Abstract
Objective
This study examined the dose-dependent effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) supplementation on heart rate variability (HRV) at rest and during standard laboratory stress tasks. We also investigated whether EPA + DHA supplementation was associated with changes in mood state.
Methods
This placebo-controlled, double-blind, randomized, three-period crossover trial (8-week treatment, 6-week washout) compared two doses of EPA + DHA supplementation (0.85 and 3.4 g/d) in 26 adults with elevated triglycerides. After each treatment period, HRV was assessed during an acute stress protocol that included a resting baseline, standard laboratory stress tasks (speech task and cold pressor), and recovery periods. In addition, mood state was assessed.
Results
Root mean square of successive differences in interbeat interval and total power increased 9.9% and 20.6%, respectively, after the high dose relative to placebo (Tukey p = .016 and .012, respectively). The low dose was not significantly different from the high dose or placebo dose. There was a trend for a treatment effect on high-frequency HRV (p = .058), with 21.0% greater power observed after the high dose compared with placebo (Tukey p = .052). Mood did not differ between treatments, and there was no association between mood state and HRV.
Conclusions
In healthy adults with elevated triglycerides, supplementation of 3.4 g/d EPA + DHA resulted in greater HRV, whereas 0.85 g/d EPA + DHA had no effect. These results indicate that EPA + DHA supplementation may improve autonomic tone in adults at increased risk for cardiovascular disease within 8 weeks.
Trial Registration
NCT00504309 (ClinicalTrials.gov).
Keywords: heart rate variability, acute stress, omega-3 fatty acids, eicosapentaenoic acid, docosahexaenoic acid
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death in the world (1). Increased sympathetic nervous system function is believed to contribute to the development of CVD, whereas increased parasympathetic activity seems to protect against sudden cardiac death (2,3). Heart rate variability (HRV) is a noninvasive method used to index autonomic influence on cardiac function by quantifying the beat-to-beat fluctuations in R-R interval (4). Spectral analysis of these fluctuations allows identification of several frequency bands, each with its own physiological determinants (5). Although there is controversy over what these parameters represent and which are most important for assessing CVD risk (6,7), there is robust evidence that less HRV is associated with greater CVD morbidity (8–10). In addition, several studies have reported that reductions in HRV at rest that persist during standardized laboratory stress tasks can predict increases in cardiovascular risk above and beyond traditional risk factors (11–13), which indicates that evaluating autonomic tone during stress may be beneficial to risk assessment.
In contrast, the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have been shown to decrease CVD morbidity and mortality, at least partially via antiarrhythmic mechanisms (14,15). EPA + DHA supplementation also has well-documented triglyceride-lowering effects, and higher doses (2–4 g/d EPA + DHA) are indicated for clinical treatment of severe hypertriglyceridemia (fasting triglycerides ≥500 mg/dl) (16). Recommendations regarding omega-3 intake for CVD prevention typically do not exceed 1 g/d EPA + DHA, and dietary sources are emphasized over nutritional supplements (17). Greater intake of EPA + DHA from fatty fish and/or fish oil supplements has been associated with increased HRV in some (18–20) but not all (21) epidemiological studies. Intervention studies provide evidence that EPA + DHA supplementation can improve or prevent a decline in HRV in individuals at increased risk for CVD (22–24) and those with established CVD (25–28). This effect on HRV may be one mechanism by which omega-3s exhibit their antiarrhythmic properties (29). However, other intervention studies reported null findings (30,31), particularly those enrolling healthy participants (32–34). Disease state could be a key factor in understanding these mixed results because healthy individuals may not respond to a CVD intervention in the same way as individuals with elevated risk or established CVD. Dose should also be considered because studies examining interventions of 3.0 to 6.0 g/d consistently observe a beneficial effect on HRV (22,23,26,27), whereas interventions testing doses of 2.0 g/d or less equally report beneficial (24,25) or null (30,31) findings.
Depression and other negative mood states also have been associated with increased CVD morbidity and mortality. The prevalence of depression in patients with coronary artery disease is approximately three-fold greater than that in the general population (35), and both major depressive episodes and subclinical depressive symptoms have been associated with cardiac events and survival rates (36–38). In addition, a recent review concluded that stress likely contributes to cardiac events and increases cardiovascular vulnerability (39). Mood state may influence cardiovascular health through stress-induced activation of the hypothalamic-pituitary-adrenal axis (40) or by depression-induced impairments of autonomic tone and reductions in HRV (41). Given the putative effect of omega-3 fatty acids on autonomic tone as described previously, it is plausible that omega-3 fatty acids may benefit mood states. Indeed, cross-sectional studies have reported inverse relationships between major depression and fish consumption (42–44), and a recent meta-analysis of 35 randomized controlled trials concluded that EPA + DHA supplementation may improve depressed mood in clinical populations with major depression (45). However, the evidence for EPA + DHA supplementation affecting subclinical depressed mood states in this meta-analysis was less conclusive, with most studies showing no effect.
Therefore, the purpose of this study was to compare the effects of EPA + DHA supplementation on HRV and mood. This protocol was conducted within a larger clinical trial that was designed to assess the effect of EPA + DHA supplementation in adults with elevated triglycerides (150–500 mg/dl) (46). Compared with healthy individuals, those with CVD risk factors have impaired HRV (47), and the effects of supplementation on autonomic activity may be more pronounced than those in individuals with normal cardiac function. We compared two doses to placebo supplementation: a low dose recommended for secondary prevention of CVD (0.85 g/d (17)) and a high dose indicated to lower high (>500 mg/dl) triglycerides (3.4 g/d (16)). In a previous publication, we reported that the high dose significantly reduced blood pressure and heart rate at rest and during acute stress (48). This study builds upon those results by investigating whether EPA + DHA supplementation affects autonomic activity, which could be related to the changes we observed in cardiovascular hemodynamics. For the primary analysis examining HRV at rest and during standard laboratory stress tasks, we hypothesized that EPA + DHA supplementation would increase HRV in a dose-dependent manner. Our secondary analysis evaluated the effects of EPA + DHA supplementation on mood state assessed at the end of each treatment period via self-report questionnaires. Finally, exploratory analyses were conducted to determine the association between HRV and mood state in this sample. The effect of these treatments on lipids, glycemic markers, endothelial function, inflammatory markers, erythrocyte fatty acid composition, blood pressure, and other hemodynamic variables has been reported previously (46,48).
METHODS
We enrolled healthy, nonsmoking men (n = 23) and postmenopausal women (n = 3) with moderate hypertriglyceridemia (150–500 mg/dl), as described in detail previously (46). Participants were required to be 21 to 65 years of age and have a body mass index of 20 to 39 kg/m2. Exclusion criteria were tobacco use; acute or chronic inflammatory conditions; hypertension (blood pressure ≥150/95 mm Hg); liver or kidney dysfunction (self-reported or abnormal screening blood work); unwillingness to discontinue nutritional supplements (except for calcium, which was allowed at a stable dose); weekly intake of two or more servings of fish, flaxseed, or walnuts; use of oral contraceptives or hormone replacement therapy; use of lipid-lowering, anti-inflammatory, anti-depressant, or blood pressure medication; and abnormal screening EKG or history of heart disease. Potential participants were advised that they would be expected to maintain low consumption of omega-3 fatty acids during the study, refrain from use of all supplements, and maintain their body weight. The final study population was, on average, middle-aged (mean age = 44 years), overweight (mean body mass index = 29 kg/m2), and normotensive (mean blood pressure = 123/82 mm Hg). The sample was predominantly non-Hispanic white and contained one participant of Asian Indian descent. Approval for the study was granted by the Pennsylvania State University institutional review board, and written informed consent was obtained from all participants. This study was registered on ClinicalTrials.gov (NCT00504309). Participant enrollment began in March 2007, and data collection finished in January 2009.
Experimental Design
The protocol used a randomized, double-blind, placebo-controlled, three-period crossover design. During each treatment period (8 weeks each, 6-week washout), participants consumed four capsules daily containing a total of either 0 g/d (four placebo capsules containing corn oil), 0.85 g/d (low dose, one active capsule and three placebo), or 3.4 g/d (high dose, four active capsules). Participants were assigned to an order of treatment periods by simple randomization. The active capsules were prescription omega-3 acid ethyl ester capsules containing EPA and DHA in a ratio of 1.2:1 (Lovaza; GlaxoSmithKline, Philadelphia, PA). At the end of each treatment period, mood state and HRV at rest and during two standard laboratory stressors were assessed (details later). Visits were scheduled in the afternoon, and time of day was held constant within participants. Four hours before testing, participants were told to consume a light (low-fat) meal and take half their daily treatment dose (two capsules) with this meal. For the low-dose treatment, this dose included the active capsule. They were instructed to avoid pain relievers and alcohol (24 hours), caffeine, decongestants, and exercise (12 hours) on the day of the visit. Compliance with previsit instructions was verified via self-report. Participants were rescheduled if they reported symptoms of acute infection.
Stress Tasks
In keeping with several previous studies (49–51), participants were given 2 minutes to prepare for a 3-minute speech on one of three hypothetical situations (being falsely accused of shoplifting, being prevented from boarding a flight, and receiving a traffic ticket). Participants were told that the speech would be videotaped and evaluated by the researchers for content and clarity. During the cold pressor task, participants immersed one foot up to the ankle into 4°C water for 2.5 minutes. Each task was followed by a 10-minute recovery period. HRV was collected continuously during the baseline rest, stress tasks, and recovery periods. Means were calculated for each of the six tasks (baseline rest, speech preparation, speech delivery, Recovery 1, cold pressor, Recovery 2). These six tasks were treated as repeated measures for each testing session.
HRV Assessment
Three electrocardiogram electrodes were placed according to the guidelines for impedance cardiography (52). The electrocardiogram was used to obtain raw interbeat intervals (R-R) recorded at a frequency of 1000 Hz. The R-R interval sequences were visually inspected, and the data considered artifactual were manually replaced by interpolated or extrapolated data. The square root of the mean squared differences of successive R-R intervals (RMSSD; in milliseconds) was calculated using commercial software (Nevrokard, Medistar Inc.). Frequency-domain measures of HRV (total power and high frequency [HF]) were calculated using autoregressive spectra and standard methods (53). Oscillations in the HF-HRV band (0.15–0.4 Hz) are thought to reflect vagal modulation of heart rate (54). HF-HRV values are presented in raw (in milliseconds squared) and normalized units (nu), the latter calculated according to standard formulae (HF-HRV/[total power − very low frequency power] (4)).
Mood State Assessment
Perceived stress, anxiety, depressed mood, and positive (POS) and negative affect (NEG) were assessed via self-administered questionnaires at the beginning of the stress testing session.
Perceived Stress Scale
Perceived stress during the last month was assessed using the 14-item Perceived Stress Scale (PSS) (55,56). Frequency of perceived stress was determined using a 5-point scale (0 = never, 4 = very often). A total perceived stress score was calculated with a possible range of 0 to 56 (PSS). In a large nonclinical sample of adults in the United States, the average PSS score was 19.6 (standard deviation [SD] = 7.5). This scale has shown excellent concurrent and predictive validity, as well as internal reliability (Cronbach α > .75) (55,56).
Spielberger State Anxiety Inventory
State anxiety was assessed using the Spielberger State Anxiety Inventory (STAI-State) (57). This 20-item questionnaire assesses anxiety at the current moment on a 4-point scale (1 = not at all, 4 = very much so). A total state anxiety score was calculated, with a possible range of 20 to 80 (STAI-State). In a large nonclinical sample of adults in the United States, the average STAI-State score was 39.6 (SD = 11.6). This scale has shown to be valid with excellent internal reliability (Cronbach α > .85) (57).
Positive and Negative Affect Scale
Affect was assessed using the Positive and Negative Affect Scale (58). This 20-item scale assesses POS and NEG in the past 2 weeks on a 5-point scale (1 = very slightly or not at all, 5 = extremely). Total scores for each dimension are calculated, with a possible range of 10 to 50 (POS, NEG). In a large nonclinical sample of adults in the United States, the average POS and NEG scores were 32.0 (SD = 7.0) and 19.5 (SD = 7.0), respectively. This scale has shown excellent concurrent validity and internal reliability (Cronbach α >.74 for both dimensions) (58).
Center for Epidemiological Studies Depression Scale
Symptoms of depressed mood were assessed using the Center for Epidemiological Studies Depression Scale (CES-D) (59). Frequencies of 20 depressive symptoms within the past week were determined on a 4-point scale (0 = rarely or none of the time, 3 = most or all of the time). A total depressed mood score was calculated, with a possible range from 0 to 60 (CES-D). Scores of 16 or greater have typically been used as the cut-point to identify high depressive symptoms. This scale has shown to be valid and reliable in screening for major depressive symptoms and has excellent internal reliability (Cronbach α = .85) (59).
Statistical Analysis
HRV data were available for 25 of the 26 participants; data from 1 participant could not be analyzed because of an inadequate signal. Statistical analyses were performed using SAS (version 9.2; Statistical Analysis System, Cary, NC). Variables were tested for normality, and all HRV variables, except HF-HRV (in normalized units), required log (base 10) transformations (skew for HF-HRV [in normalized units], 0.23). The NEG scale and the depressed mood scale (CES-D) also required log (base 10) transformations (skew, 1.52 and 1.75, respectively).
Repeated-measures analysis of variance (via the mixed-models procedure in SAS) was used to test the effects of treatment on HRV and mood state. Models included treatment (0.85 g/d, 3.4 g/d, placebo), task (e.g. baseline, speech, etc), period (first, second, or third treatment period), their interactions, and demographic covariates (age, sex, body mass index). Model fit was evaluated with the Bayesian Information Criteria using several variance-covariance matrix structures, and compound symmetry was selected as the optimal structure. This statistical method has many advantages including the ability to model a variety of variance-covariance structures, its use of the correct error term in repeated measures, the ease of obtaining post hoc tests for designs with two repeated factors, and its robustness to occasional missing data (60). Significant effects (p ≤ .05) were further examined using the Tukey post hoc test. When interaction effects were nonsignificant, only the main effects of treatment and task were interpreted. Results in the tables and figures are least squares means (standard error).
The correlation procedure in SAS was used to assess the association between HRV and mood state. Owing to the large number of correlations tested, the Bonferroni adjustment for multiple comparisons was applied by dividing our prespecified significance level (.05) by the number of relationships tested (n = 20), which resulted in an adjusted significance threshold of p < .0025 for the correlation analyses.
RESULTS
Effects of Stress Tasks on HRV
As expected, the stressors induced significant changes in HRV compared with the resting condition (Table 1). Compared with the baseline rest period, the preparation task significantly reduced RMSSD (F(5,328) = 9.8, Tukey p < .001, Cohen d = −0.75) HF-HRV (in milliseconds squared) (F(5,328) = 6.8, Tukey p < .001, Cohen d = −0.62), HF-HRV (in normalized units) (F(5,329) = 13.5, Tukey p = .024, Cohen d = −0.54), and total power, F(5,328) = 9.3, Tukey p < .001, Cohen d = −0.63). The speech task significantly reduced RMSSD (F(5,328) = 9.8, Tukey p < .001, Cohen d = −0.66), HF-HRV (in milliseconds squared), F(5,328) = 6.8, Tukey p < .001, Cohen d = −0.54), and HF-HRV (in normalized units) (F(5,329) = 13.5, Tukey p < .001, Cohen d = −1.14). The cold pressor significantly reduced HF-HRV (in normalized units) (F(5,329) = 13.5, Tukey p = .029, Cohen d = −0.55). We also observed some variability in how HRV responded to the different stressors (Table 1). Compared with the preparation period, the speech task significantly reduced HF-HRV (in normalized units) (F(5,329) = 13.5, Tukey p = .010, Cohen d = −0.59) and increased total power (F(5,328) = 9.3, Tukey p < .001, Cohen d = 0.70), whereas the cold pressor significantly reduced both RMSSD (F(5,328) = 9.8, Tukey p = .002, Cohen d = 0.46) and total power (F(5,328) = 9.3, Tukey p < .001, Cohen d = 0.69). Compared with the speech task, the cold pressor significantly reduced both RMSSD (F(5,328) = 9.8, Tukey p = .048, Cohen d = 0.37) and HF-HRV (in normalized units) (F(5,329) = 13.5, Tukey p = .013, Cohen d = 0.59).
TABLE 1.
Effect of Acute Stress on HRVa
| Task | RMSSD, msb | HF-HRV, ms2b | HF-HRV, nub | Total Power, ms2b |
|---|---|---|---|---|
| Baseline rest | 32.2 (2.1) | 417.7 (51.9) | 45.2 (2.9) | 2008.0 (179.1) |
| Preparation | 24.1 (2.1)c | 257.3 (50.8)c | 37.2 (2.9)c | 1440.6 (174.5)c |
| Speech | 25.2 (2.1)c,d | 279.1 (51.1)c | 28.7 (2.9)c,d | 2049.3 (175.8) |
| Recovery | 27.5 (2.1)c | 303.0 (51.1)c | 40.3 (2.9) | 1706.3 (175.9) |
| Cold | 29.0 (2.1) | 321.3 (51.6) | 37.2 (2.9)c | 2056.2 (178.0) |
| Recovery | 27.7 (2.2)c | 327.2 (52.4) | 47.8 (3.0) | 1595.3 (181.3)c |
HRV = heart rate variability; RMSSD = square root of the mean squared differences in R-R interval; HF-HRV = high-frequency heart rate variability; nu = normalized unit.
Data (adjusted means [standard error]) and p values were obtained from the mixed-models procedure (n = 25).
Main effect of task, p < .001.
Significantly different from baseline rest, Tukey p < .05.
Significantly different from cold, Tukey p < .05.
Effects of EPA + DHA on HRV
High-dose EPA + DHA supplementation was associated with changes in RMSSD and total power across the testing session (Table 2). Relative to placebo, mean RMSSD throughout the resting and stressor tasks was 2.6 milliseconds (9.9%) higher after the high-dose treatment (F(2,340) = 3.9, Tukey p = .016, Cohen d = 0.13) (Fig. 1), whereas mean total power was 339.4 ms2 (20.6%) higher after the high-dose treatment (F(2,344) = 4.2, Tukey p = .012, Cohen d = 0.26) (Fig. 1). RMSSD and total power after the low-dose treatment were not significantly different from either the high dose or the placebo dose. There was also a trend toward a significant effect of treatment of HF-HRV (F(2,341) = 2.9, p = .058), with post hoc analysis revealing 21.0% greater HF-HRV after the high-dose treatment compared with placebo (Tukey p = .052, Cohen d = 0.12). EPA + DHA supplementation did not significantly affect normalized values of HF-HRV. The treatment × task interaction was nonsignificant for all HRV variables.
TABLE 2.
Effect of EPA + DHA on HRVa
| HRV | 3.4 g/d (High) | 0.85 g/d (Low) | Placebo (P) |
p
|
|||
|---|---|---|---|---|---|---|---|
| Main Effect of Treatment | High versus Low | High versus P | Low versus P | ||||
| RMSSD, ms | 28.9 (2.0) | 27.6 (2.0) | 26.3 (2.0) | .02 | .52 | .016 | .26 |
| HF-HRV, ms2 | 346.5 (46.1) | 320.0 (48.0) | 286.3 (47.2) | .058 | .86 | .052 | .21 |
| HF-HRV, nu | 40.0 (2.6) | 39.2 (2.7) | 39.0 (2.7) | .86 | .93 | .86 | .99 |
| Total power, ms2 | 1988.0 (153.8) | 1791.2 (162.2) | 1648.6 (158.3) | .02 | .23 | .012 | .50 |
EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid; HRV = heart rate variability; RMSSD = square root of the mean squared differences in R-R interval; HF-HRV = high-frequency heart rate variability; nu = normalized unit.
Data (adjusted means [standard error]) and p values were obtained from the mixed-models procedure (n = 25). HRV was averaged across baseline rest, stress tasks, and recovery periods because the task × treatment effects were nonsignificant.
Figure 1.
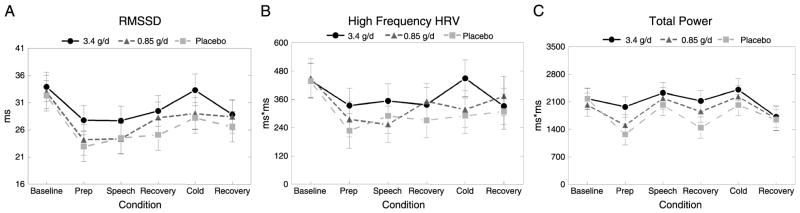
Effect of EPA + DHA on HRV at rest and during acute stress. Data (adjusted means [standard error]) were obtained from the mixed-models procedure (n = 25). A, RMSSD averaged across the six tasks was significantly affected by EPA + DHA supplementation (p = .022). Mean RMSSD was higher after the 3.4-g/d treatment compared with placebo (Tukey p = .016); the 0.85-g/d dose did not significantly differ from the 3.4-g/d dose or placebo. B, High-frequency HRV (in milliseconds squared) averaged across the six tasks trended toward a significant effect of treatment (p = .058). Mean high-frequency HRV (in milliseconds squared) was higher after the 3.4-g/d dose compared with placebo (Tukey p = .052). C, Total power averaged across the six tasks was significantly affected by EPA + DHA supplementation (p = .016). Mean RMSSD was higher after the 3.4-g/d treatment compared with placebo (Tukey p = .012); the 0.85-g/d dose did not significantly differ from the 3.4-g/d dose or placebo. EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid; RMSSD = square root of the mean squared differences in R-R interval; HRV = heart rate variability.
Effects of EPA + DHA on Mood State
Mood state after each treatment period is summarized in Table 3. There was no significant effect of EPA + DHA supplementation on any of the mood state measures.
TABLE 3.
Effect of EPA + DHA on Mood Statea
| Measure | 3.4 g/d | 0.85 g/d | Placebo |
|---|---|---|---|
| PSS | 25 (1) | 26 (1) | 24 (1) |
| STAI-State | 47 (1) | 48 (1) | 46 (1) |
| POS | 36 (1) | 36 (1) | 36 (1) |
| NEG | 15 (1) | 17 (1) | 15 (1) |
| CES-D | 7 (2) | 8 (2) | 8 (2) |
EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid; PSS = Perceived Stress Scale; STAI-State = Spielberger State Anxiety Inventory; POS = positive dimension from Positive and Negative Affect Scale; NEG = negative dimension from Positive and Negative Affect Scale; CES-D = Center for Epidemiological Studies Depression Scale.
Data (adjusted means [standard error]) were obtained from the mixed-models procedure (n = 26). There were no significant differences between treatments.
Association Between HRV and Mood State
Because mood variables did not vary by treatment, cross-sectional associations between mood state and HRV were examined with data collected on the placebo dose only. Exploratory correlations between both baseline (resting) HRV and mean HRV (averaged across the stress tasks) with mood state yielded no significant results. POS was positively correlated with resting HF-HRV (in normalized units; Pearson coefficient = 0.46, p = .028); however, this correlation did not reach the adjusted significance threshold (p > .0025).
DISCUSSION
This study examined the dose-response effects of EPA + DHA supplementation on HRV and mood state. As expected, RMSSD, a measure of parasympathetically mediated HRV (61), was reduced during the stress tasks after all treatments. However, compared with placebo supplementation, the high dose of 3.4 g/d EPA + DHA resulted in a 9.9% increase in mean RMSSD that was evident during both resting and stressor tasks. Values after the low dose of 0.85 g/d were not significantly different from either the high dose or the placebo dose. Similarly, total power was significantly greater after the high-dose treatment compared with placebo, whereas the low dose did not differ from either the high dose or placebo dose. We observed a trend toward significance for HF-HRV (in milliseconds squared) that mirrored the effect of EPA + DHA supplementation on RMSSD and total power.
To our knowledge, this is the first study to examine the effects of EPA + DHA supplementation on HRV during standard laboratory stressors. Previous studies have assessed HRV during periods of rest (22–25,30,34) and 24-hour ambulatory monitoring periods (26–28,31–33). Although lower levels of resting (8–10) and ambulatory (62,63) HRV have been associated with increased risk of CVD, assessment of HRV during standard laboratory stressors provides a unique opportunity to examine HRV during conditions of heightened sympathetic activity (64). Reduced HRV during acute stress has been prospectively associated with an increased risk of CVD (11–13). For example, in healthy middle-aged adults, lower RMSSD during stress predicted higher diastolic blood pressure 3 years later (12). Our study demonstrated that high-dose EPA + DHA supplementation may increase HRV not only under resting conditions but also during periods of autonomic activation. Although we observed some variability in how HRV responded to the different stress tasks, the treatment × task interaction was nonsignificant for all HRV variables; therefore, we only interpret the overall effect of treatment averaged across the resting baseline, stress tasks, and recovery periods. The improvements we observed in mean HRV during the stress protocol after EPA + DHA supplementation suggest the possibility of reduction in cardiovascular risk; however, it is unlikely that short-term supplementation could be expected to result in long-term benefit. Future studies should assess whether improvements in HRV during acute stress are maintained over longer periods of EPA + DHA supplementation.
Our results suggest that it is possible that EPA + DHA supplementation may reduce CVD risk in adults with elevated triglycerides by influencing the autonomic nervous system. Compared with healthy individuals, those with risk factors or established CVD have greater sympathetic activity and impaired HRV (65), and the effects of supplementation may be more pronounced than in individuals with normal cardiac function. Although previous studies examining healthy populations did not find a beneficial effect of EPA + DHA supplementation on HRV (32–34), most studies that enrolled individuals with CVD risk factors or established CVD have reported autonomic benefits of 0.8 to 5.1 g/d EPA + DHA supplementation for 1 to 3 months, consistent with our results (22–27). In overweight or obese adults without existing CVD, Ninio et al. (22) observed an 18% increase in resting HF-HRV after 12 weeks of EPA + DHA supplementation, reflecting enhanced parasympathetic modulation of heart rate. Two studies assessing EPA + DHA supplementation in patients after myocardial infarction reported improvements in HRV owing to increases in vagally mediated HRV (measured by HF-HRV (25) and the SD of all normal R-R intervals (26)). Conversely, Hamaad et al. (30) reported no change in HRV after 12 weeks of supplementation in patients after myocardial infarction. It is possible that EPA + DHA supplementation occurred at too low of a dose (0.8 g/d) to have an effect in this study, or HRV may have already stabilized post–myocardial infarction because of standard therapies that also affect autonomic activity.
Consistent with previous findings for healthy populations, we did not observe relationships between mood state, EPA + DHA supplementation, and HRV. Mood state scores after all treatment periods were within the reference range (55,57, 59,66). Thus, our findings are in agreement with a recent meta-analysis that concluded that there is no effect of EPA + DHA supplementation on depressive symptoms in adults without clinical depression (45). Similarly, our results are consistent with studies reporting that the association between reduced HRV, clinical depression, and documented CVD (67,68) likely does not apply to physically and psychologically healthy individuals.
Our study had several limitations. Our sample size was modest (n = 26) and consisted mainly of white men (3 post-menopausal women). To explore the effect of the imbalance in the numbers of men and women on the results, we conducted a subanalysis that excluded the women. In this model, the effect of treatment on HF-HRV (in milliseconds squared) reaches statistical significance (p = .04), with post hoc analyses indicating greater HF-HRV (in milliseconds squared) after the high dose compared with placebo (Tukey p = .03). The results for RMSSD, HF-HRV (in normalized units), and total power (in milliseconds squared) did not change when women were excluded. Given the sex and age differences reported in previous studies of HRV (69,70), we are unable to generalize our results to all women, particularly premenopausal women. To fully elucidate the effects of EPA + DHA supplementation on HRV according to sex, our study would need to be repeated in a larger, sex-balanced sample. A second limitation is that the duration of supplementation was only 8 weeks per treatment period, which does not allow for maximal uptake of EPA + DHA into the cell membrane. Six months of supplementation may be required to reach a steady state of EPA and DHA concentrations in red blood cell membranes (71). However, by incorporating a 6-week washout between treatments, we were able to separate the testing sessions by 14 weeks, which provides an adequate washout to compare these two doses and did result in statistically significant findings after only 8 weeks of active supplementation. We also tested just two doses of EPA + DHA; therefore, our results cannot be generalized to other doses. However, our doses were specifically chosen to reflect current recommendations for secondary prevention of CVD (≈1 g/d EPA + DHA) and treatment of elevated triglycerides (2–4 g/d EPA + DHA) (17), which increases the clinical relevance of our findings. Furthermore, the Lovaza capsules used in this study are well characterized and Food and Drug Administration approved for the treatment of hypertriglyceridemia. Other potential limitations involve HRV methodology. First, HRV was only assessed after each treatment period and not at study entry; however, treatment order was randomized and mixed modeling adjusted for habituation effects when they occurred. Second, we assessed HRV at rest and during acute stress, a model that has been associated with increased risk of CVD but has not been previously used in trials assessing the effects of EPA + DHA. Therefore, we were not able to directly compare our results with previous studies that examined only resting or 24-hour ambulatory HRV.
In conclusion, we demonstrated that 3.4 g/d of EPA + DHA supplementation for 8 weeks improves HRV in healthy adults with elevated triglycerides at rest and during stress. The lower dose of 0.85 g/d EPA + DHA had no effect on HRV, and neither dose was associated with changes in mood state in this sample of nondepressed individuals. We previously reported that the high dose significantly reduced triglycerides, heart rate, and blood pressure in this population (46,48). Taken together, our findings support existing evidence that populations at risk for CVD with low omega-3 intake might achieve modest benefits on cardiovascular risk factors by supplementing their diet with high doses (3.4 g/d) of EPA + DHA.
Acknowledgments
The authors appreciate the services provided by the General Clinical Research Center of The Pennsylvania State University.
Source of Funding: This research was funded, in part, by Grants M01 RR 10732 (Pennsylvania State University) and F31 AG 043224 (K.A.S.) from the National Institutes of Health, by Grant 12 PRE 11530001 (K.A.S.) from the American Heart Association, and by a scholarship grant from the National Fisheries Institute (A.C.S.-R.). Study materials (capsules) and additional financial support were provided by Reliant Pharmaceuticals (now GlaxoSmithKline).
Glossary
- CVD
cardiovascular disease
- HRV
heart rate variability
- EPA
eicosapentaenoic acid
- DHA
docosahexaenoic acid
- RMSSD
square root of the mean squared differences in R-R interval
- HF-HRV
high-frequency heart rate variability
- nu
normalized units
- PSS
Perceived Stress Scale
- STAI-State
Spielberger State Anxiety Inventory
- POS
positive affect
- NEG
negative affect
- CES-D
Center for Epidemiological Studies Depression Scale
Footnotes
Conflicts of Interest: The authors are investigators on studies supported by Dow Agrosciences (P.M.K.-E. and S.G.W.), the US Department of Agriculture (A.C.S.-R. and P.M.K.-E.), and Nordic Naturals (A.C.S.-R. and P.M.K.-E.) to test the effects of omega-3 fish oil on cardiovascular risk factors. The other authors (K.A.S., T.S.C., and J.A.J.) report no conflicts of interest.
References
- 1.World Health Organization. [Accessed June 15, 2012];Cardiovascular Diseases (CVDs) Fact Sheet 2011. Available at: http://www.who.int/mediacentre/factsheets/fs317/en/index.html.
- 2.Christensen JH, Svensson M, Strandhave C, Madsen T, Schmidt EB. N-3 fatty acids and cardiac autonomic function in humans. Cell Mol Biol (Noisy-le-grand) 2010;56:131–9. [PubMed] [Google Scholar]
- 3.Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens. 1999;17:719–34. doi: 10.1097/00004872-199917060-00001. [DOI] [PubMed] [Google Scholar]
- 4.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 5.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–2. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 6.Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation. 1997;96:3224–32. doi: 10.1161/01.cir.96.9.3224. [DOI] [PubMed] [Google Scholar]
- 7.Moak JP, Goldstein DS, Eldadah BA, Saleem A, Holmes C, Pechnik S, Sharabi Y. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm. 2007;4:1523–9. doi: 10.1016/j.hrthm.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao D, Cai J, Barnes RW, Tyroler HA, Rautaharju P, Holme I, Heiss G. Association of cardiac autonomic function and the development of hypertension: the ARIC study. Am J Hypertens. 1996;9:1147–56. doi: 10.1016/s0895-7061(96)00249-x. [DOI] [PubMed] [Google Scholar]
- 9.Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, Rautaharju P, Heiss G. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC Study. Am J Epidemiol. 1997;145:696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–5. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 11.Matthews KA, Salomon K, Brady SS, Allen MT. Cardiovascular reactivity to stress predicts future blood pressure in adolescence. Psychosom Med. 2003;65:410–5. doi: 10.1097/01.psy.0000057612.94797.5f. [DOI] [PubMed] [Google Scholar]
- 12.Steptoe A, Marmot M. Impaired cardiovascular recovery following stress predicts 3-year increases in blood pressure. J Hypertens. 2005;23:529–36. doi: 10.1097/01.hjh.0000160208.66405.a8. [DOI] [PubMed] [Google Scholar]
- 13.Gianaros PJ, Salomon K, Zhou F, Owens JF, Edmundowicz D, Kuller LH, Matthews KA. A greater reduction in high-frequency heart rate variability to a psychological stressor is associated with subclinical coronary and aortic calcification in postmenopausal women. Psychosom Med. 2005;67:553–60. doi: 10.1097/01.psy.0000170335.92770.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and Reinfarction Trial (DART) Lancet. 1989;2:757–61. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 15.GISSI-Prevenzione. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005;13:322–7. [PubMed] [Google Scholar]
- 17.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 18.Christensen JH, Skou HA, Fog L, Hansen V, Vesterlund T, Dyerberg J, Toft E, Schmidt EB. Marine n-3 fatty acids, wine intake, and heart rate variability in patients referred for coronary angiography. Circulation. 2001;103:651–7. doi: 10.1161/01.cir.103.5.651. [DOI] [PubMed] [Google Scholar]
- 19.Christensen JH, Skou HA, Madsen T, Torring I, Schmidt EB. Heart rate variability and n-3 polyunsaturated fatty acids in patients with diabetes mellitus. J Intern Med. 2001;249:545–52. doi: 10.1046/j.1365-2796.2001.00841.x. [DOI] [PubMed] [Google Scholar]
- 20.Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS. Dietary fish and omega-3 fatty acid consumption and heart rate variability in US adults. Circulation. 2008;117:1130–7. doi: 10.1161/CIRCULATIONAHA.107.732826. [DOI] [PubMed] [Google Scholar]
- 21.Park SK, Tucker KL, O’Neill MS, Sparrow D, Vokonas PS, Hu H, Schwartz J. Fruit, vegetable, and fish consumption and heart rate variability: the Veterans Administration Normative Aging Study. Am J Clin Nutr. 2009;89:778–86. doi: 10.3945/ajcn.2008.26849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ninio DM, Hill AM, Howe PR, Buckley JD, Saint DA. Docosahexaenoic acid–rich fish oil improves heart rate variability and heart rate responses to exercise in overweight adults. Br J Nutr. 2008;100:1097–103. doi: 10.1017/S0007114508959225. [DOI] [PubMed] [Google Scholar]
- 23.Sjoberg NJ, Milte CM, Buckley JD, Howe PR, Coates AM, Saint DA. Dose-dependent increases in heart rate variability and arterial compliance in overweight and obese adults with DHA-rich fish oil supplementation. Br J Nutr. 2010;103:243–8. doi: 10.1017/S000711450999153X. [DOI] [PubMed] [Google Scholar]
- 24.Holguin F, Tellez-Rojo MM, Lazo M, Mannino D, Schwartz J, Hernandez M, Romieu I. Cardiac autonomic changes associated with fish oil vs soy oil supplementation in the elderly. Chest. 2005;127:1102–7. doi: 10.1378/chest.127.4.1102. [DOI] [PubMed] [Google Scholar]
- 25.O’Keefe JH, Jr, Abuissa H, Sastre A, Steinhaus DM, Harris WS. Effects of omega-3 fatty acids on resting heart rate, heart rate recovery after exercise, and heart rate variability in men with healed myocardial infarctions and depressed ejection fractions. Am J Cardiol. 2006;97:1127–30. doi: 10.1016/j.amjcard.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 26.Christensen JH, Gustenhoff P, Korup E, Aaroe J, Toft E, Moller J, Rasmussen K, Dyerberg J, Schmidt EB. Effect of fish oil on heart rate variability in survivors of myocardial infarction: a double blind randomised controlled trial. BMJ. 1996;312:677–8. doi: 10.1136/bmj.312.7032.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villa B, Calabresi L, Chiesa G, Rise P, Galli C, Sirtori CR. Omega-3 fatty acid ethyl esters increase heart rate variability in patients with coronary disease. Pharmacol Res. 2002;45:475. doi: 10.1006/phrs.2002.0989. [DOI] [PubMed] [Google Scholar]
- 28.Carney RM, Freedland KE, Stein PK, Steinmeyer BC, Harris WS, Rubin EH, Krone RJ, Rich MW. Effect of omega-3 fatty acids on heart rate variability in depressed patients with coronary heart disease. Psychosom Med. 2010;72:748–54. doi: 10.1097/PSY.0b013e3181eff148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christensen JH, Schmidt EB. Autonomic nervous system, heart rate variability and n-3 fatty acids. J Cardiovasc Med (Hagerstown) 2007;8(Suppl 1):S19–22. doi: 10.2459/01.JCM.0000289276.10675.a1. [DOI] [PubMed] [Google Scholar]
- 30.Hamaad A, Kaeng Lee W, Lip GY, MacFadyen RJ. Oral omega n3-PUFA therapy (Omacor) has no impact on indices of heart rate variability in stable post myocardial infarction patients. Cardiovasc Drugs Ther. 2006;20:359–64. doi: 10.1007/s10557-006-0295-z. [DOI] [PubMed] [Google Scholar]
- 31.Svensson M, Schmidt EB, Jorgensen KA, Christensen JH. The effect of n-3 fatty acids on heart rate variability in patients treated with chronic hemodialysis. J Ren Nutr. 2007;17:243–9. doi: 10.1053/j.jrn.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Christensen JH, Christensen MS, Dyerberg J, Schmidt EB. Heart rate variability and fatty acid content of blood cell membranes: a dose-response study with n-3 fatty acids. Am J Clin Nutr. 1999;70:331–7. doi: 10.1093/ajcn/70.3.331. [DOI] [PubMed] [Google Scholar]
- 33.Dyerberg J, Eskesen DC, Andersen PW, Astrup A, Buemann B, Christensen JH, Clausen P, Rasmussen BF, Schmidt EB, Tholstrup T, Toft E, Toubro S, Stender S. Effects of trans- and n-3 unsaturated fatty acids on cardiovascular risk markers in healthy males. An 8 weeks dietary intervention study. Eur J Clin Nutr. 2004;58:1062–70. doi: 10.1038/sj.ejcn.1601934. [DOI] [PubMed] [Google Scholar]
- 34.Geelen A, Zock PL, Swenne CA, Brouwer IA, Schouten EG, Katan MB. Effect of n-3 fatty acids on heart rate variability and baroreflex sensitivity in middle-aged subjects. Am Heart J. 2003;146:E4. doi: 10.1016/S0002-8703(03)00441-1. [DOI] [PubMed] [Google Scholar]
- 35.Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994;151:979–86. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- 36.Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA. 1993;270:1819–25. [PubMed] [Google Scholar]
- 37.Anda R, Williamson D, Jones D, Macera C, Eaker E, Glassman A, Marks J. Depressed affect, hopelessness, and the risk of ischemic heart disease in a cohort of U.S. adults. Epidemiology. 1993;4:285–94. doi: 10.1097/00001648-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Bush DE, Ziegelstein RC, Tayback M, Richter D, Stevens S, Zahalsky H, Fauerbach JA. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol. 2001;88:337–41. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- 39.Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51:1237–46. doi: 10.1016/j.jacc.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simpson EE, McConville C, Rae G, O’Connor JM, Stewart-Knox BJ, Coudray C, Strain JJ. Salivary cortisol, stress and mood in healthy older adults: the Zenith study. Biol Psychol. 2008;78:1–9. doi: 10.1016/j.biopsycho.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Carney RM, Saunders RD, Freedland KE, Stein P, Rich MW, Jaffe AS. Association of depression with reduced heart rate variability in coronary artery disease. Am J Cardiol. 1995;76:562–4. doi: 10.1016/s0002-9149(99)80155-6. [DOI] [PubMed] [Google Scholar]
- 42.Tanskanen A, Hibbeln JR, Hintikka J, Haatainen K, Honkalampi K, Viinamaki H. Fish consumption, depression, and suicidality in a general population. Arch Gen Psychiatry. 2001;58:512–3. doi: 10.1001/archpsyc.58.5.512. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Dai Q, Ekperi LI, Dehal A, Zhang J. Fish consumption and severely depressed mood, findings from the first national nutrition follow-up study. Psychiatry Res. 2011;190:103–9. doi: 10.1016/j.psychres.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 44.Bountziouka V, Polychronopoulos E, Zeimbekis A, Papavenetiou E, Ladoukaki E, Papairakleous N, Gotsis E, Metallinos G, Lionis C, Panagiotakos D. Long-term fish intake is associated with less severe depressive symptoms among elderly men and women: the MEDIS (MEDiterranean ISlands Elderly) epidemiological study. J Aging Health. 2009;21:864–80. doi: 10.1177/0898264309340693. [DOI] [PubMed] [Google Scholar]
- 45.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91:757–70. doi: 10.3945/ajcn.2009.28313. [DOI] [PubMed] [Google Scholar]
- 46.Skulas-Ray AC, Kris-Etherton PM, Harris WS, Vanden Heuvel JP, Wagner PR, West SG. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr. 2011;93:243–52. doi: 10.3945/ajcn.110.003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palatini P, Julius S. The role of cardiac autonomic function in hypertension and cardiovascular disease. Curr Hypertens Rep. 2009;11:199–205. doi: 10.1007/s11906-009-0035-4. [DOI] [PubMed] [Google Scholar]
- 48.Skulas-Ray AC, Kris-Etherton PM, Harris WS, West SG. Marine-derived omega-3 fatty acids dose-dependently reduce heart rate and blood pressure and alter impedance cardiography outcomes. Ann Behav Med. 2012;44:301–308. doi: 10.1007/s12160-012-9393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sauder KA, Johnston ER, Skulas-Ray AC, Campbell TS, West SG. Effect of meal content on heart rate variability and cardiovascular reactivity to mental stress. Psychophysiology. 2012;49:470–7. doi: 10.1111/j.1469-8986.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.West SG, Krick AL, Klein LC, Zhao G, Wojtowicz TF, McGuiness M, Bagshaw DM, Wagner P, Ceballos RM, Holub BJ, Kris-Etherton PM. Effects of diets high in walnuts and flax oil on hemodynamic responses to stress and vascular endothelial function. J Am Coll Nutr. 2010;29:595–603. doi: 10.1080/07315724.2010.10719898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.West SG, Gebauer SK, Kay CD, Bagshaw DM, Savastano DM, Diefenbach C, Kris-Etherton PM. Diets containing pistachios reduce systolic blood pressure and peripheral vascular responses to stress in adults with dyslipidemia. Hypertension. 2012;60:58–63. doi: 10.1161/HYPERTENSIONAHA.111.182147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 53.Boardman A, Schlindwein FS, Rocha AP, Leite A. A study on the optimum order of autoregressive models for heart rate variability. Physiol Meas. 2002;23:325–36. doi: 10.1088/0967-3334/23/2/308. [DOI] [PubMed] [Google Scholar]
- 54.Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–48. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 55.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 56.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The Social Psychology of Health. Newbury Park, CA: Sage; 1988. pp. 31–67. [Google Scholar]
- 57.Spielberger C. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologist Press; 1983. [Google Scholar]
- 58.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 59.Radloff LS. The CES-D Scale. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 60.Bagiella E, Sloan RP, Heitjan DF. Mixed-effects models in psychophysiology. Psychophysiology. 2000;37:13–20. [PubMed] [Google Scholar]
- 61.Stein PK, Bosner MS, Kleiger RE, Conger BM. Heart rate variability: a measure of cardiac autonomic tone. Am Heart J. 1994;127:1376–81. doi: 10.1016/0002-8703(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 62.May O, Arildsen H. Long-term predictive power of heart rate variability on all-cause mortality in the diabetic population. Acta Diabetol. 2011;48:55–9. doi: 10.1007/s00592-010-0222-4. [DOI] [PubMed] [Google Scholar]
- 63.Singh JP, Larson MG, Tsuji H, Evans JC, O’Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension. 1998;32:293–7. doi: 10.1161/01.hyp.32.2.293. [DOI] [PubMed] [Google Scholar]
- 64.Lovallo WR, Gerin W. Psychophysiological reactivity: mechanisms and pathways to cardiovascular disease. Psychosom Med. 2003;65:36–45. doi: 10.1097/01.psy.0000033128.44101.c1. [DOI] [PubMed] [Google Scholar]
- 65.Greiser KH, Kluttig A, Schumann B, Swenne CA, Kors JA, Kuss O, Haerting J, Schmidt H, Thiery J, Werdan K. Cardiovascular diseases, risk factors and short-term heart rate variability in an elderly general population: the CARLA study 2002–2006. Eur J Epidemiol. 2009;24:123–42. doi: 10.1007/s10654-009-9317-z. [DOI] [PubMed] [Google Scholar]
- 66.Crawford JR, Henry JD. The Positive and Negative Affect Schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. 2004;43:245–65. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- 67.Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67:1067–74. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 68.Guinjoan SM, de Guevara MS, Correa C, Schauffele SI, Nicola-Siri L, Fahrer RD, Ortiz-Fragola E, Martinez-Martinez JA, Cardinali DP. Cardiac parasympathetic dysfunction related to depression in older adults with acute coronary syndromes. J Psychosom Res. 2004;56:83–8. doi: 10.1016/S0022-3999(03)00043-6. [DOI] [PubMed] [Google Scholar]
- 69.Huikuri HV, Pikkujamsa SM, Airaksinen KE, Ikaheimo MJ, Rantala AO, Kauma H, Lilja M, Kesaniemi YA. Sex-related differences in autonomic modulation of heart rate in middle-aged subjects. Circulation. 1996;94:122–5. doi: 10.1161/01.cir.94.2.122. [DOI] [PubMed] [Google Scholar]
- 70.Liao D, Barnes RW, Chambless LE, Simpson RJ, Jr, Sorlie P, Heiss G. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability—the ARIC study. Atherosclerosis Risk in Communities. Am J Cardiol. 1995;76:906–12. doi: 10.1016/s0002-9149(99)80260-4. [DOI] [PubMed] [Google Scholar]
- 71.Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 1997;38:2012–22. [PubMed] [Google Scholar]


