their association with prostatic diseases. Urology. 2004;64(4):707–711. doi: 10.1016/j.urology.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 40.Guy L, Védrine N, Urpi-Sarda M, Gil-Izquierdo A, Al-Maharik N, Boiteux J, et al. Orally administered isoflavones are present as glucuronides in the human prostate. Nutr Cancer. 2008;60(4):461–468. doi: 10.1080/01635580801911761. [DOI] [PubMed] [Google Scholar]
- 41.Hong S, Kim S, Kwon S, Lee J, Chung B. Comparative study of concentration of isoflavones and lignans in plasma and prostatic tissues of normal control and benign prostatic hyperplasia. Yonsei Med J. 2002;43(2):236–241. doi: 10.3349/ymj.2002.43.2.236. [DOI] [PubMed] [Google Scholar]
- 42.Messina M, Nagata C, Wu AH. Estimated asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55(1):1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- 43.Lindshield BL, Canene-Adams K, Erdman JW., Jr Lycopenoids: Are lycopene metabolites bioactive? Arch Biochem Biophys. 2007;458(2):136–140. doi: 10.1016/j.abb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Wang S, DeGroff VL, Clinton SK. Tomato and soy polyphenols reduce insulin-like growth factor-I-stimulated rat prostate cancer cell proliferation and apoptotic resistance in vitro via inhibition of intracellular signaling pathways involving tyrosine kinase. J Nutr. 2003;133(7):2367–2376. doi: 10.1093/jn/133.7.2367. [DOI] [PubMed] [Google Scholar]
- 45.Guo Y, Wang S, Hoot D, Clinton S. Suppression of VEGF-mediated autocrine and paracrine interactions between prostate cancer cells and vascular endothelial cells by soy isoflavones. J Nutr Biochem. 2007;18(6):408–417. doi: 10.1016/j.jnutbio.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Kim H-, Bowen P, Chen L, Duncan C, Ghosh L, Sharifi R, et al. Effects of tomato sauce consumption on apoptotic cell death in prostate benign hyperplasia and carcinoma. Nutr Cancer. 2003;47(1):40–47. doi: 10.1207/s15327914nc4701_5. [DOI] [PubMed] [Google Scholar]
- 47.Hedlund T, van Bokhoven A, Johannes W, Nordeen S, Ogden L. Prostatic fluid concentrations of isoflavonoids in soy consumers are sufficient to inhibit growth of benign and malignant prostatic epithelial cells in vitro. Prostate. 2006;66(5):557–566. doi: 10.1002/pros.20380. [DOI] [PubMed] [Google Scholar]
- 48.Vaishampayan U, Hussain M, Banerjee M, Seren S, Sarkar F, Fontana J, et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59(1):1–7. doi: 10.1080/01635580701413934. [DOI] [PubMed] [Google Scholar]
- 49.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary guidelines for americans. Washington, DC: U.S. Government Printing Office; 2010. 2010; Report No.: 7th edition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.WCRF Panel. Food, nutrition, physical activity, and the prevention of cancer:A global perspective. Washington (DC): American Institute for Cancer Research; 2007. [Google Scholar]


