Abstract
Endogenous signaling lipids (“endocannabinoids”) functionally related to Δ9-tetrahydrocannabinol, the psychoactive ingredient of marijuana (Cannabis), are important biomediators and metabolic regulators critical to mammalian (patho)physiology. The growing family of endocannabinoids, along with endocannabinoid biosynthetic and inactivating enzymes, transporters, and at least two membrane-bound, G-protein coupled receptors, comprise collectively the mammalian endocannabinoid signaling system. The ubiquitous and diverse regulatory actions of the endocannabinoid system in health and disease have supported the regulatory approval of natural products and synthetic agents as drugs that alter endocannabinoid-system activity. More recent data support the concept that the endocananbinoid system may be modulated for therapeutic gain at discrete pharmacological targets with safety and efficacy. Potential medications based on the endocannabinoid system have thus become a central focus of contemporary translational research for varied indications with important unmet medical needs. One such indication, obesity, is a global pandemic whose etiology has a pathogenic component of endocannabinoid-system hyperactivity and for which current pharmacological treatment is severely limited. Application of high-affinity, selective CB1 cannabinoid receptor ligands to attenuate endocannabinoid signaling represents a state-of-the-art approach for improving obesity pharmacotherapy. To this intent, several selective CB1 receptor antagonists with varied chemical structures are currently in advanced preclinical or clinical trials, and one (rimonabant) has been approved as a weight-management drug in some markets. Emerging preclinical data suggest that CB1 receptor neutral antagonists may represent breakthrough medications superior to antagonists/inverse agonists such as rimonabant for therapeutic attenuation of CB1 receptor transmission. Since obesity is a predisposing condition for the cluster of cardiovascular and metabolic derangements collectively known as the metabolic syndrome, effective endocannabinoid-modulatory anti-obesity therapeutics would also help redress other major health problems including type-2 diabetes, atherothrombosis, inflammation, and immune disorders. Pressing worldwide healthcare needs and increasing appreciation of endocannabinoid biology make the rational design and refinement of targeted CB1 receptor modulators a promising route to future medications with significant therapeutic impact against overweight, obesity, obesity-related cardiometabolic dysregulation, and, more generally, maladies having a reward-supported appetitive component.
Keywords: Addiction, Antagonist, Appetitive behavior, Cardiometabolic risk, Cardiovascular disease, Diet, Drugs, Endocannabinoid, Inverse agonist, Ligands, Metabolic syndrome, Neutral antagonist, Obesity, Receptor, Rimonabant, Weight management
1. Introduction Cannabinoid pharmacology
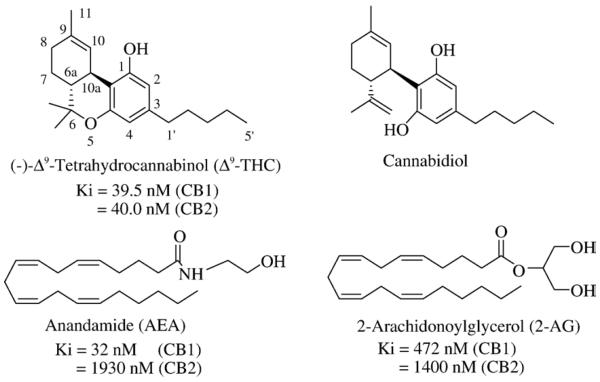
Human recreational use of the hemp plant Cannabis sativa (“marijuana”) and anecdotal attempts to exploit it for potential therapeutic benefit have been documented throughout millennia [1]. Some of marijuana's popularity as a recreational substance and medicament reflects its ability to alter sensory perception and relieve anxiety. Other medicinal effects of marijuana un-related to its psychoactive properties, such as pain relief, have also been recorded in ancient texts. Yet systematic study of marijuana biochemistry and pharmacology was not undertaken until much more recently. Initial attempts at isolating individual plant cannabinoids (phytocannabinoids) from Cannabis, first reported in the mid-20th century, led only to limited pharmacological investigation of these often crude extracts [1,2]. In the 1960's, the correct stereochemical structures of both Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (Fig. 1) were elucidated, their complete chemical syntheses were achieved, and Δ9-THC was identified as the major phytocannabinoid among the approximately 60 present in Cannabis [2].
Fig. 1.
Chemical structures of the phytocannabinoids Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol and the endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG). In this and all other structure figures (i.e., Figs. 3–6), comparative Ki or IC50 values for ligand binding to CB1 and CB2 receptors are given (when available) from literature references cited in the relevant text.
Although Δ9-THC, cannabidiol, and some other phytocannabinoids are bioactive with, for example, intriguing anti-inflammatory, anti-convulsive, and anti-emetic effects of potential therapeutic value, Δ9-THC is regarded as the sole psychotropic cannabinoid in Cannabis [3]. Largely because of its psychoactivity as well as its prevalence and early availability in synthetic form as a research tool, Δ9-THC quickly attained the status of prototypic cannabinoid and became the focus of many pharmacological and mechanistic studies through the 1970's and 1980's. Much of this research in vivo was aimed at elucidating the effects of Δ9-THC in experimental animals with the aid of newly-synthesized Δ9-THC analogs, some of which were radiolabeled as molecular probes of cannabinoid-tissue interactions. Given Δ9-THC's psychotropic effects, many biological investigations employed brain and brain plasma membranes as study-objects. Consensus data describing several key characteristics of cannabinoid action emerged: Δ9-THC and synthetic analogs elicit biological effects in a stereo- and structurally selective manner. Their binding to brain plasma membranes is avid, saturable, stereospecific, concordant with in vitro and in vivo bioresponses (e.g., adenylyl cyclase inhibition, analgesia), and nonrandom in select brain regions [3,4]. These characteristics strongly implied that cannabinoid pharmacology is receptor-mediated, spurring the search for discrete mammalian cannabinoid receptors whose activation by Δ9-THC would elicit psychotropic effects. The search led to the discovery and cloning of two G protein-coupled receptors (GPCRs) for cannabinoids (CB), designated CB1 and CB2, which in humans share ≈44% sequence homology [5,6]. The CB1 receptor subtype is localized primarily in the central nervous system (CNS), reflecting its prevalence as the most abundant GPCR in brain. CB1 receptors are distributed among the cortex, cerebellum, hippocampus, and basal ganglia, brain regions that control motor, cognitive, emotional, and sensory functions. Hence, central CB1 receptor activation mediates most cannabinoid psychotropic and behavioral effects. The CB1 receptor is also present in high density in the brainstem, hypothalamus, and pituitary gland, loci influencing pain perception; hormonal activity; thermoregulation; and cardiovascular, gastrointestinal, and respiratory physiology. CB1 receptors at peripheral sites (e.g., adipocytes, liver, uterus) help regulate such basic physiological processes as energy balance and reproduction. Although detectable at exceedingly low levels in brain [7], CB2 receptors are expressed mainly by immune and hematopoietic cells, osteoclasts, and osteoblasts and mediate immune responses, inflammation, inflammatory and neuropathic pain, and bone remodeling [5,6].
2. Endogenous cannabinoids and the endocannabinoid signaling system
The discordance between the presence of cannabinoid receptors in mammalian brain and the absence of intrinsic tissue phytocannabinoids invited the search for cannabinoid-receptor ligands that are produced and metabolized as endogenous bioactive tissue constituents (“endocannabinoids”). By the mid-1990's, the first two endocannabinoids, N-arachidonoylethanolamine (“anandamide”) (AEA) and 2-arachidonoylglycerol (2-AG) (Fig. 1), were identified and characterized as derivatives of the polyunsaturated fatty acid, arachidonic acid (AA) [8–10]. AEA and 2-AG are members of a growing family of natural amide and ester derivatives of long-chain fatty acids ubiquitous in humans and other mammals that act as CB1 and/or CB2 receptor agonists [11]. AEA itself is as a partial CB1 receptor agonist and a relatively weak CB2 receptor ligand with low overall efficacy, whereas 2-AG, produced in significantly greater amounts than AEA, is a full agonist at both receptors [6,12]. “Classical” CB1 and CB2 receptor transmission is coupled through inhibitory G-proteins (Gi/o) to inhibit adenylyl cyclase (i.e., cyclic AMP formation) and L-, N-, and P/Q-type calcium channels and activate potassium channels and mitogen-activated protein kinase [13]. When coupled to other G-protein classes, receptor-mediated cannabinoid signaling influences diverse transduction systems to elicit, for example, cellular calcium uptake [13,14]. Furthermore, endocanabinoids may signal through G protein-independent systems involving messengers such as phosphatidylinositol-3-kinase or nitric oxide, and receptor-independent endocannabinoid effects on excitable cells have been reported [15].
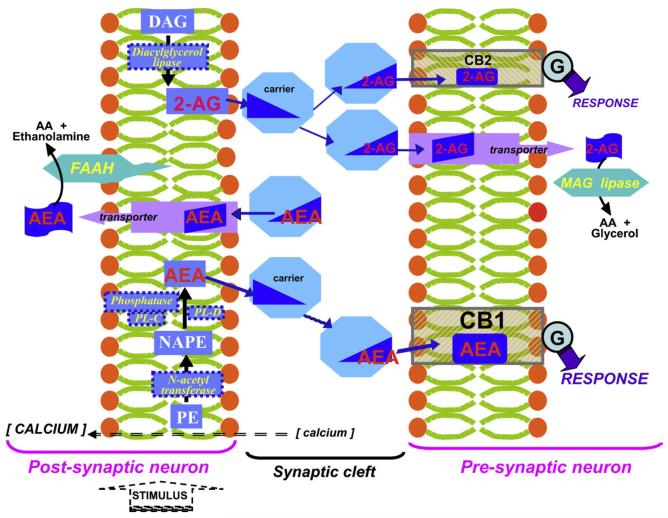
The endocannabinoids and their cellular receptors, along with carriers and transporters that assist in the transit of these hydrophobic mediators between and into cells and the enzymes directly responsible for endocannabinoid biosynthesis and metabolism, collectively comprise the endocannabinoid signaling system [16,17]. In concert with the hydrophobic nature of the endocannabinoids themselves and the selective tissue disposition of their target receptors, endocannabinoid metabolism and the distribution of endocannabinoid metabolizing enzymes help delimit the broad influence these lipid mediators may exert on mammalian physiology. The balanced actions of endocannabinoid biosynthetic and metabolizing enzymes are key to the stringent homeostatic regulation of endocannabinoid-system signaling [11,12,18]. In marked contrast to many neurotransmitters that are stored within intracellular vesicles awaiting mobilization in bioactive form, endocannabinoids are synthesized “on demand” in response to stimulus-induced intracellular calcium elevation. The precursor for AEA synthesis is the membrane phospholipid N-arachidonoyl-phosphatidylethanolamine (NAPE). Acylamides such as AEA are synthesized by hydrolytic liberation of polyunsaturated fatty acid by a specific phospholipase D (PL-D); through the phospholipase C-mediated formation of phosphor-AEA, which itself is dephosphorylated by a specific phosphatase; or through sequential NAPE deacylation by α,β-hydrolase 4 and the subsequent cleavage of glycerophosphate [19]. NAPE itself is synthesized by an N-acetyl transferase (NAT) that may be rate-limiting to AEA biosynthesis [19]. The major pathway for 2-AG formation is through 2-arachidonoyl-phosphatidylinositol hydrolysis by phospholipase C (PL-C) to diacylglycerol (DAG), which is further hydrolyzed to 2-AG by DAG lipase or, in select tissues, by phospholipase A1 and subsequent lysophospholipase activity [20]. Endocannabinoids are efficiently removed from their sites of action by cellular uptake, the mechanism of which largely remains to be characterized, as well as by distinct intracellular enzymes [12,16,21]. In most tissues, AEA is metabolized by a membrane-bound amidase, fatty acid amide hydrolase (FAAH), and 2-AG by soluble monoacylglycerol (MAG) lipase, although FAAH can also act on 2-AG. A number of oxidative enzymes including lipoxygenases, cytochrome P450s, and cyclooxygenase-2 can transform endocannabinoids into eicosanoid-related bioactive products whose physiological roles remain to be appreciated [22]. Within most brain areas, MAG lipase and CB1 receptors (and far fewer CB2 receptors) are localized presynaptically, whereas FAAH is predominantly postsynaptic in somata and dendrites of principal neurons [5,6,18]. This distribution of endocannabinoid-system components defines topographically a CNS signaling paradigm depicted in Fig. 2 by which, following stimulus-dependent intracellular calcium increase and membrane phospholipid hydrolysis, endocannabinoids are synthesized in post-synaptic neurons. The endocannabinoids are then released/transported into the synaptic cleft to act on pre-synaptic neurons. In this retrograde manner, endocannabinoids regulate the synaptic transmission of excitatory and inhibitory neural circuits by modulating neurotransmitter release, the net effect depending upon receptor localization within the circuit [24].
Fig. 2.
Diagrammatical representation of central nervous system (CNS) endocannabinoid signaling. In response to a stimulus-induced intracellular calcium increase, the endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are synthesized in post-synaptic neurons. AEA synthesis starts with N-acylation of phosphatidylethanolamine (PE) by enzyme-catalyzed transfer of an arachidonic-acid (AA) moiety from a donor membrane phospholipid (such as phosphatidylcholine) to form N-arachidonoyl-phosphatidylethanolamine (NAPE), which may then hydrolyzed by, for example, phospholipase D (PL-D) or acted upon by phospholipase C (PL-C) followed by phosphatase action on the resulting phosphoAEA to yield AEA. 2-AG is synthesized in many tissues by lipolysis of diacylglycerol (DAG). The endocannabinoids are then released from post-synaptic neurons and traverse the synaptic cleft (aided, perhaps, by carrier proteins) to pre-synaptic neurons, where they function as retrograde messengers by binding to cannabinoid receptors: AEA has the greater affinity for the CB1 receptor, whereas 2-AG binds preferentially to the relatively minute population of CB2 receptors in the CNS. The signaling through both receptors is coupled to G-proteins (Ⓖ) to elicit a bioresponse. Pre-synaptic monoacylglycerol (MAG) lipase inactivates 2-AG, and post-synaptic fatty acid amide hydrolase (FAAH) inactivates AEA. Endocannabinoid uptake may be facilitated by transmembrane protein transporters. In this manner, endocannbinoids regulate synaptic transmission of excitatory and inhibitory circuits.
3. Therapeutic targets within the endocannabinoid system
Alterations in endocannabinoid signaling accompany a variety of disorders and have been interpreted as either attempts to counteract a disease process/compromised homeostasis (e.g., pain sensing, inflammation) or as pathological contributors (e.g., many psychobehavioral and affective problems) [11,23]. Consequently, several components of the endocannabinoid signaling system continue to be evaluated for their “drugability.” Some have already been validated as therapeutic targets through cannabinoid-based therapeutics that have attained regulatory approval. Theoretically, any system component requisite to either endocannabinoid tone or function could be targeted by an intervention that alters the associated signal transmission by modifying the formation, action, half-life, or tissue residency of these lipid mediators. At present, though, not all endocannabinoid-system components are sufficiently well-characterized either (bio)chemically or functionally to be considered viable candidates for large-scale drug-discovery. Some endocannabinoid metabolizing enzymes, such as cyclooxygenase-2 and lipoxygenases, have indeed been the focus of intense proprietary biopharmaceutical efforts [25]. However, these enzymes fulfill constitutive roles well beyond endocannabinoid metabolism, making their pharmacological modulation to alter only endocannabinoid signaling an invitation for unwanted collateral effects. The means for and consequences of modulating selectively the activities of most enzymes involved in endocannabinoid metabolism (e.g., DAG and MAG lipases, N-acetyl transferase) are also poorly realized at present [23,26,27]. Future detailed characterization of the molecular nature of endocannabinoid transit and internalization may afford the opportunity to identify discrete intercellular carriers or plasma-membrane transporters as drug targets [16,28]. In contrast, extensive data support FAAH's role in generating the transmembrane concentration gradient for cellular AEA uptake and catalyzing AEA degradation [29]. These unique FAAH actions critical to terminating endocannabinoid signaling have made FAAH a favored therapeutic target among known endocannabinoid-system enzymes [18,26]. The availability of human-recombinant FAAH and synthetic FAAH inhibitors [30,31] and clinical associations between FAAH polymorphisms and human disease [32] further enhance FAAH's drug-discovery appeal.
The multiplicity of signaling pathways downstream from cannabinoid-receptor transmission, their myriad effects, and the complexities of endocannabinoid metabolism have placed the most intense interest on CB1 and CB2 receptors as discrete therapeutic targets for a variety of maladies involving most mammalian organ systems [33,34]. Putative non-CB1/CB2 cannabinoid receptors [35], when adequately characterized, may likewise become attractive drug targets. The indications for which cannabinoid-receptor ligands could serve as drugs fall into two broad classes. One class consists of diseases whose pathology and/or symptomology may be ameliorated through endocannabinoid-system activation, as exemplified by the mounting contemporary interest in the discovery and development of synthetic CB2 receptor agonists as anti-inflammatory medicines and analgesics for chronic neuropathic pain [36]. The virtual absence of CB2 receptors from brain makes selective CB2 receptor ligands intrinsically devoid of CNS and psychobehavioral side-effects [33]. The other class of indications thought amenable to endocannabinoid-system modulation appears to reflect heightened endocannabinoid signaling. These include a host of addictive disorders (e.g., smoking and drug and alcohol abuse) whose etiology has an appetitive component and which may be treatable with selective CB1 receptor antagonists. Much contemporary research is aimed at defining the pharmacophore requirements within the CB1 and CB2 receptor binding domains and synthesizing and profiling novel ligands whose selective affinity for either receptor results in discrete modulation of endocannabinoid signaling and a well-defined, salutary pharmacotherapeutic activity [34,37]. The availability of recombinant CB1 and CB2 receptors from rodent and human sources facilitates uncovering species-selectivity in binding motifs and signaling responses to inhibitors/ligands [38,39].
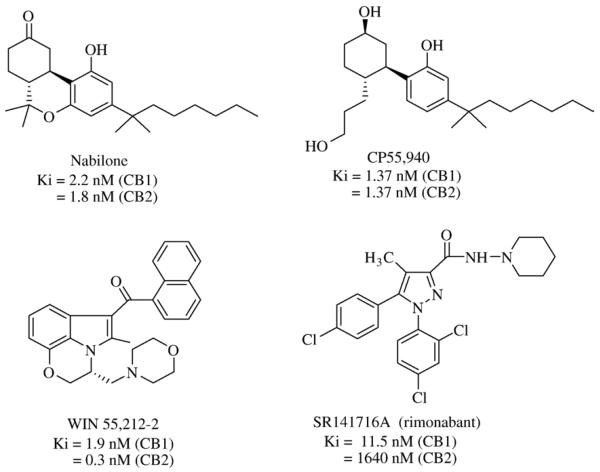
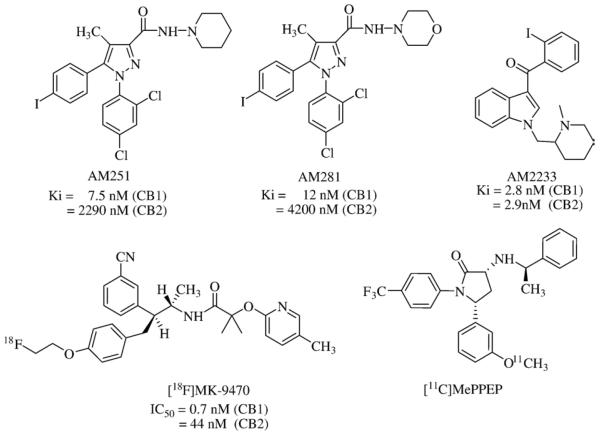
Ethical medicines that act as ligands for CB1 and CB2 receptors have further validated these receptors as viable endocannabinoid-system drug targets. Virtually all cannabinoid-related medications granted regulatory approval thus far are directly related to Cannabis and, hence, act as nonselective agonists at both the CB1 and CB2 receptors [1,40]. Assorted extracts and tinctures of Cannabis had been available commercially in Britain until the 1970's. Δ9-THC (dronabinol, Marinol®) (United Pharmaceuticals) and its synthetic analog, nabilone (Cesamet®) (Valeant Pharmaceuticals) (Fig. 3), are licensed as anti-nausea and anti-emetic medications for chemotherapy patients. Nabilone is also approved as an appetite stimulant to treat acquired immune deficiency syndrome-related cachexia. Sativex® (GW Pharmaceuticals), a standardized Cannabis extract containing an approximately equal mixture of two phytocannabinoids (Δ9-THC and cannabidiol) formulated as a sublingual spray, was first licensed in Canada in April, 2005, for alleviation of neuropathic pain in multiple sclerosis patients and given a qualifying notice in June, 2007, for its approval in Canada for cancer pain relief [41]. A European regulatory approval for Sativex® as treatment for muscular-dystrophy spasticity is expected pending additional data [42]. The low reported frequency of abuse and neurocongnitive side-effects of Cannabis extracts/Δ9-THC derivatives has invited their continued clinical evaluation. For example, although Marinol® is currently the only cannabinoid with approval for marketing in the United States, the United States Food and Drug Administration has permitted Sativex® (under agreement between GW Pharmaceuticals and Otsuka Pharmaceuticals) to enter late-stage development for treatment of pain in advanced cancer patients not adequately relieved by opioids [43]. Aside from nabilone, other synthetic cannabinoid-receptor activators such as CP55,940 (Pfizer) and WIN 55,212-2 (Sterling-Winthrop) (Fig. 3) have been studied clinically for alleviation of emesis; motor-related symptoms in patients with multiple sclerosis, Tourette's syndrome, or Parkinson's disease; intraocular pressure in glaucoma patients; neuropathic pain; or post-trauma brain damage [40]. In general, no such trials have yet shown compelling primary-endpoint results leading to licensed drugs, and the psychoactive potential of mixed CB1/CB2 receptor ligands and consequent dose/efficacy limitations have been somewhat market-adverse. As rationally targeted to a specific component of the endocannabinoid pathway, SR141716A (rimonabant; Acomplia®) (Sanofi-Aventis) (Fig. 3) stands alone as the first (and thus far only) selective CB1 receptor antagonist to have reached medical practice [33,44] (vide infra). Given such market validation of therapeutic endocannabinoid-system modulation through attenuation of CB1 receptor transmission, great current interest rests with synthetic, selective CB1 receptor antagonists as promising drugs for disorders whose underlying etiology has an appetitive component [45,46]. Indeed, CB1 receptor antagonists such as AM251 and AM281 (Fig. 4) have been synthesized that retain rimonabant's biarylpyrazole motif but show markedly greater selectivity for the CB1 vs. CB2 receptor [47,48]. The cutting-edge therapeutic approach of selective CB1-receptor antagonism will now be considered as a potential route to medicines for obesity and the interrelated cardiometabolic diseases it helps incite.
Fig. 3.
Chemical structures of synthetic cannabinoid receptor ligands that serve as ethical medications.
Fig. 4.
Chemical structures of cannabinoid receptor ligands widely used as experimental agents.
4. Obesity: A global pandemic and a predisposing factor for the metabolic syndrome
Body mass index (BMI) is the body weight of an individual divided by the square of his height. The resulting quotient correlates with the amount of body fat and has been used as a surrogate for body fat percentage [49]. A BMI in the range of 18.5–24.9 kg/m2 is considered normal. A BMI between 25 and 29.9 kg/m2 is defined as overweight, and BMIs of 30 kg/m2 and above, as obese. The BMI of 30 kg/m2 is considered a critical cut-off beyond which the cardiometabolic disease risk increases exponentially, and even this figure is likely too high for certain subpopulations [50]. Obesity itself is a metabolic disorder strongly related to environmental and lifestyle factors that results from a sustained imbalance between energy intake and energy expenditure [51]. As highlighted by the June 11, 2007, issue of a leading news periodical (Time) [52], neither behavioral adjustments in physical activity and food consumption nor public health interventions per se have been sufficient to check what has grown into a global obesity pandemic since 1980 [51,53]. This state of affairs invites—if not mandates—adjunctive pharmacological approaches for anti-obesity treatment. Indeed, the United States National Institutes of Health have announced research initiatives to accelerate the development of interventions for the prevention or treatment of overweight or obesity in children and adults [54]. Several compelling reasons support the discovery and development of novel weight-management therapeutics [51,55]. Bariatric surgery consistently results in appreciable and persistent weight loss, yet surgical intervention of any type can hardly be considered a feasible, population-based attack against the obesity pandemic [56]. The history of obesity pharmacotherapy is checkered with notoriety, be it from drugs with serious side-effect/addiction liability (e.g., amphetamine-like agents) or high-profile, agency-mandated market withdrawals due to serious adverse events (e.g., fenfluramine and dexfenfluramine) [51]. Currently available prescription drugs studied in large, randomized, placebo-controlled clinical trials and approved for long-term obesity treatment are only three in number: phentermine [Adipex-P® (Gate Pharmaceuticals) or Ionamin® (Medeva Pharmaceuticals)], an amphetamine and phenethylamine appetite suppressant that increases catecholamine levels and produces a feeling of satiety; orlistat (Xenical®) (Roche), a gastrointestinal lipase inhibitor that reduces fat uptake; and sibutramine (Meridia®, Reductil®) (Abbott Laboratories), a centrally-acting noradrenalin-serotonin-dopamine reuptake inhibitor that suppresses appetite and may also increase energy expenditure [55,57–59]. These agents are hampered by serious gastrointestinal (orlistat) or cardiovascular (sibutramine) side-effects and the limited maximal reduction in body mass achievable, only 3–5% above that elicited by the dietary-restriction and physical-activity components of the overall treatment regimen. This modest efficacy, coupled to low rates of sustained compliance, often leads to weight regain, inviting psychological comorbidities, particularly depression [51,55,57,58].
Perhaps the most compelling reason for the import being accorded translational anti-obesity research is the relationship between obesity and cardiometabolic risk. Specifically, abdominal obesity predisposes individuals to the metabolic syndrome. As defined by Adult Treatment Panel III guidelines from the United States National Cholesterol Educational Program, the metabolic syndrome is a cluster of serious cardiovascular and metabolic derangements that includes type-2 diabetes, hyper-tension, atherogenic dyslipidemia, thrombosis, cancer, inflammatory syndromes such as arthritis, and immune disorders [60–62]. Despite the availability of drugs to treat various aspects of the metabolic syndrome, the position of abdominal obesity at the syndrome's very foundation means that only truly effective weight-management agents would have the pharmacotherapeutic potential to benefit the entire cardiometabolic risk profile [51,61,62]. Given estimates that one in four adults is affected by the cardiometabolic syndrome and obesity [61,62] and nearly 20% of children and adolescents in the United States are overweight [63], the potential impact of effective anti-obesity therapy on the leading public health issue in the industrialized world, cardiovascular disease risk, is obvious and decisive.
5. The endocannabinoid system and feeding behavior
The instinct for food consumption is one of the most elemental from the standpoint of survival, yet one of the most complex biologically and behaviorally, being influenced by myriad cultural, genetic, emotional, and social factors [64,65]. It is thus unremarkable that several interactive and somewhat redundant endogenous mediators and information-transducing networks exist in mammals to regulate food-related behaviors [66,67]. Physiologically, the endocannabinoid signaling system is one such network and controls feeding at two levels. It can tonically accentuate or dampen the motivation for food search and intake, possibly by interacting with mesolimbic pathways involved in reward mechanisms. Additionally, endocannabinoid-system activation in the hypothalamus after a period of food deprivation induces appetite by modulating transiently the levels/actions of other orexigenic (neuropeptide Y, orexins, ghrelin) and anorexigenic (leptin, corticotrophin-releasing hormone, α-melanocyte-stimulating hormone) mediators [51,53,68]. Endocannabinoid regulation of feeding responses may influence or be influenced by cross-talk among these and other mediators affecting energy balance. As examples, leptin reduces endocannabinoid levels in the hypothalamus, whereas a positive, direct correlation exists between endocannabinoid tone and circulating ghrelin levels following a period of food deprivation [68]. Similar to other hedonistic stimuli including some drugs of abuse, bi-directional interactions between cannabinoid and opioid circuits reinforce the rewarding properties of and cravings for food [69]. Thus, endocannabinoid signaling is involved orexigenically in both the homeostatic and the hedonistic control of food intake.
As first recorded in antiquity and now known in the vernacular as “the munchies,” appetite stimulation (particularly for sweet and palatable foods) with eventual weight-gain is one of the most notable effects of Cannabis in humans [1]. Cannabis-induced hyperphagia occurs at low doses not eliciting sedation and motor impairment that may suppress feeding behavior and has been observed consistently in laboratory animals and man [68,69]. The appetite-stimulatory property of Cannabis has been clinically applied as dronabinol (Marinol®), an oral formulation of the CB1/CB2 receptor antagonist Δ9-THC (Fig. 1) approved by the United States Food and Drug Administration in 1992 for the treatment of acquired immune deficiency syndrome-related anorexia. Dronabinol has also been proposed as an orexigenic drug to combat the anorexia and cachexia in Alzheimer's and cancer patients [1,40].
6. The CB1 receptor: A target for anti-obesity pharmacotherapy
Experimental and clinical data have validated the CB1 receptor as a target for treating appetitive disorders and, specifically, obesity [23,70,71]. Numerous laboratory studies using potent natural or synthetic endocannabinoid agonists selective for the CB1 receptor or transgenic animals in which endocannabinoid signal transduction through the CB1 receptor has been disrupted afford consensus data on the critical role of CB1 receptor transmission in the positive physiological modulation of food intake, appetite, and, consequently, energy metabolism [45,68–71]. Obesity appears to be associated with endocannabinoid-system overactivation mediated primarily through CB1 receptor transmission [68,70,71]. Endocannabinoid-system hyperactivity contributing to obesity may be supported by: a fat-rich diet, which increases polyunsaturated fatty acid substrate for endocannabinoid synthesis and upregulates the CB1 receptor in the specialized cells primarily responsible for fat storage (adipocytes) and lipogenesis (hepatocytes); insulin resistance; and genetic malfunction of the endocannabinoid-inactivating enzyme FAAH [69–71]. Preclinical trials, particularly in genetically obese animal models, show consistently that CB1 receptor antagonism exerts an anorectic effect against hyperphagia, reducing food intake by acting on both the appetitive and consummatory aspects of ingestive behavior, regardless of food type. Although not maintained after treatment suspension, the anti-obesity effect of selective CB1 receptor antagonists is recoverable by reinstituting treatment, suggesting a pharmacologically viable strategy of chronic therapy [12,23,44,70]. The precise mechanism by which CB1 receptor antagonists exert their anti-obesity effect is not fully understood. The mechanism is likely multifactorial, given the inherent multiplicities and redundancies in appetite-controlling neurochemical and signaling circuits and the involvement of peripheral targets (e.g., adipocytes, hepatocytes) in both CB1 receptor-mediated endocannabinoid signaling and bioenergetics [65–71]. Coordinate physiological regulation of energy intake and utilization is reflected in the peripheral activity of factors involved in the central regulation of appetite. For example, feeding modulation by the endocannabinoid system may involve CB1 receptors on peripheral afferent terminals in the gastrointestinal tract [68].
Development of effective weight-control drugs requires particular attention to safety and efficacy, since such agents will be taken by both symptom-free individuals and obese patients with serious comorbidities over the duration of their lives [51]. Likewise, an important translational consideration for CB1 receptor ligands as effective anti-obesity drugs is their accurate in vivo pharmacological profiling. Available radiolabeled CB1 receptor ligands have been used as tools to help improve the efficiency and economy of the anti-obesity drug-development effort aimed at safe, effective CB1 receptor antagonists. Attempts to correlate CB1 receptor antagonism in vitro with predicted/realized pharmacological activity in vivo and the general pharmacology concept of receptor affinity as a determinant of drug dose have made it useful to incorporate radiolabeled CB1 receptor ligands into both preclinical and human studies. Molecular and functional nuclear imaging techniques such as positron emission tomography (PET) and single photon emission computed tomography (SPECT) afford quantitative insight into the relationships among CB1 receptor occupancy, drug dose, circulating plasma levels, and brain uptake of CB1 receptor ligands. Labeled ligands for SPECT/PET must meet several criteria: good oral bioavailability, ready permeation across the blood-brain barrier, high CB1 receptor selectivity and binding affinity, and rapid washout/elimination [72]. Among the first candidate SPECT/PET radioligands targeted to cannabinoid receptors, [123I]AM251, [123/124/131I] AM281, and [125/131I]AM2233 (Fig. 4) have enabled quantification of CB1 receptor distribution and occupancy, biokinetic study of drug and drug candidate distribution, and mapping of pathological tissue changes at the basis of human behavioral and brain disorders [48,73–75]. [18F]MK-9470 (Fig. 4) is a selective, high-affinity, CB1 receptor inverse agonist based on MK-0364 (taranabant) (Merck) (vide infra) with lower lipophilicity and avid, specific CB1 receptor binding that is readily reversible by taranabant. This latter property accords well with the dose-related reduction in [18F]MK-9470 following taranabant administration in man [76]. [11C]MePPEP, a selective CB1 receptor radiotracer with a pyrrolidinone core structure (Fig. 4), is considered suitable for in vivo CB1 receptor PET imaging in human brain and will be employed in a clinical trial to visualize cannabinoid receptors [77]. Pharmacia-Upjohn (Pfizer) has previously described compounds similar to [11C]MePPEP as CB1 receptor ligands [78]. Pfizer has also named CP-945,598 (structure undisclosed) as a drug candidate entering phase-III clinical weight-loss trials [79].
The availability of quantitative tools such as radiolabeled cannabinoid-receptor ligands that aid translational research and the central role of CB1 receptor transmission in the orexigenic control of feeding have promoted study of CB1 receptor antagonists as adjunctives to lifestyle modifications such as diet and exercise in overall weight-control strategies against obesity. The promise of translational success in the context of a growing global anti-obesity market has prompted increased intellectual and financial investment by both academia and the biopharmaceutical industry aimed at commercializing proprietary, selective CB1 receptor antagonists as safe, effective anti-obesity drugs. The investment has already produced novel CB1 receptor antagonists with sufficient preclinical efficacy as modulators of feeding behavior to hold anti-obesity therapeutic potential. Other selective CB1 receptor antagonists are the subjects of advanced clinical study, many as part of controlled, multi-center obesity trials. The current positioning of such agents in drug discovery and clinical development will next be discussed, starting with a detailed assessment of completed and ongoing human trials involving rimonabant, to-date the only targeted endocannabinoid-system modulator in the marketplace as a CB1 receptor selective drug. Newer chemical entities will then be profiled that have sufficient depth and breath of reported evidence to support their experimental, and in some cases clinical, effectiveness as selective CB1 receptor antagonists and weight-control agents.
7. Rimonabant
Based upon the concept that various appetitive disorders and self-reinforcing behaviors including food intake and nicotine addiction share a pathogenic component of endocannabinoid-system (hyper)activity mediated by CB1 receptor transmission [40,45,80], rimonabant (Fig. 3) was initially intended as a dual-purpose drug to aid weight management and smoking cessation [44,81]. The former indication effectively superseded the latter such that most pharmacological data on rimonabant relate to food intake, food-reinforced behaviors, weight-loss, and cardiometabolic parameters [44,82]. As summarized in several recent authoritative reviews [44,45,51,55,80–82], rimonabant is a potent CB1 receptor antagonist with a greater than 100-fold selectivity for the CB1 vs. CB2 receptor. Rimonabant can also act as a CB1 receptor inverse agonist such that it affects endocannabinoid signal transduction in the absence of CB1 receptor stimulation. The ability of rimonabant to suppress food intake and lower body mass has been demonstrated preclinically in various rodent models. Rimonabant's efficacy as a weight-loss agent appears to involve central as well as peripheral mechanisms mediated by the CB1 receptor. Major central contributors include the inhibition of CB1 receptor transmission in the mesolimbic and central melanocortin systems and the potentiation of cholecystokinin and vagal satiety signals in the brainstem. Candidate peripheral mechanisms supporting rimonabant's anti-obesity effect include inhibition of hepatic and adipose-tissue lipogenesis, enhancement of skeletal-muscle fatty acid oxidation, and augmentation of circulating adiponectin, an autocrine/paracrine adipokine that modulates adipocyte formation with pleiotropic effects on inflammation and atherosclerosis protective against obesity-related metabolic disease [82–84].
Whereas a 5-mg daily dose exhibited little clinical efficacy, multiple large, randomized, placebo-controlled international trials in which overweight or obese patients with or without associated comorbidities (e.g., diabetes, dyslipidemia, hypertension) were enrolled provide clear and compelling evidence that rimonabant facilitates weight loss at a therapeutic dose of 20 mg/day [44,82,85–88]. The so-called RIO (“Rimonabant In Obesity”) Program trials demonstrate that rimonabant induces over a two-year period a placebo-subtracted weight loss of greater than 5% in 30–40% of patients and greater than 10% in 10–20% of patients above that from a modest-calorie diet and physical exercise. Significantly decreased hunger, caloric intake, body weight, and waist circumference were observed in the treatment group. The phase-III “RIO-lipids” [85] and “RIO-Europe” [86] trials showed that rimonabant reduces the placebo-subtracted incidence of metabolic syndrome and ameliorates many associated cardiometabolic symptoms. Rimonabant increased HDL (i.e., “good”) cholesterol by 8–10%, reduced triglycerides by 10–30%, reduced C-reactive protein (an inflammation and predictive cardiovascular-risk marker), and improved insulin resistance and glycemic control. Circulating LDL cholesterol was not significantly affected, and a slight, if any, hypotensive effect was noted. In the RIO-North America trial [87], rimonabant-treated subjects randomized to placebo in year two regained weight, whereas those who continued to receive the drug maintained their weight loss. Rimonabant's two-year clinical effectiveness in obesity appears to be based on longer-term metabolic effects (e.g., suppression of hepatic lipogenesis, enhanced lipid oxidation), whereas its attenuation of food consumption is transient. This partial independence between improved metabolic profile and a behavior sharing similarities with the craving aspects of drugs of abuse has caused some speculation as to rimonabant's anti-obesity effectiveness over very long-term treatment [44,82,89]. Rimonabant also prevented the secondary weight-gain often associated with smoking cessation and improved the chances of quitting smoking [44,90]. Thus, rimonabant has the potential to reduce the risks of cardiovascular disease and type-2 diabetes associated with the cardiometabolic phenotype and help alleviate the socioeconomic and healthcare burdens from the single largest cause of avoidable death and disease in developed countries, smoking [45].
A few predominant adverse effects were reported in the one-year RIO-Europe trial [86], the most frequent being nausea (12.9 vs. 3.4%, treatment vs. placebo), dizziness (8.7 vs. 4.9%), and diarrhea (7.2 vs. 3.0%). Hypoglycemia was reported in 5.3% (treatment) vs. 1.7% (placebo) of diabetic subjects [88]. Rimonabant was well tolerated, most side-effects considered mild-to-moderate and often limited to the first few months of use [44,82]. At two years, rimonabant's side-effects were similar, with an excess of psychiatric disturbance (overall psychiatric disturbances: 26 vs. 14%, treatment vs. placebo; depression alone: 8.8 vs. 5.9%) and nausea (3.8 vs. 1.0%) [82,87].
These and other human data have supported rimonabant's regulatory approval in 38 countries and marketing in 18 as the weight-loss drug, Acomplia® [51,82]. Based upon a review of 59 completed clinical studies enrolling more than 15,000 patients, an advisory committee to the United States Food and Drug Administration voted unanimously not to recommend rimonabant (under the trade name Zimulti®) for use in overweight or obese patients with associated risk factors. The major concern was that rimonabant caused neurological and psychiatric problems, for it approximately doubled the risk of anxiety, depression, aggression, and psychosis and increased suicidal thinking in select patients. Sanofi-Aventis then withdrew the rimonabant New Drug Application in the United States on June 29, 2007 [91]. Consequently, reviews of recent rimonabant clinical data are now underway in the European Union, which originally restricted its original rimonabant approval to be used in combination with diet and exercise for the treatment of obese or overweight patients with associated risk factors, such as type-2 diabetes or dyslipidemia— not for indications such as smoking cessation [92]. Because patients with significant mental illness were excluded from the RIO trials [85–88], current estimates of riomonabant's potential psychiatric side effects may be conservative. Somewhat ironically, the United States Food and Drug Administration advisory committee recommendation against rimonabant approval as an anti-obesity drug was coincident with the launch in the United States of the gastric and pancreatic lipase inhibitor orlistat as an over-the-counter weight-control medication at reduced dose (brand name Allī™) (GlaxoSmithKline) [93].
Additional safety data on rimonabant are likely to emerge starting in 2–3 years upon completion of several human studies now underway that assess endpoints and surrogate measures of cardiovascular and atherothrombotic burden. The largest, the CRESCENDO trial, is examining whether rimonabant reduces stroke, myocardial infarction, and cardiovascular mortality due to either stroke or heart attack in 17,000 patients with abdominal obesity and other cardiovascular risk factors [94]. The STRADIVARIUS trial is designed to test whether improvements in metabolic syndrome are accompanied by reductions in atherogenesis after long-term CB1-receptor blockade with rimonabant [95]. Three more limited trials are currently recruiting patients. The VENUS trial has as its primary objective to assess rimonabant's efficacy on body-weight and triglyceride changes over a 52-week period [96]. The SYMPHONY trial is designed primarily to assess rimonabant's anti-obesity efficacy over 52 weeks in obese type-2 diabetic patients inadequately controlled with oral anti-diabetic drug monotherapy [97]. The SOLO trial will evaluate rimonabant's anti-obesity efficacy in obese type-2 diabetic patients inadequately controlled with diet and exercise alone [98].
8. Candidate CB1 receptor antagonists identified for potential development as anti-obesity drugs
Rimonabant's successful development encouraged medicinal chemists to search for selective CB1 receptor ligands as new chemical entities with similar or better pharmacological and safety profiles as compared to rimonabant. Bioisosteric replacements and substituent modifications of the rimonabant pyrazole pharmacophore as well as de novo and high-throughput screening approaches have indeed generated many novel CB1 receptor ligands. Some of these compounds have been identified as being actively pursued in the biopharmaceutical industry as leads for the pharmacotherapy of obesity and related metabolic disorders. In general, such lead, selective CB1 receptor antagonists for weight-management have demonstrated acute preclinical activity in reducing food intake and body weight and prophylactic and longer-term efficacy with chronic feeding in non-obese rodents and standard dietary and genetic obesity models. Measurement of meal counts, adipose-tissue mass and distribution, brain uptake, and metabolic parameters (e.g., plasma glucose and lipids, energy expenditure) have also been routine components of the lead evaluation process. To test for CNS-related effects, task learning, spatial memory, locomotor activity, and drug dependence usually have been evaluated as well.
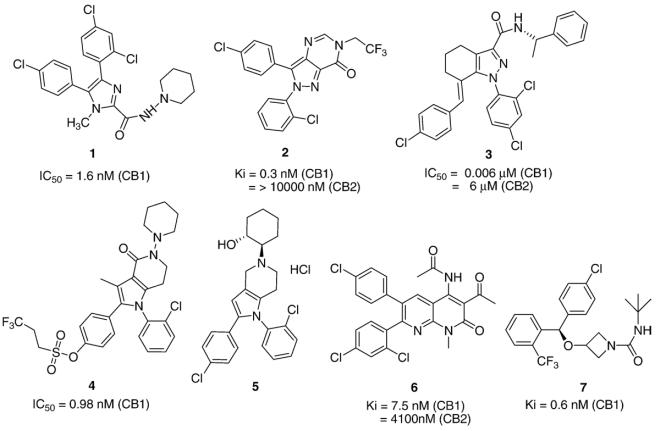
The rimonabant-related chemotype and selective CB1-receptor antagonist, compound 1 (Merck) (Fig. 5), is orally bioavailable and evidences rapid in vivo brain penetration. When administered ad libitum to rats with diet-induced obesity, compound 1 immediately caused a dose-dependent, cumulative, and prolonged reduction of food intake within the circadian clock very similar to rimonabant's effects [99]. To explore structure–activity relationships at the pyrazole scaffold, a variety of ring-constrained rimonabant analogs was synthesized. Compound 2 (Pfizer) (Fig. 5), wherein the pyrazole and pyrimidine rings are fused, exhibits very high selectivity and affinity for the CB1 receptor, antagonizes CB1 receptor mediated analgesia and hypothermia, and reduces food intake in a fasting-induced re-feeding rat model, but with suboptimal pharmacokinetics [100]. With their high CB1 receptor affinity and selectivity, 4–5 position bridged systems represented by the tetrahydroindazole, compound 3 (Janssen) (Fig. 5), have purported pharmacological properties in animal models suggestive of their stated potential as preclinical anti-obesity leads [101].
Fig. 5.
Chemical structures of candidate CB1 receptor antagonists identified in the literature for their development potential as anti-obesity drugs.
Rimonabant computational studies have suggested the importance of the C-3 amide substituent in eliminating agonist activity [102] and the carboxamide moiety for inducing inverse agonist activity at the CB1 receptor [103]. Based on the hypothesis that the carboxamide oxygen is involved in hydrogen-bonding with lysine K3.28(192) of the CB1 receptor [104], constrained analogs of rimonabant were designed and synthesized by joining the pyrazole ring at position 2 with the carboxamide nitrogen via an ethylene bridge. These pyrrolopyridinones exhibited CB1 receptor antagonist potency similar to rimonabant and elicited an anorexigenic effect in fasted-re-fed rats and a chronic reduction in weight-gain in genetically obese Zucker rats [105]. Sulphonic acid analogs of the pyrrolopyridinones such as compound 4 (Astra-Zeneca) (Fig. 5) have been described as selective CB1 receptor therapeutic agents for treating not only obesity, but psychiatric and neurological disorders as well [106]. Pyrrolopyridines lacking the carboxamide nitrogen, such as compound 5 (Bayer) (Fig. 5), have also been reported as agents used specifically to treat obesity and related disorders [107]. Compound 6 (Merck) (Fig. 5), a 1,8-naphthyridinone, has been reported to be a high-affinity, CB1 receptor inverse agonist that inhibited feeding in a dose-dependent manner and significantly decreased overnight body weight-gain and with good oral bioavailability and brain penetration in mouse, rat, dog and monkey [108]. Azetidine analogs, such as compound 7 (Vernalis) (Fig. 5), have very high affinity for the CB1 receptor and purportedly block the hypolocomotion induced by Δ9-THC in alcohol-preferring C57BL/6 mice and modulate feeding behaviors in experimental animals [109].
9. CB1 receptor antagonists in clinical development as anti-obesity pharmacotherapeutics
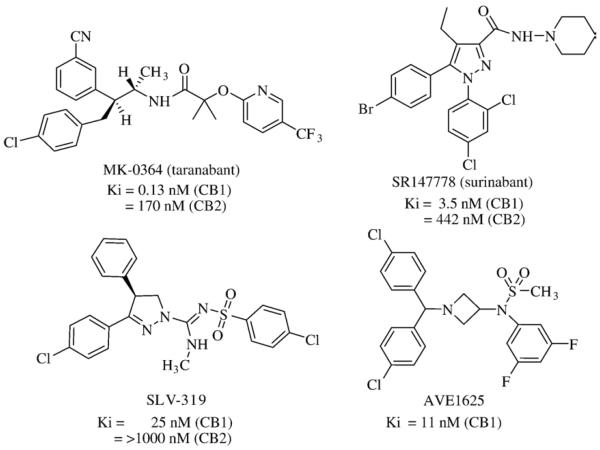
A few selective CB1 receptor antagonists appear to be progressing through clinical development following extensive laboratory and early human profiling that demonstrated their potential as anti-obesity drugs worthy of marketplace consideration. Pharmacological studies have characterized the acyclic diphenylamide, taranabant (Fig. 6), as a potent, orally-active weight-loss agent and high-affinity, selective inverse agonist to the human CB1 receptor [110,111]. A study in diet-induced obese rats demonstrated that partial (30%) occupancy of the CB1 receptor by taranabant was sufficient for significant chronic weight loss. The reported anti-obesity efficacy of taranabant was comparable to the weight-loss elicited by AM251 at equivalent CB1 receptor occupancy. A possible direct competitor to rimonabant, taranabant is currently in phase-III human obesity trials [112].
Fig. 6.
Chemical structures of CB1 receptor antagonists reported to be in clinical development as anti-obesity pharmacotherapeutics.
The rimonabant analog, SR147778 (surinabant) (Sanofi-Aventis) (Fig. 6), is a potent, selective CB1 receptor antagonist with enhanced oral activity [113]. Surinabant inhibits mitogen-activated protein kinase activity induced through CB1 receptor transmission. In vivo, surinabant dose-dependently decreases sucrose consumption and food intake in both fed and fasted rats. Surinabant suppresses alcohol preference and motivational properties in Sardinian alcohol-preferring rats [114] and is more effective than rimonabant in chronic rodent models of alcoholism [115]. Although surinabant is currently in phase-II clinical trials to treat nicotine addiction, its pharmacological properties as a potent, selective CB1 receptor antagonist with in vivo activity against appetitive disorders of alcohol and nicotine addiction suggest its potential development as a drug to aid weight-loss, pending further safety and efficacy data [116].
The 3,4-diarylpyrazoline CB1 receptor antagonist SLV-319 (Solvay) (Fig. 6) [117] reduces highly-palatable food consumption by Wistar rats at lower (11%) CB1-receptor occupancy relative to SR141716A (65%), whereas both agents dose-dependently increase extracellular dopamine at CB1 receptor occupancies >65% [118]. Compounds structurally and pharmacologically similar to SLV-319 have been reported [119]. SLV-319 itself is being evaluated in phase-IIB clinical trials as an anti-obesity and weight-loss agent [120].
Another novel, orally-active, highly-selective CB1 receptor antagonist, AVE1625 (Sanofi-Aventis) (Fig. 6), belongs to the benzhydrylazetidine class [121]. AVE1625 exhibits anti-aggression and antidepressant activity with few side-effects in animal models [122] and increases rodent acetylcholine levels coincident with improved memory-test performance [123]. AVE1625 reverses the impairment caused by scopolamine, a cholinergic antagonist that disrupts learning performance, in the standard mouse eyeblink conditioning model [124] and is antidyskinetic and prokinetic in rodent and primate models of Parkinson's disease [125]. As with some other known, potent CB1-receptor ligands, AVE1625 penetrates the blood-brain barrier and antagonizes Δ9-THC [126]. AVE1625 is currently being evaluated in a phase-II clinical trial for the treatment of cognitive impairment, as an adjunct to antipsychotic therapy in schizophrenia (“CONNECT trial”), and for its tolerance in mild-to-moderate Alzheimer's patients [127,128]. Without influence on locomotor activity, AVE1625 increases lipid oxidation and beneficially influences glucose control, circulating lipid profiles, hepatic glycogen stores, and energy expenditure independent of weight loss in male Wistar rats while markedly reducing food intake [129]. These effects imply peripheral sites of action for AVE1625 and suggest market potential as a drug to help redress the metabolic syndrome and lower obesity-induced cardiometabolic risk. AVE1625 is indeed being evaluated in a phase-II clinical study to assess its 24-week weight-loss effect in abdominally obese subjects with atherogenic dyslipidemia [130].
10. Conclusion: Perspectives on the pharmacotherapeutic modulation of endocannabinoid signaling for treating overweight, obesity, and their cardiometabolic and psychiatric comorbidities
The etiology of many appetitive disorders is characterized by a pathogenic component of reward-supported craving, be it for substances of abuse (including alcohol and nicotine) or food. Such maladies affect large numbers of people as prevalent socioeconomic and healthcare burdens. Yet in most instances drugs for their safe and effective pharmacotherapeutic management are lacking despite the attendant medical needs, collateral adverse physical and psychological effects, and enormous global market potential. The endocannabinoid signaling system plays a critical role in motivational homeostasis as a conduit for reward stimuli and a positive modulator of brain reward circuits. Endocannabinoid-system hyperactivity through CB1 receptor transmission is considered contributory to a range of appetitive disorders and, hence, is a major focus of contemporary pharmaceutical research [11,16,23,34,37,40,45,80]. At the drug-discovery stage, researchers continue to design and synthesize novel CB1 receptor antagonists and optimize their physical properties, intrinsic receptor affinity, oral bioavailability, pharmacokinetics, and safety. Subsequent demonstration of attenuated CB1 receptor transmission with positive pharmacological outcome in relevant preclinical trials has already endowed some highly-selective CB1 receptor antagonists with promise for lead-candidate status, if not actual clinical study in humans, as potential anti-obesity therapeutics (Figs. 5 and 6). Hence, the CB1 receptor has been accepted as an eminently “drugable” target for which new chemical entities are being profiled in the laboratory and clinic — and in one case (rimonabant), prescribed as an antiobesity therapeutic. Future-generation, selective CB1 receptor antagonists are expected to provide a valuable pharmacotherapeutic approach to obesity therapy and help redress this fundamental underlying factor of the metabolic syndrome, thereby lowering overall cardiovascular risk.
Endocannabinoid-related candidate drugs for obesity have therapeutic implications beyond weight management and the metabolic syndrome. Rimonabant itself is being studied clinically as potential treatment for alcohol addiction [131]. Modulators of CB1 receptor transmission may occupy therapeutic niches to help combat some serious and often disabling eating disorders. Both anorexia and bulimia nervosa are associated with CB1 receptor gene polymorphisms [132]. Subjects with anorexia nervosa or binge-eating disorders, but not bulimia nervosa, have increased circulating AEA levels [133]. Rimonabant increases insulin-responsive glucose transporter 4 expression [44,82,89], suggesting that CB1 receptor antagonists may be attractive diabetes drugs whose anti-lipogenic effect could also help prevent/reverse hepatic steatosis and nonalcoholic liver disease. The CB1 receptor antagonist AVE1625 (Fig. 6) is being studied in the clinic as treatment for schizophrenia, as are cannabidiol and Δ9-THC (Fig. 1) for schizophrenia and bipolar affective disorder [127,134,135]. The results of these latter studies have great relevance to obesity pharmacotherapy, since depression and schizophrenia are recognized comorbidities of substance abuse [136], and obesity is strongly correlated with a major depressive disorder [137].
New CB1 receptor ligands are on the horizon that may prove even more attractive for clinical development as anti-obesity agents, particularly if they exhibit increased antagonist potency with reduced potential for psychotropic side-effects. Recent detailed pharmacological profiling of the novel, CB1 receptor neutral antagonist, AM4113, demonstrates that it is possible to resolve in vivo the appetite-suppressant effects of CB1 receptor antagonism from the principal clinical side-effects (nausea and malaise) of CB1 receptor antagonists/inverse agonists such as rimonabant [138]. In contrast to rimonabant and other CB1 receptor antagonists/inverse agonists which stimulate signal transduction that opposes the activity of endogenous CB1 receptor agonists, neutral CB1 receptor antagonists may affect feeding by reducing endocannabinoid tone [139]. CB1 receptor neutral antagonists such as AM4113 that suppress feeding without producing nausea-or malaise-induced food aversion in humans could offer significant clinical and market advantages over inverse agonists as anti-obesity drugs [139].
Current focus on selective CB1 receptor ligands notwith-standing, two alternative modalities for anti-obesity pharmacotherapy related to the endocannabinoid system may be entertained: combination therapy and FAAH upregulation. The etiology of many chronic diseases is multifactorial such that they cannot be treated effectively by one drug, even when that drug has broad-spectrum activity against several targets/pathogenic processes [140]. In man and other mammalian species, the complex, interactive, and somewhat redundant nature of the information-transducing networks that regulate feeding behavior makes the existence of a “magic bullet” pharmacotherapy to solve the overweight and obesity problems unlikely [49,51,53]. The contemporary view of obesity as an underlying element in the metabolic syndrome predisposing to associated cardiometabolic risk – rather than as an isolated pathophysiological state – invites consideration of combination therapy in optimizing obesity and weight-loss management by CB1 receptor antagonists. Opioid antagonists decrease short-term food intake and the pleasurable nature of palatable foods and thus could be considered in weight-loss therapy [69]. Specifically, a CB1 receptor antagonist and an opioid antagonist might synergize for a superior therapeutic effect in obese humans, particularly those with “sweet” or “snacking” eating disorders [141]. Nalmefene, an opioid antagonist, has indeed been shown to reduce food intake in lean and diet-induced obese mice when administered with the CB1 receptor antagonist AM251 [142]. As yet, there are no reported clinical trials demonstrating significant weight loss by even high doses of opioid antagonists and no positive human studies with selective opioid-receptor antagonists in obese subjects or binge eaters [69]. Other polytherapy approaches for the treatment of obesity and associated cardiometabolic comorbidities have proposed a combination of a CB1 receptor antagonist with sibutramine, a potassium channel opener, a lipase inhibitor, a sterol absorption inhibitor (β-lactam), a dipeptidyl-peptidase IV inhibitor, or an intestinal-acting microsomal triglyceride transfer protein inhibitor [51,59,143–149]. One such microsomal triglyceride transfer protein inhibitor, dirlotapide (Slentrol®) (Pfizer) [150], has recently been accorded the status of the first agent granted United States Food and Drug Administration approval for the treatment of canine obesity [151]. Insights are emerging into the molecular and pharmacological interactions between the CB1 receptor and other receptors whose transmission may influence feeding behavior and energy balance (e.g., opioid [152] and orexin [153] receptors). Therapeutic targeting of this interaction may redefine the concept of “polypharmacological” weight-control therapy by using a single agent to modulate signal transmission from multiple receptors.
Genetic variation data suggest FAAH upregulation could be a weight-management therapeutic modality. A missense polymorphism in the FAAH gene resulting in reduced cellular FAAH expression and activity has been linked to obesity [154] and undercuts the salutary effect of a low-fat diet on dyslipidemia in obese subjects [155]. However, oleoylethanolamine, an anorectic endogenous lipid that stimulates lipolysis and fatty-acid oxidation, is also a FAAH substrate [156]. So any anti-obesity effect of pharmacological FAAH upregulation (i.e., enhanced anandamide hydrolysis) might be counteracted by potentiation of oleoylethanolamine degradation, as might the enhancement of long-term rimonabant metabolic benefit by oleoylethanolamine administration [157].
Future research will undoubtedly identify new endocannabinoid signaling elements, define their (patho)physiological roles, better integrate endocananbinoid function with related mediator networks at the systems-biology level, and uncover additional therapeutic targets within the endocannabinoid system for overweight, obesity, and the metabolic syndrome. Continued application of ligand-based molecular and biophysical approaches to characterize the binding motifs of cannabinoid receptors [158] and the fabrication of new metabolomic/lipidomic tools tailored to the endocannabinoid system [159] will undoubtedly contribute to future successes in translational cannabinoid research. The resulting data should facilitate the design and profiling of new chemical entities as therapeutic endocannabinoid modulators. They may also enable identification of endocannabinoid-system dynamics that could serve clinically as predictive and/or diagnostic biomarkers useful in patient selection, perhaps enabling an era of endocannabinoid-based personalized medicine.
Acknowledgements
The authors thank the National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH), and Northeastern University for support.
Abbreviations
- Δ9-THC
Δ9-tetrahydrocannabinol
- GPCR
G-protein-coupled receptor
- CB
cannabinoid
- CNS
central nervous system
- AEA
anandamide (N-arachidonoylethanolamine)
- 2-AG
2-arachidonoylglycerol
- AA
arachidonic acid
- NAPE
N-arachidonoyl-phosphatidylethanolamine
- NAT
N-acetyl transferase
- DAG
diacylglycerol
- FAAH
fatty acid amide hydrolase
- MAG
monoacylglycerol
- SR141716A
rimonabant (Acomplia®, Zimulti®)
- BMI
body mass index
- PET
positron emission tomography
- SPECT
single photon emission computed tomography
- PL-D
phospholipase D
- PL-C
phospholipase C
References
- [1].Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006;147:S163–71. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mechoulam R. Discovery of endocannabinoids and some random thoughts on their possible roles in neuroprotection and aggression. Prostaglandins Leukot Essent Fat Acids. 2002;66:93–9. doi: 10.1054/plef.2001.0340. [DOI] [PubMed] [Google Scholar]
- [3].Grotenhermen F. Pharmacology of cannabinoids. Neuro Endocrinol Lett. 2004;25:14–23. [PubMed] [Google Scholar]
- [4].Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1999;58:315–48. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- [5].Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- [6].Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes. 2006;30:S13–8. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- [7].Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann NY Acad Sci. 2006;1074:514–36. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- [8].Devane WA, Hanhus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–9. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- [9].Mechoulam R, Benshabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- [10].Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- [11].Di Marzo V, Petrosino S. Endocannabinoids and the regulation of their levels in health and disease. Curr Opin Lipidol. 2007;18:129–40. doi: 10.1097/MOL.0b013e32803dbdec. [DOI] [PubMed] [Google Scholar]
- [12].Matias I, Di Marzo V. Endocannabinoid synthesis and degradation, and their regulation in the framework of energy balance. J Endocrinol Invest. 2006;29:15–26. [PubMed] [Google Scholar]
- [13].Demuth DG, Molleman A. Cannabinoid signaling. Life Sci. 2006;78:549–63. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- [14].Childers SR. Activation of G-proteins in brain by endogenous and exogenous cannabinoids. AAPS J. 2006;8:E112–7. doi: 10.1208/aapsj080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Oz M. Receptor-independent actions of cannabinoids on cell membranes: focus on endocannabinoids. Pharmacol Ther. 2006;111:114–44. doi: 10.1016/j.pharmthera.2005.09.009. [DOI] [PubMed] [Google Scholar]
- [16].Fowler CJ. The cannabinoid system and its pharmacological manipulation—a review, with emphasis upon the uptake and hydrolysis of anandamide. Fundam Clin Pharmacol. 2006;20:549–62. doi: 10.1111/j.1472-8206.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- [17].Mackie K, Stella N. Cannabinoid receptors and endocannabinoids: evidence for new players. AAPS J. 2006;8:E298–306. doi: 10.1007/BF02854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Basavarajappa BS. Critical enzymes involved in endocannabinoid metabolism. Protein Pept Lett. 2007;14:237–46. doi: 10.2174/092986607780090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu J, Wang L, Harvey-White J, Huang BX, Kim HY, Luquet S, Palmiter RD, Krystal G, Rai R, Mahadevan A, Razdan RK, Kunos G. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology. doi: 10.1016/j.neuropharm.2007.05.020. in press. doi:10.1016/j.neuropharm.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sugiura T, Kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res. 2006;45:405–46. doi: 10.1016/j.plipres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- [21].Ligresti A, Cascio MG, Di Marzo V. Endocannabinoid metabolic pathways and enzymes. Curr Drug Targets CNS Neurol Disord. 2005;4:615–23. doi: 10.2174/156800705774933104. [DOI] [PubMed] [Google Scholar]
- [22].Ho WS, Randall MD. Endothelium-dependent metabolism by endocannabinoid hydrolases and cyclooxygenases limits vasorelaxation to anandamide and 2-arachidonoylglycerol. Br J Pharmacol. 2007;150:641–51. doi: 10.1038/sj.bjp.0707141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pacher P, Bátaki S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007;13:127–37. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- [25].Khanapure SP, Garvey DS, Janero DR, Letts LG. Eicosanoids in inflammation: biosynthesis, pharmacology, and therapeutic frontiers. Curr Topics Med Chem. 2007;7:311–40. doi: 10.2174/156802607779941314. [DOI] [PubMed] [Google Scholar]
- [26].Bari M, Battista N, Fezza F, Gasperi V, Maccarrone M. New insights into endocannabinoid degradation and its therapeutic potential. Mini Rev Med Chem. 2006;6:257–68. doi: 10.2174/138955706776073466. [DOI] [PubMed] [Google Scholar]
- [27].Bisogno T, Cascio MG, Saha B, Mahadevan A, Urbani P, Minassi A, et al. Development of the first potent and specific inhibitors of endocannabinoid biosynthesis. Biochim Biophys Acta. 2006;1761:205–12. doi: 10.1016/j.bbalip.2005.12.009. [DOI] [PubMed] [Google Scholar]
- [28].Karanian DA, Brown QB, Makriyannis A, Kosten TA, Bahr BA. Dual modulation of endocannabinoid transport and fatty acid amide hydrolase protects against excitotoxicity. J Neurosci. 2005;25:7813–20. doi: 10.1523/JNEUROSCI.2347-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kaczocha M, Hermann A, Glaser ST, Bojesen IN, Deutsch DG. Anandamide uptake is consistent with rate-limited diffusion and is regulated by the degree of its hydrolysis by fatty acid amide hydrolase. J Biol Chem. 2006;281:9066–75. doi: 10.1074/jbc.M509721200. [DOI] [PubMed] [Google Scholar]
- [30].Puffenbarger RA. Molecular biology of the enzymes that degrade endocannabinoids. Curr Drug Targets CNS Neurol Disord. 2005;4:625–31. doi: 10.2174/156800705774933050. [DOI] [PubMed] [Google Scholar]
- [31].Karanian DA, Karim SL, Wood JT, Williams JS, Lin S, Makriyannis A, Bahr BA. Endocannabinoid enhancement protects against kainic acid-induced seizures and associated brain damage. J Pharmacol Exp Ther. 2007;322:1059–66. doi: 10.1124/jpet.107.120147. [DOI] [PubMed] [Google Scholar]
- [32].Flanagan JM, Gerber Al, Cadet JL, Beutler E, Sipe JC. The fatty acid amide hydrolase 385 A/A (P129T) variant: haplotype analysis and validation of risk for drug addiction. Hum Genet. 2006;120:581–8. doi: 10.1007/s00439-006-0250-x. [DOI] [PubMed] [Google Scholar]
- [33].Makie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–22. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- [34].Pavlopoulos S, Thakur GA, Nikas SP, Makriyannis A. Cannabinoid receptors as therapeutic targets. Curr Pharm Des. 2006;12:1751–69. doi: 10.2174/138161206776873743. [DOI] [PubMed] [Google Scholar]
- [35].Wilkinson JD, Williamson EM. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J Dermatol Sci. 2007;45:87–92. doi: 10.1016/j.jdermsci.2006.10.009. [DOI] [PubMed] [Google Scholar]
- [36].Ibrahim MM, Rude ML, Stagg NJ, Mata HP, Lai J, Vanderah TW, et al. CB2 cannabinoid receptor mediation of antinociception. Pain. 2006;122:36–42. doi: 10.1016/j.pain.2005.12.018. [DOI] [PubMed] [Google Scholar]
- [37].Thakur GA, Duclos RI, Jr, Makriyannis A. Natural cannabinoids: templates for drug discovery. Life Sci. 2005;78:454–66. doi: 10.1016/j.lfs.2005.09.014. [DOI] [PubMed] [Google Scholar]
- [38].Xu W, Filppula SA, Mercier R, Yaddanapudi S, Pavlopoulos S, Cai J, et al. Purification and mass spectroscopic analysis of human CB1 cannabinoid receptor functionally expressed using the baculovirus system. J Pept Res. 2005;66:138–50. doi: 10.1111/j.1399-3011.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- [39].Zvonok N, Yaddanapudi S, Williams J, Dai S, Dong K, Rejtar T, et al. Comprehensive proteomic mass spectrophotometric characterization of human cannabinoid CB2 receptor. J Proteome Res. 2007;6:2068–79. doi: 10.1021/pr060671h. [DOI] [PubMed] [Google Scholar]
- [40].Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nature Rev Drug Dis. 2004;3:771–84. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- [41]. [August, 2007 Accessed];Sativex receives qualifying notice for approval in Canada for the relief of cancer pain. Available at http://www.gwpharma.com/news_press_releases.asp.
- [42]. [August, 2007 Accessed];GW EU regulatory withdrawal. Available at http://www.gwpharma.com/news_press_releases.asp.
- [43]. [August, 2007 Accessed];GW and Otsuka enter into global cannabinoid research collaboration. Available at http://www.gwpharma.com/news_press_releases.asp.
- [44].Henness S, Robinson DM, Lyseng-Williamson KA. Rimonabant. Drugs. 2006;66:2109–19. doi: 10.2165/00003495-200666160-00006. [DOI] [PubMed] [Google Scholar]
- [45].Tucci SA, Halford JC, Harrold JA, Kirkham TC. Therapeutic potential of targeting the endocannabinoids: implications for the treatment of obesity, metabolic syndrome, drug abuse and smoking cessation. Curr Med Chem. 2006;13:2669–80. doi: 10.2174/092986706778201512. [DOI] [PubMed] [Google Scholar]
- [46].Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonist as promising newmedications fordrugdependence. JPharmacol ExpTher. 2005;312:875–83. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- [47].Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, et al. Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem. 1999;42:769–76. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- [48].Lan R, Gatley J, Lu Q, Fan P, Fernando SR, Volkow ND, et al. Design and synthesis of the CB1 selective cannabinoid antagonist AM281: a potential human SPECT ligand. AAAP PharmSci. 1999;1:E4. doi: 10.1208/ps010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Baumer JH. Obesity and overweight: its prevention, identification, assessment and management. Arch Dis Child Educ Pract Ed. 2007;92:92–6. doi: 10.1136/adc.2007.118448. [DOI] [PubMed] [Google Scholar]
- [50].Mascie-Taylor CG, Goto R. Human variations and body mass index: a review of the universality of BMI cut-offs, gender and urban–rural differences, and secular changes. J Physiol Anthropol. 2007;26:109–12. doi: 10.2114/jpa2.26.109. [DOI] [PubMed] [Google Scholar]
- [51].Hofbauer KG, Nicholson JR, Boss O. The obesity epidemic: current and future pharmacological treatments. Annu Rev Pharmacol Toxicol. 2007;47:565–92. doi: 10.1146/annurev.pharmtox.47.120505.105256. [DOI] [PubMed] [Google Scholar]
- [52]. [August, 2007 Accessed];The Science of Appetite. Time, June 11, 2007. Available at http://www.time.com/magazine/article/0,9171,1627006,00.html.
- [53].Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr Rev. 2006;27:750–61. doi: 10.1210/er.2006-0032. [DOI] [PubMed] [Google Scholar]
- [54]. [Accessed August, 2007];PA-06-256: Exploratory/developmental clinical research grants in obesity (R21) Posted March 17, 2006. Available at http://grants.nih.gov/grants/guide/pa-files/PA-06-256.html.
- [55].Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine and rimonabant. Lancet. 2007;369:71–7. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- [56].Brethauer SA, Chand B, Schauer PR. Risks and benefits of bariatric surgery: current evidence. Cleve Clin J Med. 2006;73:993–1007. doi: 10.3949/ccjm.73.11.993. [DOI] [PubMed] [Google Scholar]
- [57].Chaput JP, St-Pierre S, Tremblay A. Currently available drugs for the treatment of obesity: sibutramine and orlistat. Mini Rev Med Chem. 2007;7:3–10. doi: 10.2174/138955707779317849. [DOI] [PubMed] [Google Scholar]
- [58].Bray GA, Ryan DH. Drug treatment of the overweight patient. Gastroenterology. 2007;132:2239–52. doi: 10.1053/j.gastro.2007.03.053. [DOI] [PubMed] [Google Scholar]
- [59].Cooke D, Bloom S. The obesity pipeline: current strategies in the development of anti-obesity drugs. Nat Rev Drug Disc. 2006;5:919–31. doi: 10.1038/nrd2136. [DOI] [PubMed] [Google Scholar]
- [60].Grundy SM, Cleeman JI, Merz NB, Brewer HB, Clark LT, Hunninghake DB, et al. The Coordinating Committee of the National Cholesterol Education Program Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- [61].Fernandez ML. The metabolic syndrome. Nutr Rev. 2007;65:S30–4. doi: 10.1111/j.1753-4887.2007.tb00325.x. [DOI] [PubMed] [Google Scholar]
- [62].Katagiri H, Yamada T, Oka Y. Adiposity and cardiovascular disorders: disturbance of the regulatory system consisting of humoral and neuronal signals. Circ Res. 2007;101:27–39. doi: 10.1161/CIRCRESAHA.107.151621. [DOI] [PubMed] [Google Scholar]
- [63].Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- [64].Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357:370–9. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- [65].Jones LR, Wilson CI, Wadden TA. Lifestyle modification in the treatment of obesity: an educational challenge and opportunity. Clin Pharmacol Ther. 2007;81:776–9. doi: 10.1038/sj.clpt.6100155. [DOI] [PubMed] [Google Scholar]
- [66].Janero DR. Nutritional spects of nitric oxide: human health implications and therapeutic opportunities. Nutrition. 2001;17:896–903. doi: 10.1016/s0899-9007(01)00647-5. [DOI] [PubMed] [Google Scholar]
- [67].Coll AP, Farooqui IS, O'Rahilly S. The hormonal control of food intake. Cell. 2007;129:251–62. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27:73–100. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- [69].Cota D, Tschöp MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Res Rev. 2006;51:85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- [70].Bellocchio L, Mancini G, Vicennati V, Pasquali R, Pagotto U. Cannabinoid receptors as therapeutic targets for obesity and metabolic diseases. Curr Opin Pharmacol. 2006;6:586–91. doi: 10.1016/j.coph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- [71].Duffy D, Rader D. Endocannabinoid antagonism: blocking the excess in the treatment of high-risk abdominal obesity. Trends Cardiovasc Med. 2007;17:35–43. doi: 10.1016/j.tcm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- [72].Ametamey SM, Honer M. Pharmacological prerequisites for PET ligands and practical issues in preclinical PET research. Ernst Schering Res Found Workshop. 2007;62:317–27. doi: 10.1007/978-3-540-49527-7_12. [DOI] [PubMed] [Google Scholar]
- [73].Gatley SJ, Gifford AN, Volkow ND, Lan R, Makriyannis A. 123I-labeled AM251: a radioiodinated ligand which binds in vivo to mouse brain cannabinoid CB1 receptors. Eur J Pharmacol. 1996;307:331–8. doi: 10.1016/0014-2999(96)00279-8. [DOI] [PubMed] [Google Scholar]
- [74].Berding G, Schneider U, Gielow P, Buchert R, Donnerstag F, Brandau W, et al. Feasibility of central cannabinoid CB1 receptor imaging with [124I] AM281 PET demonstrated in a schizophrenic patient. Psychiatry Res. 2006;147:249–56. doi: 10.1016/j.pscychresns.2006.02.002. [DOI] [PubMed] [Google Scholar]
- [75].Dhawan J, Deng H, Gatley SJ, Makriyannis A, Akinfeleye T, Bruneus M, et al. Evaluation of the in vivo receptor occupancy for the behavioral effects of cannabinoids using a radiolabeled cannabinoid receptor agonist, R-[125/131I]AM2233. Synapse. 2006;60:93–101. doi: 10.1002/syn.20277. [DOI] [PubMed] [Google Scholar]
- [76].Burns HD, Van Laere K, Sanabria-Bohorquez S, Hamill TG, Bormans G, Eng WS, et al. [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc Natl Acad Sci USA. 2007;104:9800–5. doi: 10.1073/pnas.0703472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77]. [August, 2007 Accessed];Imaging of cannabinoid receptors using new radioactive tracer. Available at http://clinicaltrials.gov/show/NCT00450021.
- [78].Carpino PA, Sanner MA. Cannabinoid receptor ligands and uses thereof. 2007/020502 WO patent 2007.
- [79]. [August, 2007 Accessed];A 2-year, randomized, double-blind, placebo controlled, phase 3 study to evaluate the long-term efficacy and safety of CP-945,598 in the treatment of obese subjects. Available at http://clinicaltrials.gov/show/NCT00375401.
- [80].Janero DR, Makriyannis A. Targeted modulators of the endogenous cannabinoid system: future medications to treat addiction disorders and obesity. Curr Psychiatry Rep. 2007;9:365–73. doi: 10.1007/s11920-007-0047-1. [DOI] [PubMed] [Google Scholar]
- [81].Boyd AT, Fremming BA. Rimonabant—a selective CB1 antagonist. Ann Pharmacother. 2005;39:684–90. doi: 10.1345/aph.1E499. [DOI] [PubMed] [Google Scholar]
- [82].Patel PN, Pathak R. Rimonabant: a novel selective cannabinoid-1 receptor antagonist for treatment of obesity. Am J Health Syst Pharm. 2007;64:481–9. doi: 10.2146/060258. [DOI] [PubMed] [Google Scholar]
- [83].Kakafika AI, Mikhailidis DP, Karagiannis A, Athyros VG. The role of endocannabinoid system blockade in the treatment of the metabolic syndrome. J Clin Pharmacol. 2007;47:642–52. doi: 10.1177/0091270007299358. [DOI] [PubMed] [Google Scholar]
- [84].Szmitko PE, Teoh H, Stewart D, Verma S. Adiponectin and cardiovascular disease: state of the art? Am J Physiol. 2007;292:H1655–63. doi: 10.1152/ajpheart.01072.2006. [DOI] [PubMed] [Google Scholar]
- [85].Despres JP, Golay A, Sjostrom L, The Rimonabant in Obesity-Lipids Study Group Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–34. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- [86].Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S, The RIO-Europe Study Group Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–97. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- [87].Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J, The RIO-North America Study Group Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients. JAMA. 2006;295:761–75. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- [88].Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF, RIO-Diabetes Study Group Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes. Lancet. 2006;368:1160–72. doi: 10.1016/S0140-6736(06)69571-8. [DOI] [PubMed] [Google Scholar]
- [89].Wierzbicki AS. Rimonabant: endocannabinoid inhibition for the metabolic syndrome. Int J Clin Pract. 2006;60:1697–706. doi: 10.1111/j.1742-1241.2006.01210.x. [DOI] [PubMed] [Google Scholar]
- [90].Reid RD, Quinlan B, Riley DL, Pipe AL. Smoking cessation: lessons learned from clinical trial evidence. Curr Opin Cardiol. 2007;22:280–5. doi: 10.1097/HCO.0b013e328236740a. [DOI] [PubMed] [Google Scholar]
- [91]. [August, 2007 Accessed];Rimonabant regulatory update in the United States. Available at http://en.sanofi-aventis.com/Images/070629_rimonabant-US_en_tcm24-17003.pdf.
- [92].SR141716 Zimulti (rimonabant) NDA 21-888. [August, 2007 Accessed];Briefing information for FDA advisory committee meeting. Available at http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4306b1-01-sponsor-backgrounder.htm.
- [93]. [August, 2007 Accessed];Allī — the OTC weight loss product — on shelves nationwide later this week. Available at http://us.gsk.com/ControllerServlet?appId=4&pageId=402&newsid=1103.
- [94].CRESCENDO [August, 2007 Accessed];Comprehensive rimonabant evaluation study of cardiovascular endpoints and outcomes. 2005 Available at http://www.clinicaltrials.gov/ct/show/NCT00263042.
- [95].STRADIVARIUS [August, 2007 Accessed];Strategy to reduce atherosclerosis development involving administration of rimonabant—the intravascular ultrasound study. 2005 Available at http://www.clinicaltrials.gov/ct/show/NCT00124332.
- [96].VENUS [August, 2007 Accessed];Japanese study of rimonabant in obese patients with dyslipidemia. 2007 Available at http://www.clinicaltrials.gov/ct/show/NCT00434096.
- [97].SYMPHONY [August, 2007 Accessed];Japanese study with rimonabant on obese type 2 diabetic patients with oral anti-diabetic drug. 2007 Available at http://www.clinicaltrials.gov/ct/show/NCT00478595.
- [98].SOLO [August, 2007 Accessed];Japanese study with rimonabant in obese type 2 diabetic patients on diet and exercise. Available at http://www.clinicaltrials.gov/ct/show/NCT00478972.
- [99].Plummer CW, Finke PE, Mills SG, Wang J, Tong X, Doss GA, et al. Synthesis and activity of 4,5-diarylimidazoles as human CB1 receptor inverse agonists. Bioorg Med Chem Lett. 2005;15:1441–6. doi: 10.1016/j.bmcl.2004.12.078. [DOI] [PubMed] [Google Scholar]
- [100].Carpino PA, Griffith DA, Sakya S, Dow RL, Black SC, Hadcock JR, et al. New bicyclic cannabinoid receptor-1 (CB1-R) antagonists. Bioorg Med Chem Lett. 2006;16:731–6. doi: 10.1016/j.bmcl.2005.10.019. [DOI] [PubMed] [Google Scholar]
- [101].Lagu B, Liotta F, Pan M, Wachter MP, Xia M. Tetrahydroindazole cannabinoid modulators. 2005/095353 WO Patent 2005.
- [102].Shim JY, Welsh WJ, Cartier E, Edwards JL, Howlett AC. Molecular interaction of the antagonist N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide with the CB1 cannabinoid receptor. J Med Chem. 2002;45:1447–59. doi: 10.1021/jm010267o. [DOI] [PubMed] [Google Scholar]
- [103].Hurst DP, Lynch DL, Barnett-Norris J, Hyatt SM, Seltzman HH, Zhong M, et al. N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-p yrazole-3-carboxamide (SR141716A) interaction with LYS 3.28(192) is crucial for its inverse agonism at the cannabinoid CB1 receptor. Mol Pharmacol. 2002;62:1274–87. doi: 10.1124/mol.62.6.1274. [DOI] [PubMed] [Google Scholar]
- [104].Hurst D, Umejiego U, Lynch D, Seltzman H, Hyatt S, Roche M, et al. Biarylpyrazole inverse agonists at the cannabinoid CB1 receptor: importance of the C-3 carboxamide oxygen/lysine3.28(192) interaction. J Med Chem. 2006;49:5969–87. doi: 10.1021/jm060446b. [DOI] [PubMed] [Google Scholar]
- [105].Smith RA, Fathi Z, Brown SE, Choi S, Fan J, Jenkins S, et al. Constrained analogs of CB-1 antagonists: 1,5,6,7-Tetrahydro-4H-pyrrolo[3,2-c]pyridine-4-one derivatives. Bioorg Med Chem Lett. 2007;17:673–8. doi: 10.1016/j.bmcl.2006.10.095. [DOI] [PubMed] [Google Scholar]
- [106].Cheng L. Preparation and use of tetrahydropyrrolo[3,2-C]pyridine-4-one derivatives for treatment of obesity, psychiatric and neurological disorders. 2007/039740 WO Patent 2007.
- [107].Smith RA, Wong WC, O'Connor SJ, Choi S, Kluender HCE, Zhang Z, Lavoie RC, Fan J, Podlogar BL. Preparation and use of 1,5,6,7-tetrahydropyrrolo[3,2-c]pyridine derivatives for treatment of obesity. 2003/027114. WO Patent 2003. 2004
- [108].Debenham JS, Madsen-Duggan CB, Walsh TF, Wang J, Tong X, Doss GA, et al. Synthesis of functionalized 1,8-napthyridiones and their evaluation as novel, orally active CB1 receptor inverse agonists. Bioorg Med Chem Lett. 2006;16:681–5. doi: 10.1016/j.bmcl.2005.10.028. [DOI] [PubMed] [Google Scholar]
- [109].Davidson JEP, Dawson CE, Harrison K, Mansell HL, Pratt RM, Sohal S, Ruston VJ. Azetidinecarboxamide derivatives and their use in the treatment of CB1 receptor-mediated disorders. 2004/096763. WO Patent. 2004
- [110].Lin LS, Lanza TJ, Jr, Jewell JP, Liu P, Shah SK, Qi H, et al. Discovery of N-[(1S,2S)-3-(4-Chlorophenyl)-2-(3-cyanophenyl)-1-methylpropyl]-2-methyl-2-{[5-(trifluoromethyl)pyridin-2-yl]oxy}propanamide (MK-0364), a novel, acyclic cannabinoid-1 receptor inverse agonist for the treatment of obesity. J Med Chem. 2006;49:7584–7. doi: 10.1021/jm060996+. [DOI] [PubMed] [Google Scholar]
- [111].Fong TM, Guan XM, Marsh DJ, Shen CP, Stribling DS, Rosko KM, et al. Antiobesity efficacy of a novel cannabinoid-1 receptor inverse agonist, N-[(1S,2S)-3-(4-chlorophenyl)-2-(3-cyanophenyl)-1-methylpropyl]-2-methyl-2-[[5-(trifluoromethyl)pyridin-2-yl]oxy]propanamide (MK-0364), in rodents. J Pharmacol Exp Ther. 2007;321:1013–22. doi: 10.1124/jpet.106.118737. [DOI] [PubMed] [Google Scholar]
- [112]. [August, 2007 Accessed];Taranabant approved as generic name for MK-0364. Available at http://www.merck.com/newsroom/press_releases/research_and_development/2007_0226.html.
- [113].Rinaldi-Carmona M, Barth F, Congy C, Martinez S, Oustric D, Perio A, et al. SR147778 [5-(4-bromophenyl)-1-(2,4-dichlorophenyl)-4-ethyl-N-(1-piper-idinyl)-1H-pyr azole-3-carboxamide], a new potent and selective antagonist of the CB1 cannabinoid receptor: biochemical and pharmacological characterization. J Pharmacol Exp Ther. 2004;310:905–14. doi: 10.1124/jpet.104.067884. [DOI] [PubMed] [Google Scholar]
- [114].Gessa GL, Serra S, Vacca G, Carai MA, Colombo G. Suppressing effect of the cannabinoid CB1 receptor antagonist, SR147778, on alcohol intake and motivational properties of alcohol in alcohol-preferring sP rats. Alcohol Alcohol. 2005;40:46–53. doi: 10.1093/alcalc/agh114. [DOI] [PubMed] [Google Scholar]
- [115].Lallemand F, De Witte P. SR147778, a CB1 cannabinoid receptor antagonist, suppresses ethanol preference in chronically alcoholized Wistar rats. Alcohol. 2006;39:125–34. doi: 10.1016/j.alcohol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- [116].SURSMOKE [August, 2007 Accessed];Efficacy and safety of surinabant treatment as an aid to smoking cessation. Available at http://www.clinicaltrials.gov/ct/show/NCT00432575.
- [117].Lange JH, Coolen HK, van Stuivenberg HH, Dijksman JA, Herremans AH, Ronken E, et al. Synthesis, biological properties, and molecular modeling investigations of novel 3,4-diarylpyrazolines as potent and selective CB(1) cannabinoid receptor antagonists. J Med Chem. 2004;47:627–43. doi: 10.1021/jm031019q. [DOI] [PubMed] [Google Scholar]
- [118].Need AB, Davis RJ, Alexander-Chacko JT, Eastwood B, Chernet E, Phebus LA, et al. The relationship of in vivo central CB1 receptor occupancy to changes in cortical monoamine release and feeding elicited by CB1 receptor antagonists in rats. Psychopharmacology (Berl) 2006;184:26–35. doi: 10.1007/s00213-005-0234-x. [DOI] [PubMed] [Google Scholar]
- [119].Lange JH, van Stuivenberg HH, Veerman W, Wals HC, Stork B, Coolen HK, et al. Novel 3,4-diarylpyrazolines as potent cannabinoid CB1 receptor antagonists with lower lipophilicity. Bioorg Med Chem Lett. 2005;15:4794–8. doi: 10.1016/j.bmcl.2005.07.054. [DOI] [PubMed] [Google Scholar]
- [120]. [August, 2006 Accessed];Solvay's obesity candidate treatment advances in phase II clinical trials. Available at http://www.solvaypharmaceuticals.com/static/wma/pdf/9/3/1/3/20061208SLV319OBESITYEN.pdf.
- [121].Diu-Hercend A, Suc-Royer I, Gallea S, Laville M, Rouyer T, Bohme GA, Benavides J. Binding profile and functional characterization of AVE1625, a novel cannabinoid CB1 receptor antagonist, Program Number 653.13. Society for Neuroscience; Washington, DC: 2005. Abstract. [Google Scholar]
- [122].Pratt J, Roux M, Stutzmann JM, Piot-Grosjean O, Benavides J. Program Number 653.16. Society for Neuroscience; Washington, DC: 2005. Effects of AVE1625, a novel cannabinoid CB1 antagonist, in models of aggression and depression. Abstract. [Google Scholar]
- [123].Piot-Grosjean O, Goniot P, Imbault F, Mazadier M, Miquet JM, Obinu MC, et al. Program Number 653.14. Society for Neuroscience; Washington, DC: 2005. In vivo effects of AVE1625, a novel cannabinoid CB1 receptor antagonist, on brain acetylcholine levels and in rodent models of learning and memory. Abstract. [Google Scholar]
- [124].Lopez-Ramos J, Bohne GA, Piot-Grosjean O, Benavides J, Delgado-Garcia J. Program Number 653.15. Society for Neuroscience; Washington, DC: 2005. Effects of AVE1625, a novel CB1 antagonist, on classical eyeblink conditioning in mice. Abstract. [Google Scholar]
- [125].Stutzmann J, Hill M, Pratt J, Bezard E, Piot-Grosjean O, Crossman A, et al. Program Number 175.5/HH8. Society for Neuroscience; Atlanta, Georgia: 2006. Antidyskinetic and prokinetic effects of AVE1625, a selective CB1 cannabinoid receptor antagonist, on rodent and primate models of Parkinson's disease. Abstract. [Google Scholar]
- [126].Zuurman L, Roy C, Schoemaker RC, Asset G, Amatsaleh A, Guimaraes L, et al. Inhibition of THC-induced effects on central nervous system effects and heart rate by a novel CB1 receptor antagonist AVE1625. Br J Clin Pharmacol. 2007;63(Supplement):769. Abstract. [Google Scholar]
- [127].CONNECT [August, 2007 Accessed];Efficacy and safety of AVE1625 as a co-treatment with antipsychotic therapy in schizophrenia. Available at http://www.clinicaltrials.gov/ct/show/NCT00439634.
- [128]. [August, 2007 Accessed];Activity of AVE1625 in mild to moderate Alzheimer's patients. Available at http://www.clinicaltrials.gov/ct/show/NCT00380302.
- [129].Herling AW, Gossel M, Haschke G, Stengelin S, Kuhlmann J, Müeller G, Schmoll D, Kramer W. The CB1 receptor antagonist AVE1625 affects primarily metabolic parameters independent of reduced food intake in Wistar rats. Am J Physiol Endocrinol Metab. 2007;293:E826–32. doi: 10.1152/ajpendo.00264.2007. [DOI] [PubMed] [Google Scholar]
- [130]. [August, 2007 Accessed];Dose-ranging study evaluating AVE1625 in abdominally obese patients with atherogenic dyslipidemia. Available at http://www.clinicaltrials.gov/ct/show/NCT00345410.
- [131]. [August, 2007 Accessed];Rimonabant to reduce alcohol consumption. Available at http://www.clinicaltrials.gov/ct/show/NCT0075205.
- [132].Siegfried Z, Kanyas K, Latzer Y, Karni O, Bloch M, Lerer B, et al. Association study of cannabinoid receptor gene (CNR1) alleles and anorexia nervosa: differences between restricting and binging/purging subtypes. Am J Med Genet B Neuropsychiatr Genet. 2004;125:126–30. doi: 10.1002/ajmg.b.20089. [DOI] [PubMed] [Google Scholar]
- [133].Monteleone P, Matias I, Martiadis V, De Petrocellis L, Maj M, Di Marzo V. Blood levels of the endocannabinoid anandamide are increased in anorexia nervosa and in binge-eating disorder, but not in bulimia nervosa. Neuropsychopharmacology. 2005;30:1216–21. doi: 10.1038/sj.npp.1300695. [DOI] [PubMed] [Google Scholar]
- [134]. [August, 2007 Accessed];A clinical trial on the antipsychotic properties of cannabidiol. Available at http://www.clinicaltrials.gov/ct/show/NCT00309413.
- [135]. [August, 2007 Accessed];Cannabinoids in bipolar affective disorder. Available at http://www.clinicaltrials.gov/ct/show/NCT00397605.
- [136].Ziedonis DM, Smelson D, Rosenthal RN, Batki SL, Green AI, Henry RJ, et al. Improving the care of individuals with schizophrenia and substance use disorders: consensus recommendations. J Psychiatr Pract. 2005;11:315–9. doi: 10.1097/00131746-200509000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ohayon MM. Epidemiology of depression and its treatment in the general population. J Psychiatr Res. 2007;41:207–13. doi: 10.1016/j.jpsychires.2006.10.006. [DOI] [PubMed] [Google Scholar]
- [138].Sink KS, McLaughlin PJ, Wood JAT, Brown C, Fan P, Vemuri VK, Pang Y, Olzewska T, Thakur GA, Makriyannis A, Parker LA, Salamone JD. The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacol. doi: 10.1038/sj.npp.1301476. in press. doi:10.1007/s00213-007-0988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007;91:383–8. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–6. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- [141].Ward SJ, Dykstra LA. The role of CB1 receptors in sweet versus fat reinforcement: effect of CB1 receptor deletion, CB1 receptor antagonism (SR141716A) and CB1 receptor agonism (CP-55,940) Behav Pharmacol. 2005;16:381–8. doi: 10.1097/00008877-200509000-00010. [DOI] [PubMed] [Google Scholar]
- [142].Chen RZ, Huang RRC, Shen CP, MacNeil DJ, Fong TM. Synergistic effects of cannabinoid inverse agonist AM251 and opioid antagonist nalmefene on food intake in mice. Brain Res. 2004;999:227–30. doi: 10.1016/j.brainres.2003.12.004. [DOI] [PubMed] [Google Scholar]
- [143].Odile P-G, Philippe P, Francois P. Combination of CB1 receptor antagonist and of sibutramine, the pharmaceutical compositions comprising them and their use in the treatment of obesity. 7148258. United States patent. 2006
- [144].Jacobson PB, Brune ME, Bush EN, Opgenorth TJ, Collins CA, Von Geldern T. Sibutramine and rimonabant combination for treatment of obesity. 2006/124506. WO Patent. 2006
- [145].Odile P-G, Philippe P, Francois P. Combination of CB1 receptor antagonist and of sibutramine, the pharmaceutical compositions comprising them and their use in the treatment of obesity. 2006/258709 United States patent application 2006.
- [146].Antel J, Krause HG, Gregory P-C, Wurl M, Waldeck H, Lange JHM, Kruse CG. Combination treatment of obesity involving 4,5-dihydro-1H-pyrazole derivatives having CB1 antagonistic activity and lipase inhibitors. 2005/039566. WO Patent. 2005
- [147].Firnges M, Gregory P-C, Antel J, Lange JHM, Waldeck H. Pharmaceutical compositions comprising CB1 cannabinoid receptor antagonists and potassium channel openers for the treatment of diabetes mellitus type I, obesity and related conditions. 2006/045799. WO Patent. 2006
- [148].Veltri EP. Combinations of substituted azetidinones and CB1 antagonists. 2006/039334. WO Patent. 2006
- [149].Amatruda JM, Fong TM, Moller DE, Thornberry NA. Combination of dipeptidyl peptidase-IV inhibitor and a cannabinoid CB1 receptor antagonist for the treatment of diabetes and obesity. 2006/119260. WO Patent. 2006
- [150].Wren JA, Ramudo AA, Campbell SL, King VL, Eagleson JS, Gossellin J, Sunderland SJ. Efficacy and safety of dirlotapide in the management of obese dogs evaluated in two placebo-controlled, masked clinical studies in North America. J Vet Pharmacol Ther. 2007;30(Supppl 1):81–9. doi: 10.1111/j.1365-2885.2007.00867.x. [DOI] [PubMed] [Google Scholar]
- [151]. [August, 2007 Accessed];FDA approves the first drug for obese dogs. Available at http://www/fda. gov/bbs/topics/NEWS/2007/NEW01542.html.
- [152].Schoffelmeer ANN, Hogenboom G, Wardeh G, De Vries TJ. Interactions between CB1 cannabinoid and μ opioid receptors mediating inhibition of neurotransmitter release in rat nucleus accumbens core. Neuropharmacology. 2006;51:773–81. doi: 10.1016/j.neuropharm.2006.05.019. [DOI] [PubMed] [Google Scholar]
- [153].Ellis J, Pediani JD, Canals M, Milasta S, Milligan G. Orexin-1 receptor-cannabinoid CB 1 receptor heterodimerization results in both ligand-dependent and -independent coordinated alterations of receptor localization and function. J Biol Chem. 2006;281:38812–24. doi: 10.1074/jbc.M602494200. [DOI] [PubMed] [Google Scholar]
- [154].Sipe JC, Waalen J, Gerber A, Beutler E. Overweight and obesity associated with a missense polymorphism in fatty acid amide hydrolase (FAAH) Int J Obes. 2005;29:755–9. doi: 10.1038/sj.ijo.0802954. [DOI] [PubMed] [Google Scholar]
- [155].Aberle J, Fedderwitz I, Klages N, George E, Beil FU. Genetic variation in two proteins of the endocannabinoid system and their influence on body mass index and metabolism under low fat diet. Horm Metab Res. 2007;39:395–7. doi: 10.1055/s-2007-977694. [DOI] [PubMed] [Google Scholar]
- [156].Fegley D, Gaetani S, Duranti A, Tontine A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-3-yl ester (URB597): effects of anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–8. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- [157].Serrano A, Del Arco I, Javier Pavón F, Macías M, Perez-Valero V, Roderíguez de Fonseca F. The cannabinoid CB1 receptor antagonist SR141716A (rimonabant) enhances the metabolic benefits of long-term treatment with oleoylethanolamide in Zucker rats. Neuropharmacology. doi: 10.1016/j.neuropharm.2007.03.007. in press. doi:10.1016/j.neuropharm.2007.03.007. [DOI] [PubMed] [Google Scholar]
- [158].Kapur A, Hurst DP, Fleischer D, Whitnell R, Thakur GA, Makriyannis A, Reggio PH, Abood ME. Mutation studies of Ser7.39 and ser2.60 in the human CB1 cannabinoid receptor: evidence for a serine-induced bend in CB1 transmembrane helix 7. Mol Pharmacol. 2007;71:1512–24. doi: 10.1124/mol.107.034645. [DOI] [PubMed] [Google Scholar]
- [159].Williams J, Wood J, Pandarinathan L, Karanian DA, Bahr BA, Vouros P, Makriyannis A. Quantitative method for the profiling of the endocannabinoid metabolome by LC-atmosphere pressure chemical ionization-MS. Anal Chem. 2007;79:5582–93. doi: 10.1021/ac0624086. [DOI] [PubMed] [Google Scholar]