Abstract
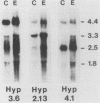
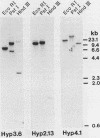
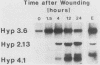
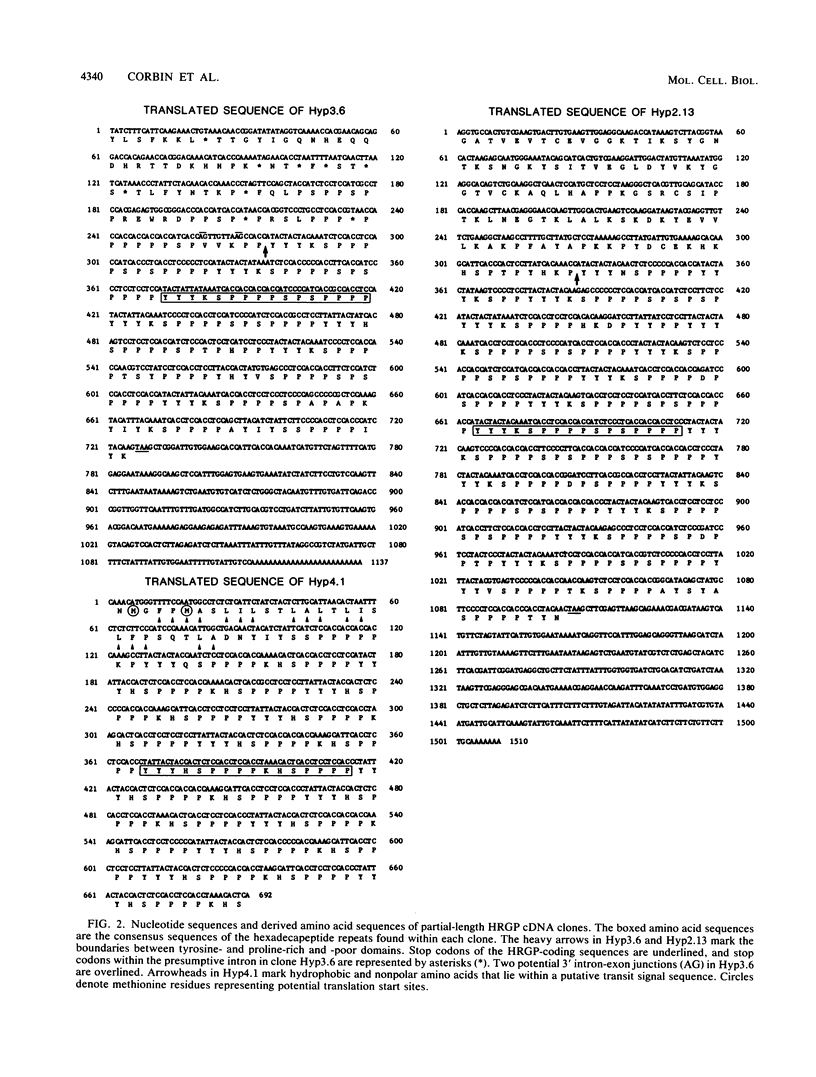
We have characterized three different transcripts induced by fungal elicitor, wounding, or infection which encode apoproteins of cell wall hydroxyproline-rich glycoproteins involved in plant defense against infection. The proteins encoded by two of these transcripts contain a proline-rich domain involving tandem repetition of the 16-amino-acid unit Tyr3-Lys-Ser-Pro4-Ser-Pro-Ser-Pro4. The third transcript encodes a protein with a proline-rich domain involving a variant of this 16-mer canonical repeat: Tyr3-His-Ser-Pro4-Lys-His-Ser-Pro4. Each transcript is encoded by a separate gene present at single or low copy number in the haploid genome. These transcripts exhibit markedly different patterns of accumulation in different stress conditions, indicating the operation of several distinct intercellular stress signal systems in higher plants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson-Prouty A. J., Albersheim P. Host-Pathogen Interactions: VIII. Isolation of a Pathogen-synthesized Fraction Rich in Glucan That Elicits a Defense Response in the Pathogen's Host. Plant Physiol. 1975 Aug;56(2):286–291. doi: 10.1104/pp.56.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. N., Ryder T. B., Wingate V. P., Bailey J. A., Lamb C. J. Differential accumulation of plant defense gene transcripts in a compatible and an incompatible plant-pathogen interaction. Mol Cell Biol. 1986 May;6(5):1615–1623. doi: 10.1128/mcb.6.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglie K. E., Gaynor J. J., Broglie R. M. Ethylene-regulated gene expression: molecular cloning of the genes encoding an endochitinase from Phaseolus vulgaris. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6820–6824. doi: 10.1073/pnas.83.18.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Varner J. E. An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J. 1985 Sep;4(9):2145–2151. doi: 10.1002/j.1460-2075.1985.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Edwards C., Chua N. H. Tissue-specific and light-regulated expression of a pea nuclear gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase. EMBO J. 1984 Aug;3(8):1671–1679. doi: 10.1002/j.1460-2075.1984.tb02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Dey P. M., Lawton M. A., Lamb C. J. Phytoalexin Induction in French Bean : Intercellular Transmission of Elicitation in Cell Suspension Cultures and Hypocotyl Sections of Phaseolus vulgaris. Plant Physiol. 1983 Feb;71(2):251–256. doi: 10.1104/pp.71.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyrberg E. A., Mahaffey J. W., Bond B. J., Davidson N. Transcripts of the six Drosophila actin genes accumulate in a stage- and tissue-specific manner. Cell. 1983 May;33(1):115–123. doi: 10.1016/0092-8674(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hightower R. C., Meagher R. B. Divergence and differential expression of soybean actin genes. EMBO J. 1985 Jan;4(1):1–8. doi: 10.1002/j.1460-2075.1985.tb02309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M. A., Lamb C. J. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol. 1987 Jan;7(1):335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach J. E., Cantrell M. A., Sequeira L. Hydroxyproline-rich bacterial agglutinin from potato : extraction, purification, and characterization. Plant Physiol. 1982 Nov;70(5):1353–1358. doi: 10.1104/pp.70.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mellon J. E., Helgeson J. P. Interaction of a hydroxyproline-rich glycoprotein from tobacco callus with potential pathogens. Plant Physiol. 1982 Aug;70(2):401–405. doi: 10.1104/pp.70.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapid switching of plant gene expression induced by fungal elicitor. Science. 1985 Mar 8;227(4691):1240–1243. doi: 10.1126/science.227.4691.1240. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira L. Mechanisms of induced resistance in plants. Annu Rev Microbiol. 1983;37:51–79. doi: 10.1146/annurev.mi.37.100183.000411. [DOI] [PubMed] [Google Scholar]
- Showalter A. M., Bell J. N., Cramer C. L., Bailey J. A., Varner J. E., Lamb C. J. Accumulation of hydroxyproline-rich glycoprotein mRNAs in response to fungal elicitor and infection. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6551–6555. doi: 10.1073/pnas.82.19.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]