Abstract
Background:
To properly assess the progression and treatment response of alopecia, one must measure the changes in hair mass, which is influenced by both the density and diameter of hair. Unfortunately, a convenient device for hair mass evaluation had not been available to dermatologists until the recent introduction of the cross-section trichometer, which directly measures the cross-sectional area of an isolated bundle of hair.
Objective:
We sought to evaluate the accuracy and sensitivity of the HairCheck® device, a commercial product derived from the original cross-section trichometer.
Materials and Methods:
Bundles of surgical silk and human hair were used to evaluate the ability of the HairCheck® device to detect and measure small changes in the number and diameter of strands, and bundle weight.
Results:
Strong correlations were observed between the bundle's cross-sectional area, displayed as the numeric Hair Mass Index (HMI), the number of strands, the silk/hair diameter, and the bundle dry weight.
Conclusion:
HMI strongly correlated with the number and diameter of silk/hair, and the weight of the bundle, suggesting that it can serve as a valid indicator of hair mass. We have given the name cross-section trichometry (CST) to the methodology of obtaining the HMI using the HairCheck® system. CST is a simple modality for the quantification of hair mass, and may be used as a convenient and useful tool to clinically assess changes in hair mass caused by thinning, shedding, breakage, or growth in males and females with progressive alopecia or those receiving alopecia treatment.
Keywords: Hair density, hair diameter, hair mass, hair mass index, hair measurement
INTRODUCTION
Hair loss affects millions worldwide. For example, the most common form of alopecia, androgenetic alopecia (AGA, pattern hair loss), affects 30% of males before the age of 30, and 50% by the age of 50.[1] By age 70, approximately 80% of Caucasian men and 60% of Caucasian women are affected by some degree of hair loss.[2]
At present, most dermatologists use simple, non-quantitative methods to detect, categorize, and monitor AGA, with an emphasis on density alone. These include the Norwood/Ludwig Scales for AGA,[3,4,5] the Savin Scale,[6,7] the Cohen Hair Loss Severity Scale (HLSS),[8] and informal clinical photography. However, small decreases in hair density are not easily detected by the naked eye; a study showed that no visible difference was appreciated until 50% of the hair was lost.[9] Therefore, comparing “before and after” global photographs has little value if the loss is less than 50%.
In addition to hair density, the diameter of the hair fibers also contributes greatly to the appearance of hair. Despite the known fact of hair miniaturization during AGA progression, few methods are available to monitor changes in hair diameter for office dermatologists besides global photography and simple magnified scalp exam (dermatoscopy),[10,11,12,12] which are not quantitative. Although sophisticated measuring methods are available, their use has been limited to research facilities and industry laboratories. These include conventional and contrast-enhanced phototrichogram,[10,11,13,14,15,16] measurement of dry hair weight,[17,18] electron microscopy, and confocal laser scanning microscopy.[13] Phototrichogram, the standard assay required by the FDA for hair growth product evaluation, measures the growth and shedding of any hair that is greater than 30 μm in diameter, but does not appreciate or reflect the range of miniaturized hairs that exists in patients with AGA [Figure 1]. Additionally, small changes in hair diameter can be very difficult to visualize. The dry hair weight measurement evaluates changes in hair density and diameters over a fixed period of time by measuring the weight of clipped hair. Whereas it is recognized as the industry gold standard for measuring hair, it is a research modality that is difficult to perform, and requires hair clipping. Furthermore, besides changes in hair density or diameter, changes in growth rate can also lead to altered dry hair weight, unless samples are corrected for length.
Figure 1.
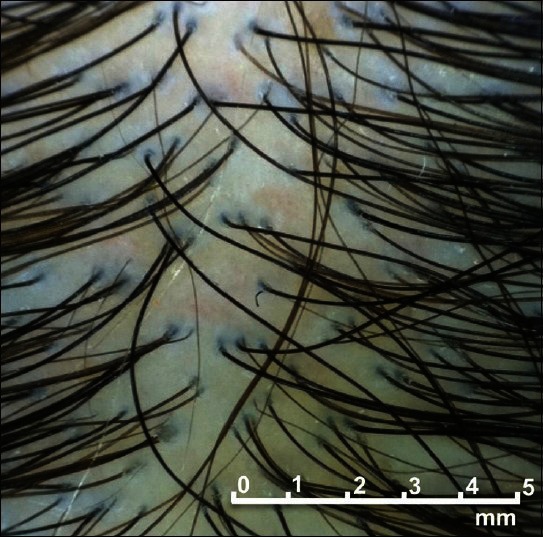
In androgenetic alopecia, the hair diameter varies greatly. But for assays of hair density, any hair with a diameter of greater than 30 μm is counted as single hair strand without consideration of the variation
The inability to quantify hair loss conveniently had significantly limited the treatment of AGA and other hair loss disorders. Managing hair loss without convenient quantification was compared with weight management without a scale. What factors should be included when quantifying hair? The hallmark of AGA is continuous miniaturization of terminal hair follicles, which leads to decrease in density and diameter, both of which influence appearance. Therefore, in circumstances such as AGA management, a meaningful assay should measure both density and diameter, which Arnold has termed the “hair mass.”[19] User-friendly devices/procedures that can measure the hair mass accurately and reliably will greatly facilitate dermatologists’ assessment of alopecia progression and treatment response.
In 2008, Cohen published a report introducing the cross-section trichometer, a hand-held device for measuring hair mass.[20] This device “grabs” the bundle of hair from a 2 × 2 cm scalp area in a J-slot and measures the cross-sectional area of the hair bundle. It then displays the Trichometric Index – which equals to bundle cross-sectional area in mm2 per cm2 of scalp surface multiplied by 100.[20] In a pre-clinical study using surgical silk and cut human hair, a direct correlation was observed between filament number, diameter, dry weight, and the cross-sectional area.[20] In addition, a direct correlation was detected between the observed hair loss severity (using the HLSS) and the Trichometric Index.[20] For clinical applications, a separate locating strip allows repeated measurement of hair from the same scalp area without the use of tattoos.[20,21] In a subsequent clinical study, the device was found to be user-friendly and showed high reproducibility.[22]
The objective of this study was to determine the accuracy, precision, and sensitivity of the HairCheck® device, a commercial version of the original trichometer prototype, in a pre-clinical setting. Bundles of surgical silk and cut human hair were measured to determine the correlation between the number and diameter of strands, the weight of the bundle, and the numeric display of the HMI (equivalent to the Trichometric Index measured by the trichometer prototype).
MATERIALS AND METHODS
The HairCheck® measuring device
The HairCheck® measuring device, together with a locating strip, comprised the HairCheck® System (Divi International Co., Miami, FL, USA), which was designed to quantitatively measure scalp hair mass.[21,22] The locating strip enabled the return to the same sample site on the scalp on subsequent visits without the application of tattoos. The measuring device was a hand-held mechanical device with paired levers that transmitted a pre-determined load to a captured bundle of hair.[21] The capture chamber has a slotted hook and anvil integrated into a disposable cartridge. Its size was designed to measure the cross-sectional area of a bundle of hair growing within a 2 × 2 cm (4 cm2) scalp area. The hair needed to be at least 2.5 cm (1 inch) in length for the device to function properly. An LED screen displayed the HMI value, expressed as mm2 of hair per cm2 of scalp × 100, rounded up to the closest integer.[21]
Obtaining the Hair Mass Index using the HairCheck® device
Bundles of 3-0, 4-0, 5-0, and 6-0 surgical silk and cut human hair were measured using the HairCheck® device. First, a disposable cartridge was calibrated using a metal key. Once the cartridge was calibrated, the bundle of surgical silk or hair was placed in the J-slot and measured. The cross-sectional area of the bundle was instantaneously displayed as the HMI on the LED screen.[21]
RESULTS
To determine the accuracy, precision, and sensitivity of the HairCheck® measuring device in a pre-clinical setting, we measured bundles of surgical silk and cut human hair to assess the correlation between the number and diameter of filaments, the weight of the bundle, and the HMI display.
Measuring surgical silk using the HairCheck® device
To determine the correlation between the number of surgical silk strands and HMI, bundles of 5-0 surgical silk (20-160 strands) were measured using the HairCheck® device. A strong correlation was observed between the number of silk strands and the HMI display (correlation coefficient r = 0.9969) [Figure 2a].
Figure 2.
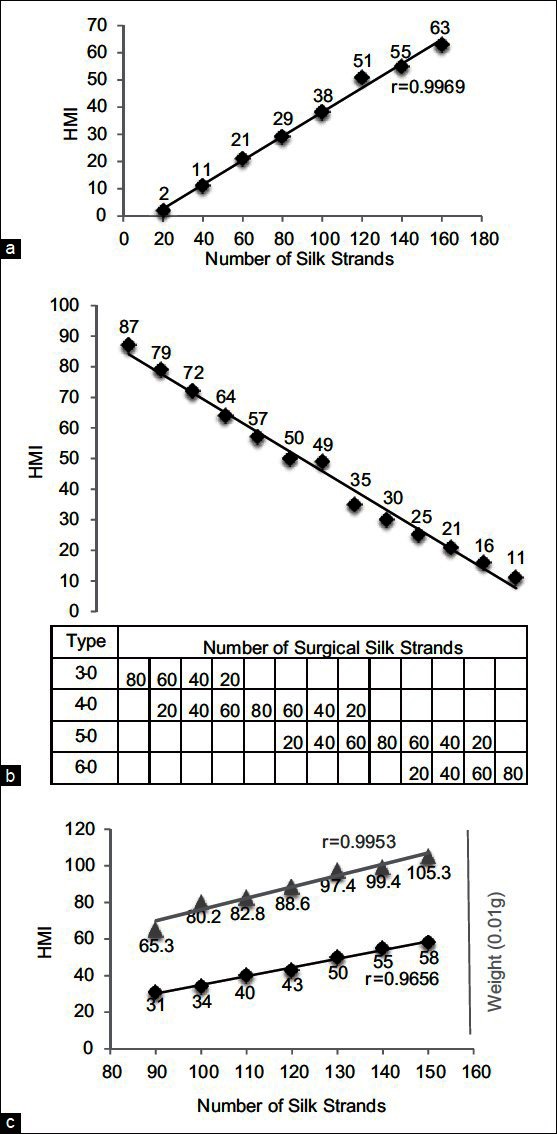
Cross-section trichometry (CST) of surgical silk. (a) Correlation between the number of strands and Hair Mass Index (HMI). (b) CST of mixed silk strands of different types/diameters. (c) Correlation between HMI, the number of strands, and bundle weight
To determine the sensitivity of the device to changes in filament diameter, bundles of 80-strand surgical silk were measured, using filaments of the same size or of two mixed sizes. A strong correlation was observed between filament diameter and the HMI; as thicker filaments were replaced with thinner filaments, HMI steadily decreased in a linear relation to the number of thicker filaments [Figure 2b]. These observations suggest that the device will be useful in evaluating progressive hair thinning, such as miniaturization in AGA.
We then examined the correlation between the number of silk strands, the HMI, and the bundle weight – hair weight being the industry standard for measuring hair mass. We observed proportionate incremental changes in the HMI and bundle weight as the number of silk strands increased (r = 0.9656 between the number of strands and HMI, r = 0.9953 between dry weight and HMI) [Figure 2c]. These results established that there was direct correlation between the cross-sectional area expressed as the HMI and the number of strands, as well as the bundle weight.
Measuring cut human hair using the HairCheck® device
When bundles of cut human hair were measured instead of surgical silk, a strong correlation was established between the number of hair strands, the hair weight, and bundle cross-sectional area expressed as the HMI (r = 0.9959 between the number of strands and HMI, r = 0.9874 between dry weight and HMI) [Figure 3]. The HairCheck® device was sensitive enough to detect the small changes in HMI when 10 hair fibers were removed at a time from a bundle of 330-400 strands [Figure 3].
Figure 3.
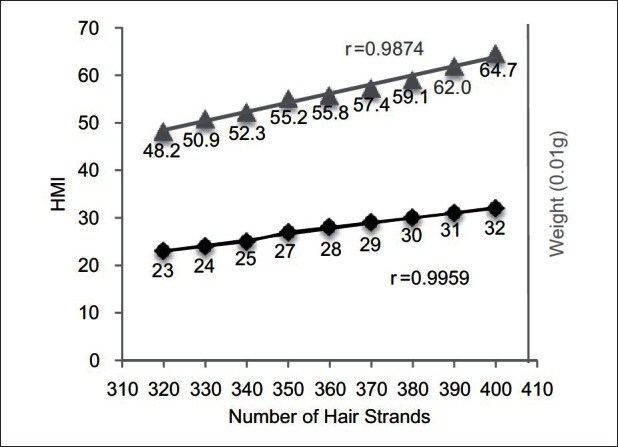
Cross-section trichometry of bundles of cut human hair using the HairCheck® device. Shown is correlation between the HMI, the number of hair strands, and bundle weight
DISCUSSION
In this study, we evaluated the accuracy, precision, and sensitivity of the HairCheck® measuring device in a pre-clinical setting. Using bundles of surgical silk and cut human hair, we observed strong correlations between the HMI display and the number and diameter of silk/hair, and the weight of the bundle. These results suggest that HMI is a valid indicator of hair mass. We have given the name cross-section trichometry (CST) to the HairCheck® system and its methodology to determine the HMI.
The hair mass index
HMI equals to the cross-sectional area of a bundle of hair (mm2) per cm2 of the sample scalp area multiplied by 100. It is equivalent to the Trichometric Index obtained with the trichometer prototype. HMI is a direct measurement of the hair mass as determined by the number of hair fibers (density) and hair diameter, and is not influenced by the length of the hair. The numeric value of HMI allows physicians and patients to monitor the hair mass over time, much like the way body mass index (BMI) is used in the management of weight.
Comparing with other methods and technologies used to measure hair, HMI allows early detection of changes in hair mass, and is a quantitative and more accurate indicator of hair mass. Global photography is only of value when hair loss is visible, and does not provide visible information when less than 50% of hair has been lost.[9] Phototrichogram measures density alone and ignores the variability in hair diameter as long as it is greater than 30 μm.[11,14,15] It is therefore not useful for measuring hair mass changes in AGA progression and its treatment when miniaturization generates a range of hair diameters less than 80 μm. Although hair weight measurement is considered the industry gold standard, it does not exclude the effects of hair length, and will reflect improvement if a test product makes hair grow faster (therefore longer and heavier) without increasing the hair density and/or diameter. However, the ability to increase growth rate is of little relevance under many circumstances, since human hair is styled, cut, and its length often determined arbitrarily. The HairCheck's HMI values are not influenced by hair length, but reflect hair mass as determined by density and diameter alone – the two anatomic hallmarks of hair loss and growth.
The HMI was shown to correlate with visible stages of balding in AGA. In a series of AGA patients with vertex balding, a direct correlation was observed between the HMI and the global assessment of hair loss via the HLSS.[20] In the absence of hair loss, normal HMI ranged from 75 for fine hair to 100 for coarse hair with an average of 87. HMIs were observed in the range of 50 in patients with minimal hair loss, and in the ranges of 40 in patients with mild hair loss. As the visible severity of the loss increased, an incremental reduction in the HMI was observed, with patients with severe hair loss having HMI values of ~20.[20]
Cross-section trichometry
CST is designed to quickly and conveniently quantify the hair mass in the same scalp area during different office visits without hair clipping and without the application of tattoos.[21,22] The HairCheck® System (Divi International Co., Miami, FL, USA) is a commercial version of the original trichometer prototype,[20,21] and consists of a locating strip and a measuring device.[21] The locating strip enables the return to the same sample site (2 × 2 cm) on the scalp on subsequent visits without the application of tattoos. The measuring device measures the cross-sectional area of the bundle of hair growing within the 2 × 2 cm (4 cm2) scalp area, and displays the HMI. In a previous study, measurement of scalp hair using the HairCheck® system was shown to be highly reproducible.[22] CST generates a precise numeric HMI score that is easy to document and compare between office visits.
CST is suitable for measuring all types of hair, from super fine hair (~40 μm in diameter) to very coarse hair (~100 μm in diameter), with the HMI values estimated at 50-120, respectively, at a density of 230 hairs/cm2 (human scalp hair density ranges from 180 to 280 hairs per cm2). The hair must be at least 2.5 cm in length for accurate measurement, but no hair clipping is required for the assay.
For the purpose of this pre-clinical study, surgical fibers and cut human hair were measured using the HairCheck® device alone. We showed that the HMI was directly proportional to the number of strands in the bundle, the diameter of fibers in the bundle, and the bundle weight – the industry gold standard. We also showed that the device was sufficiently sensitive to detect small changes in hair number/density and filament diameter, including filaments of mixed diameters. Our results suggest that CST is a valuable tool for the quantitative assessment of hair mass and hair loss disorders.
Applications of cross-section trichometry
CST was designed for quick and convenient quantification of scalp hair mass by office clinicians. The measurement did not require hair clipping, took only a few minutes to complete, and generated a precise and meaningful numeric HMI score that was easy to document and compare between office visits. The locating strip enabled measurement of the same sample area with high reproducibility, without the application of tattoos.[21,22] CST can measure all types of hair, from super fine to very coarse, so far as the hair is at least 2.5 cm (1 inch) in length. CST takes into account both hair density and diameter, the two anatomic hallmarks of hair loss and growth. Because the scalp is not tattooed, its use as a research modality is limited.
The sensitivity of CST for small changes in the hair mass (caused by changes in diameter or density) makes it a valuable tool in a wide range of hair loss situations regardless of etiology. The early detection and measurement of non-visible AGA offers a significant advantage to the patient. It alerts the physician and patient to consider minoxidil, finasteride, or laser treatment, 10-15 years in advance of visible signs of loss. CST in the occiput (permanent fringe) during the initial visit will establish a baseline HMI value that will serve as a reference. By comparing the HMI in the suspected area of loss with the baseline HMI, non-visible AGA can be easily detected and quantified. If treatment is started before severe follicular miniaturization ensues, the treatment can be profoundly more effective. Additionally, for hair transplantation patients, CST in the donor area during initial consultation will help the surgeon and patient develop realistic expectations of the outcome. In addition to male and female AGA, CST can be used to manage additional hair loss conditions unique to women, such as post-partum effluvium and traction-induced alopecia, assessing their clinical course and treatment efficacy.
CST can also be used to evaluate drugs such as minoxidil and finasteride, and detect the response to dosage changes and combination treatments. Similarly, it can be used to evaluate devices and over-the-counter products (lasers, food supplements, etc.) that promise to grow hair. It can measure the hair's response to thyroid and iron therapy in patients with deficiencies. The HMI is measured in the affected area before initiating hair loss treatment, and measured again every 6-12 months to determine its precise effect. On the other hand, CST can be used to measure hair loss as a side effect of patient's response to drugs. Until now, it has been impossible to objectively evaluate the efficacy in many of these circumstances.
In addition to measuring hair loss and growth, CST can be used to measure hair breakage. When breakage is present, the bundle cross-sectional area decreases from the proximal portion toward the tip, and the ratio of the distal HMI to the proximal HMI can be used as an indicator of breakage severity.[23]
In summary, CST provides a quick and accurate assessment of the hair mass in a wide range of hair loss situations in both male and female patients that include thinning, shedding, and breakage. Repeated evaluation of hair mass with CST by office dermatologists over time will generate quantitative information on the progression of alopecia or treatment response, which will lead to optimized treatment plans that will result in improved efficacy.
Footnotes
Source of Support: TCW is the recipient of a Career Development Award from NIH/NIAMS (AR050487)
Conflict of Interest: None declared.
REFERENCES
- 1.Ellis JA, Sinclair R, Harrap SB. Androgenetic alopecia: Pathogenesis and potential for therapy. Expert Rev Mol Med. 2002;4:1–11. doi: 10.1017/S1462399402005112. [DOI] [PubMed] [Google Scholar]
- 2.Yip L, Rufaut N, Sinclair R. Role of genetics and sex steroid hormones in male androgenetic alopecia and female pattern hair loss: An update of what we now know. Australas J Dermatol. 2011;52:81–8. doi: 10.1111/j.1440-0960.2011.00745.x. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247–54. doi: 10.1111/j.1365-2133.1977.tb15179.x. [DOI] [PubMed] [Google Scholar]
- 4.Norwood OT. Male pattern baldness: Classification and incidence. South Med J. 1975;68:1359–65. doi: 10.1097/00007611-197511000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Norwood OT. Incidence of female androgenetic alopecia (female pattern alopecia) Dermatol Surg. 2001;27:53–4. [PubMed] [Google Scholar]
- 6.Price VH, Roberts JL, Hordinsky M, Olsen EA, Savin R, Bergfeld W, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43:768–76. doi: 10.1067/mjd.2000.107953. [DOI] [PubMed] [Google Scholar]
- 7.Savin R. Kalamazoo, MI: The Upjohn Company; 1994. Evaluating androgenetic alopecia in male and female patients: An improved visual method of classifying and tracking hair loss using computer-generated male and female pattern and density scales. [Google Scholar]
- 8.Cohen B. Hair loss index, profile, and severity scale. In: Haber SD, editor. Hair Transplantation. Philadelphia: Elesevier; 2006. p. 12. [Google Scholar]
- 9.Marritt E. The death of the density debate. Dermatol Surg. 1999;25:654–60. doi: 10.1046/j.1524-4725.1999.99067.x. [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain AJ, Dawber RP. Methods of evaluating hair growth. Australas J Dermatol. 2003;44:10–8. doi: 10.1046/j.1440-0960.2002.t01-1-00631.x. [DOI] [PubMed] [Google Scholar]
- 11.Dhurat R. Phototrichogram. Indian J Dermatol Venereol Leprol. 2006;72:242–4. doi: 10.4103/0378-6323.25795. [DOI] [PubMed] [Google Scholar]
- 12.Micali G, Lacarrubba F, Massimino D, Schwartz RA. Dermatoscopy: Alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64:1135–46. doi: 10.1016/j.jaad.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Hillmann K, Blume-Peytavi U. Diagnosis of hair disorders. Semin Cutan Med Surg. 2009;28:33–8. doi: 10.1016/j.sder.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann R. TrichoScan: Combining epiluminescence microscopy with digital image analysis for the measurement of hair growth in vivo. Eur J Dermatol. 2001;11:362–8. [PubMed] [Google Scholar]
- 15.Leroy T, Van Neste D. Contrast enhanced phototrichogram pinpoints scalp hair changes in androgen sensitive areas of male androgenetic alopecia. Skin Res Technol. 2002;8:106–11. doi: 10.1034/j.1600-0846.2002.00329.x. [DOI] [PubMed] [Google Scholar]
- 16.Scheede S, Herpens A, Burmeister F, Oltrogge B, Saenger K, Schmidt-Rose T, et al. Qualification of a new and precise automatic tool for the assessment of hair diameters in phototrichograms. Skin Res Technol. 2011;17:186–95. doi: 10.1111/j.1600-0846.2010.00482.x. [DOI] [PubMed] [Google Scholar]
- 17.Das-Chaudhuri AB. Genetic basis of human scalp hair weight: A twin study. Ann Hum Biol. 1980;7:77–81. doi: 10.1080/03014468000004061. [DOI] [PubMed] [Google Scholar]
- 18.Price VH, Menefee E, Strauss PC. Changes in hair weight and hair count in men with androgenetic alopecia, after application of 5% and 2% topical minoxidil, placebo, or no treatment. J Am Acad Dermatol. 1999;41:717–21. doi: 10.1016/s0190-9622(99)70006-x. [DOI] [PubMed] [Google Scholar]
- 19.Neidel FG, Bretschneider P. Measuring hair density and mass. In: Unger WP, Shapiro R, editors. Hair Transplantation. New York: Marcel Dekker; 2004. p. 876. [Google Scholar]
- 20.Cohen B. The cross-section trichometer: A new device for measuring hair quantity, hair loss, and hair growth. Dermatol Surg. 2008;34:900–10. doi: 10.1111/j.1524-4725.2008.34175.x. [DOI] [PubMed] [Google Scholar]
- 21.Cohen B. The cross-section trichometer. In: Unger WP, Shapiro R, Unger R, Unger M, editors. Hair Transplantation. Colchester, Essex, UK: Informa Healthcare; 2010. pp. 340–3. [Google Scholar]
- 22.Hendriks MA, Geerts PA, Dercksen MW, van den Hurk CJ, Breed WP. Evaluation of Cohen's cross-section trichometer for measuring hair quantity. Dermatol Surg. 2012 doi: 10.1111/j.1524-4725.2011.02176.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Mhaskar S, Kalghatgi B, Chavan M, Rout S, Gode V. Hair breakage index: An alternative tool for damage assessment of human hair. J Cosmet Sci. 2011;62:203–7. [PubMed] [Google Scholar]


