Abstract
Oculo-auriculo-vertebral (OAV) spectrum summarizes a continuum of ocular, auricular, and vertebral anomalies. Goldenhar syndrome is a variant of this spectrum and is characterized by pre-auricular skin tags, microtia, facial asymmetry, ocular abnormalities, and vertebral anomalies of different sizes and shapes. Most cases are thought to be sporadic. However, a few families were reported to have an autosomal recessive inheritance and other families’ presentation of the syndrome strongly supported an autosomal dominant inheritance. We report OAV in a female infant presenting with tracheomalacia, diaphragmatic hernia, encephalomeningocele, sacral neural tube defect, and cardiac defect and her brother having no more than dysmorphic features. The mode of inheritance in this family supports an autosomal recessive inheritance where the transmission was from normal first-degree consanguineous parents to one of the sons and to the daughter. This report further broadens the clinical presentation and symptoms of OAV and supports the hypothesis advancing OAV as a genetically heterogeneous disorder.
Keywords: oculo-auriculo-vertebral spectrum, Goldenhar syndrome, diaphragmatic hernia, neural tube defect
Introduction
Goldenhar syndrome was first described in 1952 in three patients with epibulbar dermoids, pre-auricular skin tags, mandibular asymmetry and cervical vertebrae abnormalities.1 Later on, Gorlin et al reported similar findings for which they suggested the term oculo-auriculo-vertebral (OAV) dysplasia, which was then changed to oculoauriculovertebral (OAV) spectrum by Cohen et al.2,3 Currently, the term OAV summarizes a continuum of ocular, auricular, and vertebral anomalies that were described previously in Goldenhar syndrome, hemifacial microsomia as well as first and second branchial arch anomalies.2,4–6 The clinical presentation can be mild or severe. The incidence of OAV has been estimated at 1/3500,7 1/5600,2,8,9 and 1/26,550 live births.10
Most cases are thought to be sporadic; however, a few families were reported to have an autosomal recessive inheritance.11–18 Other families’ presentation of the syndrome strongly supported an autosomal dominant inheritance.6,8,19–27
We describe OAV in a female infant and in her brother. In this family, we report an autosomal recessive transmission from normal first-degree consanguineous parents to one of the sons and to the daughter, both of which presented with OAV but with variable genetic expression. This case report broadens the clinical presentation and symptoms of OAV and supports the hypothesis advancing OAV as a genetically heterogeneous disorder.
Case report
The proband was a full-term female infant born at 37 + 3 weeks of gestation (birth weight: 3045 g) to a 27-year-old healthy woman (G3P3SAb0) by cesarean section for fetal distress. Apgar scores at 1 and 5 minutes were 8 and 8, respectively. Maternal antenatal history was clear for any medication intake, alcohol ingestion, or drug usage. The proband had two older brothers born to first-degree consanguineous parents of Lebanese Middle East ascendants. The first, a 6-year-old, was diagnosed with OAV. He reportedly was noticed at birth to have right microtia, right mandibular hypoplasia, and two anterior left ear skin tags with no other anomalies. He was operated on for ear malformations but still has growth delay.
The second child was found to be developmentally normal with no detectable malformations. Family history revealed two interrupted pregnancies from the paternal side from equally consanguineous parents (Figure 1).
Figure 1.
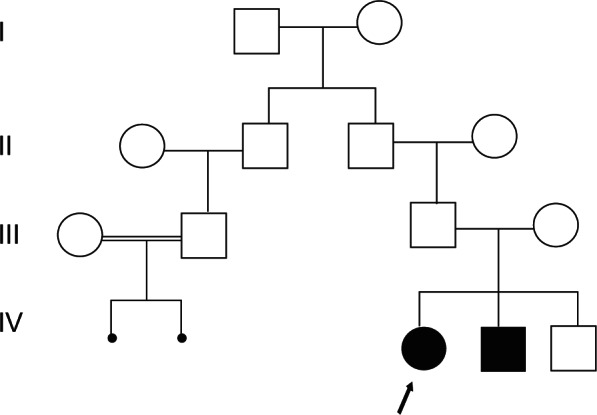
The pedigree shows two interrupted pregnancies from the paternal side from equally consanguineous parents.
At birth, the proband developed tachypnea and was transferred to the Neonatal Intensive Care Unit (NICU). Blood gases and O2 saturation were stable. Physical examination was remarkable for the following findings: short neck, widely spaced nipples, dysmorphic features including hypertelorism, upslanting palpebral fissures, flat nasal bridge, right mandibular hypoplasia, and facial asymmetry more obvious when the newborn cried. Her ears were normal-looking except for one preauricular left ear skin tag.
Soon, the respiratory condition of the newborn deteriorated, necessitating the placement of continuous positive airway pressure with the presumptive diagnosis of pneumonia. On day 5, a CT scan of chest revealed right diaphragmatic hernia with two-thirds of the liver protruding into the right chest and lower lobe consolidation. CT scan of brain and neck showed occipital encephalomeningocele. Echocardiography detected moderate perimembraneous ventricular septal defect, sinus venous arterial septal defect, and mild pulmonary hypertension. There was no evidence of congestive heart failure. Diuretic management with furosemide and spironolactone was started.
On day 16, she underwent an uneventful surgical repair of diaphragmatic hernia.
On day 24, surgical correction of the encephalomeningocele was performed with no intra-operative complications.
At 5 months of age, the infant suffered profound oxygen desaturation and bradycardia followed by cardiac arrest. Cardiopulmonary resuscitation was initiated with no pick-up. The infant was declared dead following a flat ECG.
The cytogenetic studies consisted of conventional karyotyping using G-banding, which revealed a 46, XX normal female profile. Molecular analysis using fluorescence in situ hybridization (FISH) with the N25 and TUPLE 1 probes in the DGS/VCFS region at 22q11.2 revealed disomy for this locus, excluding any deletions in this particular region. Although array-CGH would be useful, it is unfortunately not possible in this case.
Discussion
OAV spectrum is characterized by pre-auricular skin tags, microtia, facial asymmetry, ocular abnormalities, and vertebral anomalies of different sizes and shapes.
Our female patient presented with facial asymmetry, diaphragmatic hernia, encephalomeningocele, and preauricular skin tag. On the other hand her sibling had facial asymmetry and abnormal ears. Although the siblings may have different conditions, they both have the phenotypic clinical features of OAV and fit the minimal criteria for OAV diagnosis. This variability attests to the wide phenotypic range and severity of OAV and supports the hypothesis advancing OAV as a genetically heterogeneous disorder.
There is no national register of congenital disorders in Lebanon to date. To the best of our knowledge, this is the second case of OAV reported from Lebanon. We have previously reported a case of OAV in a male infant born to a woman with a history of prenatal fluoxetine ingestion throughout her pregnancy. The infant was born from nonconsanguinous parents and had facial asymmetry with right microtia and mandibular hypoplasia; bilateral hypoplastic macula, scoliotic deformity of the thoracic spine; and ventricular septal defect.28
Cardiovascular anomalies have also been associated with OAV.29–34 The frequency of cardiovascular heart defects in OAV ranged from 5% to 58% with conotruncal defects and septal defects as the most common among patients, as it was manifested in our patient.33,34
Of important note is that our patient also presented with diaphragmatic hernia that was repaired surgically. Congenital diaphragmatic hernias, as well as encephalocele, have been associated previously with Goldenhar syndrome.25,35
The clinical presentation of OAV varies among patients. Stoll et al reported OAV in a mother and two of her children. The mother had only auricular anomalies. Her first child, a female, presented with many anomalies including: bilateral cleft lip and palate, a coloboma of the upper eyelid, facial asymmetry, and posteriorly angulated ears. Her second child, a male, presented with cleft lip and palate, clubfeet, hypoplasia of the left ear and of the left maxillary and mandibular arches, and left microphthalmia.25
Symptoms of OAV were also described in two infants (a male and a female) who also shared features of the CHARGE association. Each had facial asymmetry, mandibular hypoplasia, ear abnormalities, hearing impairment, microphthalmia, heart defects, and developmental delay. In addition, both had plagiocephaly and torticollis and the boy presented with cleft lip, heminostril, and tracheoesophageal fistula.36 Later on, studies revealed a variety of symptoms in addition to the general characteristics of the OAV such as dysplastic ectopic, or bilobed caruncles, nasal sinus, nasal-ocular cleft,37 ocular colobomas,38 facial nerve paralysis and mental retardation,39 speech impairment, and difficulties in feeding/eating.40 Growth hormone deficiency was also reported in a patient with OAV but no association was proved.41
Most cases of OAV reported are sporadic but familial cases have been described. Kaye et al rejected the hypothesis of no genetic transmission and reported an autosomal dominant inheritance after examining families of 74 probands.5 In 1963, Saraux et al described the condition in two sisters born of healthy unrelated parents with normal karyotype, as reported in our case.11 Three years later, the same condition was reported in a son and daughter to consanguineous parents where the father was also affected.12 Recently, Tasse et al described the transmission of OAV from a mother to her daughter.6 The mode of inheritance was autosomal dominant. Tasse et al also concluded after reviewing the literature that patients with autosomal dominant inheritance of OAV present more often with bilateral anomalies.6
Amalnath et al described OAV in three brothers. The authors suggested that the occurrence of this condition in all three male siblings and its absence in the parents suggests an X-linked mode of inheritance in the family.42
On the other hand, Ellwood et al reported an autosomal recessive transmission in an Arabic family presenting with bilateral anotia and meatal atresia and in a Jewish family presenting with unilateral microtia and meatal atresia.13 Strisciuglio et al postulated a recessive inheritance in a sibship with a wide variation of expression.43 The family that was described had two brothers presenting with unilateral right microtia and bilateral meatal atresia. The sister presented with preauricular left appendage with absence of other anomalies. The absence of any ear anomalies in the parents suggested an autosomal recessive inheritance.43 The difference in severity of the clinical presentation in the children reveals a high variation in the manifestations in this syndrome. The above mentioned studies further elucidate the role of an autosomal recessive inheritance.
Different reports have described OAV along with different cytogenetic findings. OAV has been reported in a patient with complete trisomy 22,44 also in a male infant who had also the cri-du-chat syndrome and was found to have, upon cytogenic examination, a deletion of chromosome 5p14.45 Descartes reported an association of Goldenhar syndrome in a patient with a 5pter-p15.33 deletion.46 Ala-Mello et al reported a complex translocation resulting in a deletion of 5pter-p15.3, deletion of 21q22.3-qter, and duplication of 21q22.11-q22.12 in a patient presenting with features of Goldenhar syndrome and hematologic anomalies.47 However, our patient had a normal karyotype.
Our patient had a brother who was diagnosed with OAV with asymmetrical face and abnormal ears. However, the brother’s clinical symptoms varied from the reported patient and the degree of severity was mild. The parents did not present any abnormalities nor did the third sibling. The mode of inheritance in this family supports an autosomal recessive inheritance.
In conclusion, this case report further broadens the clinical presentation and symptoms of OAV and supports the hypothesis advancing OAV as a genetically heterogeneous disorder. While the corresponding genetic defect underlying OAV remains poorly understood, this report could at some point prove to be useful in improving our understanding of the disorder and its underlying genetic mechanisms.
Footnotes
Disclosure
The authors declare no conflicts of interest.
References
- 1.Goldenhar M. Associations malformatives do l’oeil et de l’oreille, en particulier le syndrome dermoïde epibulbaire-appendices auriculaires-fistula auris congenita et ses relations avec la dysostose mandibulo-faciale. J Genet Hum. 1952;1:243–282. French. [Google Scholar]
- 2.Gorlin RJ, Jue KL, Jacobsen U, Goldschmidt E. Oculoauriculovertebral dysplasia. J Pediatr. 1963;63:991–999. doi: 10.1016/s0022-3476(63)80233-4. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MM, Jr, Rollnick BR, Kaye CI. Oculoauriculovertebral spectrum: an updated critique. Cleft Palate J. 1989;26(4):276–286. [PubMed] [Google Scholar]
- 4.Rollnick BR, Kaye CI, Nagatoshi K, Hauck W, Martin AO. Oculoauriculovertebral dysplasia and variants: phenotypic characteristics of 294 patients. Am J Med Genet. 1987;26(2):361–375. doi: 10.1002/ajmg.1320260215. [DOI] [PubMed] [Google Scholar]
- 5.Kaye CI, Martin AO, Rollnick BR, et al. Oculoauriculovertebral anomaly: segregation analysis. Am J Med Genet. 1992;43(6):913–917. doi: 10.1002/ajmg.1320430602. [DOI] [PubMed] [Google Scholar]
- 6.Tasse C, Majewski F, Bohringer S, et al. A family with autosomal dominant oculo-auriculo-vertebral spectrum. Clin Dysmorphol. 2007;16(1):1–7. doi: 10.1097/MCD.0b013e328010d313. [DOI] [PubMed] [Google Scholar]
- 7.Poswillo D. Otomandibular deformity: pathogenesis as a guide to reconstruction. J Maxillofac Surg. 1974;2(2–3):64–72. doi: 10.1016/s0301-0503(74)80018-4. [DOI] [PubMed] [Google Scholar]
- 8.Grabb WC. The first and second branchial arch syndrome. Plast Reconstr Surg. 1965;36(5):485–508. doi: 10.1097/00006534-196511000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Hartsfield JK. Review of the etiologic heterogeneity of the oculo-auriculo-vertebral spectrum (Hemifacial Microsomia) Orthod Craniofac Res. 2007;10(3):121–128. doi: 10.1111/j.1601-6343.2007.00391.x. [DOI] [PubMed] [Google Scholar]
- 10.Melnick M. The etiology of external ear malformations and its relation to abnormalities of the middle ear, inner ear, and other organ systems. Birth Defects: Original Article Series. 1980;16(4):303–331. [PubMed] [Google Scholar]
- 11.Saraux H, Grignon JL, Dhermy P. A propos d’une observation familiale de syndrome de Franceschetti-Goldenhar. Bull Soc Ophtal Franc. 1963;63:705–707. French. [Google Scholar]
- 12.Proto F, Scullica L. Contributo allo studio della ereditarieta dei dermoidi epibulbari. Acta Genet Med Gemellol. 1966;15:351–363. doi: 10.1017/s1120962300014943. Italian. [DOI] [PubMed] [Google Scholar]
- 13.Ellwood LC, Winter ST, Dar H. Familial microtia with meatal atresia in two sibships. J Med Genet. 1968;5(4):289–291. doi: 10.1136/jmg.5.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirke DK. Goldenhar’s syndrome: two cases of oculo-auriculo-vertebral dysplasia occurring in full-blood Australian aboriginal sisters. Aust Paediatr J. 1970;6(4):213–214. [PubMed] [Google Scholar]
- 15.Krause U. The syndrome of goldenhar affecting two siblings. Acta Ophthalmol. 1970;48(3):494–499. doi: 10.1111/j.1755-3768.1970.tb03749.x. [DOI] [PubMed] [Google Scholar]
- 16.Konigsmark BW, Nager GT, Haskins HL. Recessive microtia, meatal atresia, and hearing loss. Report of a sibship. Arch Otolaryngol. 1972;96(2):105–109. doi: 10.1001/archotol.1972.00770090179002. [DOI] [PubMed] [Google Scholar]
- 17.Schmid M, Schroder M, Langenbeck U. Familial microtia, meatal atresia, and conductive deafness in three siblings. Am J Med Genet. 1985;22(2):327–332. doi: 10.1002/ajmg.1320220216. [DOI] [PubMed] [Google Scholar]
- 18.Witters I, Schreurs J, Van Wing J, Wouters W, Fryns JP. Prenatal diagnosis of facial clefting as part of the oculo-auriculo-vertebral spectrum. Prenat Diagn. 2001;21(1):62–64. doi: 10.1002/1097-0223(200101)21:1<62::aid-pd969>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Summitt RL. Familial Goldenhar syndrome. Birth Defects. 1969;5:106–109. [Google Scholar]
- 20.Parving A. Progressive hearing loss in Goldenhar’s syndrome. Scand Audiol. 1978;7(2):101–103. doi: 10.3109/01050397809043138. [DOI] [PubMed] [Google Scholar]
- 21.Setzer ES, Ruiz-Castaneda N, Severn C, Ryden S, Frias JL. Etiologic heterogeneity in the oculoauriculovertebral syndrome. J Pediatr. 1981;98(1):88–90. doi: 10.1016/s0022-3476(81)80547-1. [DOI] [PubMed] [Google Scholar]
- 22.Regenbogen L, Godel V, Goya V, Goodman RM. Further evidence for an autosomal dominant form of oculoauriculovertebral dysplasia. Clin Genet. 1982;21(3):161–167. doi: 10.1111/j.1399-0004.1982.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 23.Rollnick BR, Kaye CI. Hemifacial microsomia and variants: pedigree data. Am J Med Genet. 1983;15(2):233–253. doi: 10.1002/ajmg.1320150207. [DOI] [PubMed] [Google Scholar]
- 24.Singer SL, Haan E, Slee J, Goldblatt J. Familial hemifacial microsomia due to autosomal dominant inheritance. Case reports. Aust Dent J. 1994;39(5):287–291. doi: 10.1111/j.1834-7819.1994.tb05564.x. [DOI] [PubMed] [Google Scholar]
- 25.Stoll C, Viville B, Treisser A, Gasser B. A family with dominant oculoauriculovertebral spectrum. Am J Med Genet. 1998;78(4):345–349. doi: 10.1002/(sici)1096-8628(19980724)78:4<345::aid-ajmg8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 26.Tasse C, Bohringer S, Fischer S, et al. Oculo-auriculo-vertebral spectrum (OAVS): clinical evaluation and severity scoring of 53 patients and proposal for a new classification. Eur J Med Genet. 2005;48(4):397–411. doi: 10.1016/j.ejmg.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Vendramini-Pittoli S, Kokitsu-Nakata NM. Oculoauriculovertebral spectrum: report of nine familial cases with evidence of autosomal dominant inheritance and review of the literature. Clin Dysmorphol. 2009;18(2):67–77. doi: 10.1097/MCD.0b013e328323a7dd. [DOI] [PubMed] [Google Scholar]
- 28.Farra C, Yunis K, Mikati M, Yazbeck N, Majdalani M, Awwad J. Goldenhar syndrome associated with prenatal maternal Fluoxetine ingestion: Cause or coincidence? Birth Defects Res A Clin Mol Teratol. 2010;88(7):582–585. doi: 10.1002/bdra.20674. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A, Friedman JM, Taylor GP, Patterson MW. Pattern of cardiac malformation in oculoauriculovertebral spectrum. Am J Med Genet. 1993;46(4):423–426. doi: 10.1002/ajmg.1320460415. [DOI] [PubMed] [Google Scholar]
- 30.van Bever Y, van den Ende JJ, Richieri-Costa A. Oculo-auriculo-vertebral complex and uncommon associated anomalies: report on 8 unrelated Brazilian patients. Am J Med Genet. 1992;44(5):683–690. doi: 10.1002/ajmg.1320440530. [DOI] [PubMed] [Google Scholar]
- 31.Lin HJ, Owens TR, Sinow RM, et al. Anomalous inferior and superior venae cavae with oculoauriculovertebral defect: review of Goldenhar complex and malformations of left-right asymmetry. Am J Med Genet. 1998;75(1):88–94. doi: 10.1002/(sici)1096-8628(19980106)75:1<88::aid-ajmg18>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 32.Derbent M, Orun UA, Varan B, et al. A new syndrome within the oculo-auriculo-vertebral spectrum: microtia, atresia of the external auditory canal, vertebral anomaly, and complex cardiac defects. Clin Dysmorphol. 2005;14(1):27–30. [PubMed] [Google Scholar]
- 33.Morrison PJ, Mulholland HC, Craig BG, Nevin NC. Cardiovascular abnormalities in the oculo-auriculo-vertebral spectrum (Goldenhar syndrome) Am J Med Genet. 1992;44(4):425–428. doi: 10.1002/ajmg.1320440407. [DOI] [PubMed] [Google Scholar]
- 34.Digilio MC, Calzolari F, Capolino R, et al. Congenital heart defects in patients with oculo-auriculo-vertebral spectrum (Goldenhar syndrome) Am J Med Genet A. 2008;146A(14):1815–1819. doi: 10.1002/ajmg.a.32407. [DOI] [PubMed] [Google Scholar]
- 35.Kita D, Munemoto S, Ueno Y, Fukuda A. Goldenhar’s syndrome associated with occipital meningoencephalocele--case report. Neurol Med Chir (Tokyo) 2002;42(8):354–355. doi: 10.2176/nmc.42.354. [DOI] [PubMed] [Google Scholar]
- 36.Van Meter TD, Weaver DD. Oculo-auriculo-vertebral spectrum and the CHARGE association: clinical evidence for a common pathogenetic mechanism. Clin Dysmorphol. 1996;5(3):187–196. [PubMed] [Google Scholar]
- 37.Nijhawan N, Morad Y, Seigel-Bartelt J, Levin AV. Caruncle abnormalities in the oculo-auriculo-vertebral spectrum. Am J Med Genet. 2002;113(4):320–325. doi: 10.1002/ajmg.b.10715. [DOI] [PubMed] [Google Scholar]
- 38.Beck AE, Hudgins L, Hoyme HE. Autosomal dominant microtia and ocular coloboma: new syndrome or an extension of the oculo-auriculo-vertebral spectrum? Am J Med Genet A. 2005;134(4):359–362. doi: 10.1002/ajmg.a.30638. [DOI] [PubMed] [Google Scholar]
- 39.Touliatou V, Fryssira H, Mavrou A, Kanavakis E, Kitsiou-Tzeli S. Clinical manifestations in 17 Greek patients with Goldenhar syndrome. Genet Couns. 2006;17(3):359–370. [PubMed] [Google Scholar]
- 40.Stromland K, Miller M, Sjogreen L, et al. Oculo-auriculo-vertebral spectrum: associated anomalies, functional deficits and possible developmental risk factors. Am J Med Genet A. 2007;143A(12):1317–1325. doi: 10.1002/ajmg.a.31769. [DOI] [PubMed] [Google Scholar]
- 41.Yusufoglu AM, Cetinkaya E, Ceylaner S, et al. Goldenhar syndrome associated with growth hormone deficiency. Genet Couns. 2008;19(2):173–176. [PubMed] [Google Scholar]
- 42.Amalnath SD, Subrahmanyam DK, Dutta TK, Shenoy P. Familial oculoauriculovertebral sequence with lymphoma in one sibling. Am J Med Genet A. 2008;146A(23):3082–3085. doi: 10.1002/ajmg.a.32258. [DOI] [PubMed] [Google Scholar]
- 43.Strisciuglio P, Ballabio A, Parenti G. Microtia with meatal atresia and conductive deafness: mild and severe manifestations within the same sibship. J Med Genet. 1986;23(5):459–460. doi: 10.1136/jmg.23.5.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobrynski L, Chitayat D, Zahed L, et al. Trisomy 22 and facioauriculo-vertebral (Goldenhar) sequence. Am J Med Genet. 1993;46(1):68–71. doi: 10.1002/ajmg.1320460111. [DOI] [PubMed] [Google Scholar]
- 45.Choong YF, Watts P, Little E, Beck L. Goldenhar and cri-du-chat syndromes: a contiguous gene deletion syndrome? J AAPOS. 2003;7(3):226–227. doi: 10.1016/s1091-8531(02)42019-8. [DOI] [PubMed] [Google Scholar]
- 46.Descartes M. Oculoauriculovertebral spectrum with 5p15.33-pter deletion. Clin Dysmorphol. 2006;15(3):153–154. doi: 10.1097/01.mcd.0000204989.46743.ad. [DOI] [PubMed] [Google Scholar]
- 47.Ala-Mello S, Siggberg L, Knuutila S, von Koskull H, Taskinen M, Peippo M. Further evidence for a relationship between the 5p15 chromosome region and the oculoauriculovertebral anomaly. Am J Med Genet A. 2008;146A(19):2490–2494. doi: 10.1002/ajmg.a.32479. [DOI] [PubMed] [Google Scholar]


