Abstract
The conversion of ammonia into urea by the human liver requires the coordinated function of the 6 enzymes and 2 transporters of the urea cycle. The initial and rate-limiting enzyme of the urea cycle, carbamylphosphate synthetase 1 (CPS1), requires an allosteric activator, N-acetylglutamate (NAG). The formation of this unique cofactor from glutamate and acetyl Coenzyme-A is catalyzed by N-acetylglutamate synthase (NAGS). An absence of NAG as a consequence of NAGS deficiency may compromise flux through CPS1 and result in hyperammonemia. The NAGS gene encodes a 528-amino acid protein, consisting of a C-terminal catalytic domain, a variable segment, and an N-terminal mitochondrial targeting signal. Only 22 mutations in the NAGS gene have been reported to date, mostly in the catalytic domain. NAGS is primarily expressed in the liver and intestine. However, it is also surprisingly expressed in testis, stomach and spleen, and during early embryonic development at levels not concordant with the expression of other urea cycle enzymes, CPS1, or ornithine transcarbamylase. The purpose of NAGS expression in these tissues, and its significance to NAGS deficiency is as yet unknown. Inherited NAGS deficiency is the rarest of the urea cycle disorders, and we review the currently reported 34 cases. Treatment of NAGS deficiency with N-carbamyglutamate, a stable analog of NAG, can restore deficient urea cycle function and normalize blood ammonia in affected patients.
Keywords: urea cycle, urea cycle disorder, N-acetyl-L-glutamate, N-acetylglutamate synthase, hyperammonemia, N-carbamyl-L-glutamate
Introduction
In humans, detoxification of ammonia occurs in the liver via the urea cycle, a biochemical pathway consisting of 6 enzymes and 2 mitochondrial membrane transporters.1,2 The metabolic consequence of a defect in any step of the urea cycle has been well documented in man.1–3 A common feature of all urea cycle disorders is elevated blood ammonia which may lead to mental retardation, coma, and possibly death.
N-acetylglutamate (NAG) is the required allosteric activator of carbamylphosphate synthetase (CPS1; EC 6.4.3.16), the first and rate limiting enzyme of urea cycle.4,5 NAG, in turn, is synthesized from glutamate and acetyl Co-enzyme A6,7 by the hepatic mitochondrial enzyme, N-acetylglutamate synthase (NAGS; EC 2.3.1.1). In the absence of NAG, the activity of CPS1 is virtually nil,8,9 thus a deficiency of NAGS (MIM #237310) may result in hyperammonemia.
Herein, we describe the clinical and biochemical phenotype of NAGS deficiency, review the current published mutations in the NAGS gene, discuss the epidemiology of NAGS deficiency and review its treatment with N-carbamylglutamate.
NAGS deficiency
In humans, the only known sequelae of NAGS deficiency result from decreased flux through the CPS1 reaction.10 Indeed, the clinical and biochemical features of NAGS deficiency are identical to those seen in CPS1 deficiency.
In published cases to date, NAGS deficiency has presented at ages ranging from the neonatal period11 to the fifth decade of life.12 Clinical features of NAGS deficiency are those resulting from hyperammonemia, and include poor feeding, vomiting, altered level of consciousness, seizures, and coma. Patients with late-onset NAGS deficiency may present with chronic headaches and nausea. In such patients, acute decompensation has been precipitated by illness,13 pregnancy,14,15 or surgery,16 and symptoms include confusion and combativeness.
Biochemical features of NAGS deficiency include an elevated plasma ammonia and glutamine, whereas the concentrations of other urea cycle intermediates are low-to-normal. As in other proximal urea cycle disorders, plasma citrulline is frequently low or undetectable.16–29 However, unlike in OTC deficiency,30 urinary orotic acid is not elevated, as the interruption in the urea cycle occurs before the formation of carbamylphosphate.
Initial diagnoses of NAGS deficiency were based on measurements of hepatic NAGS activity,11,31 but in some cases, enzymatic assays were not reliable.32–35 Cloning of the human NAGS gene in 200236 has allowed molecular testing to become the primary method of diagnosis. Mutations in the coding region of the NAGS gene have been identified in all but 1 reported case of NAGS deficiency since 2002 (Table 1).
Table 1.
Clinical presentation, genotype, N-carbamylglutamate (NCG) treatment and outcome of 34 patients reported to have NAGS deficiency
| Family | Patient | Onset of symptoms | Presentation | Genotype | Onset of NCG therapy | Initial NCG dose | Chronic NCG dose | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1* | 1 | FH, 3d | Asymptomatic | 10 d | 100 mg/kg/d | 180 mg/kg/d at 13 m | Ataxia, spasticity psychomotor retardation, death at 9 y | Bachman et al5 Bachman et al1 Schubiger et al45 |
|
| 2 | 2 | 6 d | Poor feeding, tachypnea, somnolence | N/A | Death at 8 d | Bachman et al17 | |||
| 3* | 3 | 13 m | Decreased level of conciousness following febrile illness | N/A | Death at 13 m | Elpeleg et al22 | |||
| 4‡ | 4 | 5 w | Seizures following a viral gastroenteritis | N/A | Normal development at 18 m | Pandya et al27 | |||
| 5 | FH, 26 d | Diarrhea | N/A | Mild psychomotor retardation and spasticity at 6 m | |||||
| 5 | 6 | 2m | Frequent episodes of vomiting and lethargy, hypotonia | N/A | Profound psychomotor retardation at 2 y | Burlina et al18 | |||
| 6 | 7 | 4 y 10 m | Vomiting, lethargy hepatomegaly | N/A | Normal development at 5 y 7 m | Vockley et al34 | |||
| 7 | 8 | 3–4 d | Poor feeding, seizures | S410P/S410P | 25 d | 220 mg/kg/d | 80 mg/kg/d at 1 y | Normal development at 1 y | Guffon et al24 Schmidt et al25 |
| 9 | FH, 1 d | Asymptomatic | 1 d | 1 14 mg/kg × 1 dose | Normal development | Guffon et al23 | |||
| 8 | 10 | 1.5 y | Confusion, combative behavior | 20 y | 60 mg/kg/d | 50 mg/kg/d at 22 y | Cerebral dysfunction, paraplegia, incontinence at 22 y | Hinnie et al25 | |
| 9 | 11 | 3–4 d | Poor feeding, vomiting, cycling movements and fist clenching | after 10 w | 100 mg/kg/d | 100 mg/kg/d at 20 m | Normal development at 20 m | Morris et al26 | |
| 10 | 12 | 13 m | Vomiting, somnolence, hypotonia | A279P/A279P | 13 y | 100 mg/kg/d | 100 mg/kg/d | IQ = 78 at 13 y | Plecko et al28 Haberle et al42 |
| 11 | 13 | 2.7 y | Paroxysmal crying, lethargy, meat and dairy aversion, "Reye syndrome" after valproate administration | 4 y | 100 mg/kg/d | 100 mg/kg/d | Mental development <2 SD below age-matched controls at 4 y | Forget et al50 | |
| 12 | 14 | 4d | Seizures, coma | c.1036insC/ c.1036insC | 36 m | 150 mg/kg/d | Not indicated | Psychomotor retardation at 4 y | Elpeleg et al22 |
| 15 | FH, 1 d | Asymptomatic | 3 m | 150 mg/kg/d | Not indicated | Psychomotor retardation at 2 y | |||
| 13 | 16 | <2 d | Tachypnea, jitteriness | W324X/W324X | N/A | Death at 4 d | Caldovic and Tuchman20 | ||
| 14 | 17 | 2 d | Lethargy, anorexia, vomiting, respiratory distress, coma, seizures | c.1025delC | N/A | Not indicated | Caldovic and Tuchman20 | ||
| 15 | 2 d | Lethargy, anorexia, vomiting, respiratory distress, coma, seizures | Not indicated | ||||||
| 15 | 19 | 3 d | Not indicated | c.1306insT/ IVS3-2A>T | N/A | Death at 3 d | Haberle et al42 | ||
| 16 | 20 | 3d | Not indicated | L430P/L430P | N/A | Not indicated | Haberle et al42 Schmidt et al25 |
||
| 17 | 21 | 6 d | Not indicated | E433S/E433S | Not indicated | Haberle et al42 | |||
| 18 | 22 | 3 d | Not indicated | W484R/W484R | N/A | Death at 6 m | Haberle et al42 Heckman et al32 |
||
| 19 | 23 | 4 d | Not indicated | Death at 22 d | Haberle et al42 | ||||
| 24 | FH, 2 d | Asymptomatic | W324X/W324X | 3 m | 250 mg/kg/d | 10–200 mg/kg/d | Normal at 13 y | Gessler et al4 | |
| 20 | 25 | 9 y | Attention deficit, learning disabilities, episodes of anxiety and irritability | I2y | 100 mg/kg/d | 15 mg/kg/d | Not indicated | Belanger-Quintana étal13 | |
| 21 | 26 | 4 w | Vomiting, irritability, lethargy | R509Q/IVS4-IG >C | N/A | NCG study 2.2 g/m2/d | Not indicated | Not indicated | Caldovic et al16 Caldovic et al" |
| 27 | 9 y | Lethargy, anorexia, vomiting | Not indicated | ||||||
| 22 | 28 | 33 y | After surgery: hypertensive, combative, confused, seizures | V173E/T4311 | N/A | Death at 33 y | Caldovic et al16 | ||
| 23 | 29 | 2.5 m | Vomiting, weight loss, hypotonia | C200R/C200R | 4 m | 180 mg/kg × 1 dose | Not indicated | Normal development, age not indicated | Schmidt et al25 Guffon et al23 |
| 24 | 30 | 2 d | Irritablity, poor feeding. By 4 d, drowsiness, tremor, hypotonia | A518T/A518T | 4 d | 200 mg/kg × 1 dose | Not indicated | Normal development, age not indicated | Schmidt et al25 Guffon et al23 |
| 25 | 21 | 27 y | Seizures, coma during pregnancy | L3I2P/T4311 | N/A | Not indicated | Grody et al15 Caldovic et al14 |
||
| 26 | 32 | 3d | Seizures, coma | R414P/R414P | 4 d | 200 mg/kg/d | 50 mg/kg/d at 3 y | Normal development at 3 y | Nordenstrom et al52 |
| 27 | 33 | 40 y | Migraine headaches, intermittent staring spells, nausea, recurrent vomiting, lethargy, ataxia, coma | V3501/L442V | N/A | NCG study 2.2 g/m2/d |
Not indicated | Normal intellect at 57 y | Tuchman et al2 |
| 28 | 34 | 3d | Vomiting, feeding intolerance, episodic confusion | c.278delC/MI67V | 6 m | 350 mg/kg/d | 23–140 mg/kg/d | Normal development at 20 y | Corne et al53 |
Notes: Familial hyperammonemia patients were identified prospectively based on a family history of hyperammonemia or N-acetylglutamate synthase deficiency (NAGS);
Older siblings died of hyperammonemia of unknown origin;
Older brother died of seizures, liver failure.
The NAGS gene and transcript
The existence of mammalian NAGS was inferred over 50 years ago after NAG was identified as an obligate cofactor required in the biosynthesis of urea.4 Nevertheless, the mammalian NAGS gene was the last urea cycle gene to be cloned,36 probably due to the poor conservation of the NAGS protein sequence compared with that of the other urea cycle enzymes.37 The human NAGS gene is located on chromosome 17q21.31 and consists of 7 exons and 6 introns covering slightly less than 5 kb.36 The human NAGS open reading frame encodes a 528-amino acid protein.36 A comparison of amino acid sequences of NAGS from 7 mammalian species revealed 3 regions with different degrees of sequence conservation. At the N-terminus is a 50-amino acid-long mitochondrial targeting signal (MTS). This is followed by a 40- to 46-amino acid-long variable segment and a C-terminus conserved segment.6,36 The MTS has approximately 60% sequence conservation in mammalian NAGS and removal of the MTS results in what is dubbed mature NAGS.38 The variable segment is poorly conserved in mammalian NAGS and is not required for NAGS enzymatic activity.39 The rest of the protein, the conserved segment, has 90% sequence identity across mammalian species, and contains the catalytic site and the binding site for the allosteric activator L-arginine.37,40
Mutations in the NAGS gene
NAGS deficiency is an autosomal recessive disorder, thus affected individuals carry a mutation in each of their NAGS alleles, whereas heterozygous carriers are unaffected. Twenty-two disease-causing mutations in the NAGS coding sequence and in intron/exon boundaries have been reported to date (Table 1). Although at present 2 mutations occurred in more than 1 family (T431I and W324X), there do not appear to be any mutational hot spots in the NAGS gene. This is particularly surprising given that the NAGS coding sequence is GC-rich (67% GC content) and contains 135 CpG dinucleotides.14 Interestingly, most single base pair replacements in the NAGS coding sequence do not occur in these dinucleotides.14 Identified deleterious mutations in the NAGS gene include 15 missense, 1 nonsense, 4 frame-shift, and 2 splice-site mutations.14
A limited genotype-phenotype correlation may be inferred from affected patients who were homozygous for mutations in the NAGS gene. Homozygosity for nonsense or frameshift mutations, predicted to cause truncation of the NAGS protein and thus complete absence of functional NAGS enzyme, resulted in a neonatal presentation in 4 patients.21,36,41,42 Homozygosity for missense NAGS mutations, depending on the effect of the single amino-acid substitution, may result in either absent NAGS function or diminished but significant residual NAGS activity. The presence of residual enzyme activity, as demonstrated in purified recombinant enzyme, is the likely explanation for a later non-neonatal presentation in some affected patients.14,16,42 In contrast, a neonatal presentation was observed in patients who were homozygous for missense mutations of conserved residues (eg, S410P) or where a hydrophobic residue was substituted with a polar or charged amino acid (eg, W484R and A518T).24,28,29,42 Four affected patients were homozygous for missense alterations involving replacement of an amino acid with proline, which is likely to disrupt the NAGS secondary structure resulting in enzyme with little or no activity.29,43–45 To date, no single amino-acid substitutions have been reported within either the mitochondrial targeting signal or the variable segment of NAGS, suggesting that these regions are perhaps tolerant to missense changes.
Mutations in splice sites were observed in 2 families. Two splice-site mutations involved changes in the consensus acceptor splice sites of introns 3 and 4, which are expected to abolish mRNA splicing.16,42
One alteration, G236C, was incidentally discovered in a patient whose DNA was used as wild-type control sample for the NAGS sequencing assay in our clinical laboratory. Whether or not this alteration is disease-causing is unknown, but it was neither identified in a study of common polymorphisms of urea cycle genes,46 nor found in dbSNP build 133.
Expression of the NAGS mRNA and protein
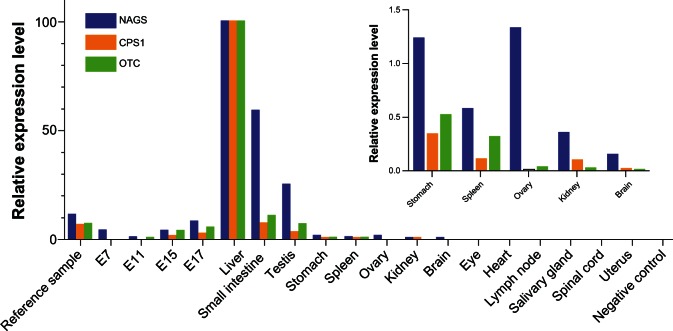
In NAGS deficiency, disruption of the NAGS gene results in reduced or absent NAGS enzyme in tissues in which it is normally expressed. NAGS mRNA is primarily expressed in the liver, but is also expressed in other tissues such as small intestine, spleen, and testis.20,36 Because the only known function of mammalian NAGS, CPS1, and OTC is to synthesize citrulline, NAGS would be expected to be expressed in the same tissues as CPS1 and OTC. To test this hypothesis, we used RT-PCR to quantify the relative expression levels of mouse NAGS, CPS1 and OTC mRNA in 14 tissues as well as at 4 stages (E7, E11, E15, and E17) of embryonic development.
As expected, liver had the highest expression of NAGS, CPS1, and OTC mRNA, followed by intestine (Figure 1). However, substantial expression of all 3 genes was also seen in the testis (between 3% and 25% of the expression in liver) and much less in the stomach and spleen (between 0.1% and 1.2% of the expression in the liver). Low levels of NAGS mRNA were also detectable in the brain, kidney, and ovary. In all other tissues, expression of NAGS, CPS1, and OTC mRNA were less than 0.1% of the expression seen in the liver. Surprisingly, NAGS mRNA was also expressed at embryonic stage E7, at levels approximately 3.7% of that seen in adult murine liver, in the absence of detectable levels of CPS1 and OTC mRNA.
Figure 1.
Relative expression levels of mouse NAGS, CPS1, and OTC mRNA in mouse tissues and stages of embryonic development. Insert shows relative expression of NAGS, CPS1 and OTC mRNA in the stomach, spleen, ovary, kidney and brain. Expression of NAGS, CPS1, and OTC mRNA was measured using quantitative real-time PCR and normalized to their mRNA abundance in liver. 1 μg of total mouse RNA from ovary, testis, brain, eye, heart, kidney, liver, lymph node, submaxillary gland, spinal cord, spleen, stomach, uterus, intestine, 7-day embryo, 11-day embryo, 15-day embryo, 17-day embryo was reverse transcribed to cDNA using random primers. Real time PCR was carried out using primers designed to anneal to different exons to avoid amplifying genomic DNA.
In contrast, western blot of a panel of 9 mouse tissues revealed the presence of NAGS, CPS1, and OTC proteins in the liver but only CPS1 and OTC in the intestine (data not shown). Although NAGS activity has previously been measured in the intestine,47,48 NAGS protein was not detectable in the small intestine most likely due to its low abundance in this tissue.
The presence of NAGS mRNA in mouse embryos at E7, as well as testis, ovary, spleen, stomach, kidney, and brain, could be due to illegitimate transcription, or more interestingly, to an as yet undiscovered novel function of NAGS. Additional studies will reveal if this expression pattern is also observed in humans, whether the expression of NAGS mRNA has physiological roles in tissues that do not express CPS1 and OTC, and whether absence of NAGS in these tissues contributes to the pathophysiology of NAGS deficiency.
Epidemiology and incidence of NAGS deficiency
Inherited NAGS deficiency is the rarest of urea cycle disorders,2 and the true incidence of NAGS deficiency is not known. To date, there are 34 reported patients from 28 families with NAGS deficiency. In the 2 decades before identification and cloning of the human NAGS gene,36 suspected diagnoses of NAGS deficiency were reported in only 11 families.11,17,18,22,24–28,34,49–51 Identification and cloning of human NAGS gene now allows accurate molecular diagnosis of the condition, and NAGS deficiency has since been reported in an additional 16 families.2,13,14,16,19–21,23,29,32,41,42,52,53 Nearly half of patients with NAGS deficiency are homozygotes, rather than compound heterozygotes, for mutations in the NAGS gene and these families indicated the existence of consanguinity22,23,25,26,29,42,52 or a known common ancestor.20
Several explanations could account for the low incidence of NAGS deficiency, compared with other urea cycle disorders.2 First, even mutations resulting in significant impairment of NAGS enzymatic function may allow for the production of sufficient CPS1 cofactor to maintain adequate flux through CPS1 and thus preclude hyperammonemia. Additionally, in a comparison of the sequences of urea cycle enzymes across phyla,54–56 NAGS is the least conserved.57 Thus, the NAGS structure may be more tolerant of amino acid substitutions. As a result, only individuals with rare amino acid substitutions that virtually abolish enzymatic function, either due to abolished substrate binding and catalysis or disruption of NAGS structure, will present with symptoms of NAGS deficiency. Alternatively, it is possible though unlikely that another enzyme is able to synthesize limited amounts of NAG, and that mutations in both NAGS and this second “moonlighting” enzyme are required to reduce CPS1 activity sufficiently to cause hyperammonemia. Finally, NAGS could potentially have other functions besides ammonia detoxification and a complete deficiency of NAGS may result in reduced embryonic survival. As described above, NAGS mRNA is curiously expressed in mouse spleen and testis (Figure 1 and Caldovic et al58) and also at mouse embryonic day 7, in the absence of significant CPS1 or OTC expression, thus positing another possible function of NAGS or NAG.
Treatment of NAGS deficiency with N-carbamylglutamate
Before the discovery of the CPS1 enzyme, Grisolia and Cohen determined that a derivative of L-glutamic acid, N-carbamylglutamate (NCG), was necessary for the biosynthesis of citrulline.59 While it was only later determined that N-acetylglutamate was the natural co-factor to the CPS1 enzyme,4 this earlier discovery was fortuitous as it would subsequently provide an important avenue of treatment for patients with NAGS deficiency.11
In contrast to NAG, which is hydrolyzed in vivo by acylamino acid acylase,60 NCG is acylase-resistant.61 Because both NAG and NCG can function as activating co-factors of CPS1, NAGS deficiency is the only inherited urea cycle disorder that can be specifically and effectively treated by a drug. In patients with NAGS deficiency, a 3-day trial of oral NCG at a dose of 2.2 g/m2/day was shown to restore ureagenesis and normalize blood ammonia, as demonstrated by [13C] and [15N] isotopic studies.2,19
Oral NCG has successfully rescued neonates with NAGS deficiency during hyperammonemic crisis.23,52 Published data on appropriate NCG dosing are limited. The initial NCG dose for treatment of acute hyperammonemia ranged in neonates from 25 mg/kg (100 mg/kg/day in 4 divided doses) to 200 mg/kg,23 compared with 15 mg/kg (60 mg/kg/day in 4 divided doses)25 to 180 mg/kg23 in those with late-onset NAGS deficiency who presented after the first month of life.
In patients receiving NCG as part of long-term chronic therapy, the lowest reported daily dose required to prevent hyperammonemia was 15 mg/kg/day in both neonatal41 and late-onset NAGS deficiency.13 NCG therapy appears to correct the metabolic defect in such patients, who no longer require ammonia-scavenging agents.13,23,25,41,49,52 In fact, dietary protein was liberalized to 2–3 g/kg/day in some patients,24,52 but 1 patient became mildly ataxic after ingestion of more than 3.5 g/kg/day.52 It is possible that a higher daily NCG dose would allow for greater protein tolerance in these patients, since in other NCG-treated patients, protein intake has been entirely liberalized, with no adverse effects.12
Extremes of NCG dosing have been associated with adverse effects. One patient, in whom NCG dosing was reduced to 10 mg/kg/day, experienced a rise in plasma ammonia from 27 to 58 μmol/L, which normalized once NCG was increased to 15 mg/kg/day.41 Another patient who received a dose of 650 mg/kg experienced tachycardia, sweating, bronchial hypersecretion, elevated body temperature, and restlessness.51
Some NAGS-deficiency patients on NCG have experienced breakthrough hyperammonemia during episodes of acute illness.26,49 Hyperammonemia while on NCG may reflect inadequate dosing. However, protein restriction during illness may be prudent if poor oral tolerance prevents the administration of NCG. Withdrawal of protein from the diet may have helped to prevent hyperammonemia in 1 patient.26
The advantage of treating NAGS deficiency with NCG is that NCG increases ammonia elimination by activating in vivo enzymes, whereas ammonia scavenging agents act stoichiometrically and response to scavengers is frequently suboptimal. All 3 affected neonates who presented with acute hyperammonemia and were administered NCG in a timely fashion along with standard therapy, had normal psychomotor development at 12 and 13 months of age.23,52 In contrast, some affected neonates who initially received conventional therapy alone, including ammonia scavenging agents and dialysis, have exhibited psychomotor retardation.21,50,51
It has been suggested that all hyperammonemic newborns with a suspected diagnosis of a urea cycle disorder should receive a therapeutic trial of NCG, which may provide a life-saving therapeutic option for patients with NAGS deficiency, and provide additional benefit in some cases of CPS1 deficiency.23 A rapid response to NCG may help to diagnose some cases of NAGS deficiency,23 though not all cases respond quickly.52
Other conditions with N-acetylglutamate deficiency
Secondary deficiencies of NAG may be observed in conditions associated with a depletion of intramitochondrial Coenzyme-A, acetyl-CoA, or glutamate, or inhibition of the NAGS reaction. A reduction of hepatic NAG has been hypothesized as the mechanism of hyperammonemia in the organic acidemias (eg, propionic academia,62,63 methylmalonic academia,62 isovaleric acidemia64), hyperinsulinism-hyperammonemia syndrome,65 and in valproic acid treatment.66,67 Exogenous benzoate may also decrease the intra-mitochondrial NAG concentration.68 Treatment with NCG may effectively treat hyperammonemia in these disorders.12,65,68–75 Indeed, 3-day administration of NCG has been shown to increase ureagenesis and decrease plasma ammonia in propionic academia.70
Acknowledgments
This work was supported by the Public Health Service grants K01DK076846, R01HD058567, R01DK064913, R01DK047870, and U54HD061221 from the National Institutes of Health. We also thank Dr Mendel Tuchman and Dr Hiroki Morizono for their continued guidance and mentorship.
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- 1.Brusilow SW, Maestri NE. Urea cycle disorders: diagnosis, pathophysiology, and therapy. Adv Pediatr. 1996;43:127–170. [PubMed] [Google Scholar]
- 2.Tuchman M, Lee B, Lichter-Konecki U, et al. Cross-sectional multicenter study of patients with urea cycle disorders in the United States. Mol Genet Metab. 2008;94(4):397–402. doi: 10.1016/j.ymgme.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Summar M. Current strategies for the management of neonatal urea cycle disorders. J Pediatr. 2001;138(Suppl 1):S30–S39. doi: 10.1067/mpd.2001.111834. [DOI] [PubMed] [Google Scholar]
- 4.Hall LM, Metzenberg RL, Cohen PP. Isolation and characterization of a naturally occurring cofactor of carbamyl phosphate biosynthesis. J Biol Chem. 1958;230(2):1013–1021. [PubMed] [Google Scholar]
- 5.Waterlow JC. The mysteries of nitrogen balance. Nutr Res Rev. 1999;12(1):25–54. doi: 10.1079/095442299108728857. [DOI] [PubMed] [Google Scholar]
- 6.Caldovic L, Ah Mew N, Shi D, Morizono H, Yudkoff M, Tuchman M. N-acetylglutamate synthase: structure, function and defects. Mol Genet Metab. 2010;100(Suppl 1):S13–S19. doi: 10.1016/j.ymgme.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shigesada K, Tatibana M. N-Acetylglutamate synthetase from ratliver mitochondria. Partial purification and catalytic properties. Eur J Biochem. 1978;84(1):285–291. doi: 10.1111/j.1432-1033.1978.tb12167.x. [DOI] [PubMed] [Google Scholar]
- 8.Metzenberg RL, Marshall M, Cohen PP. Purification of carbamyl phosphate synthetase from frog liver. J Biol Chem. 1958;233(1):102–105. [PubMed] [Google Scholar]
- 9.Metzenberg RL, Marshall M, Paik WK, Cohen PP. The synthesis of carbamyl phosphate synthetase in thyroxin-treated tadpoles. J Biol Chem. 1961;236:162–165. [PubMed] [Google Scholar]
- 10.Caldovic L, Tuchman M. N-acetylglutamate and its changing role through evolution. Biochem J. 2003;372(Pt 2):279–290. doi: 10.1042/BJ20030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachmann C, Colombo JP, Jaggi K. N-acetylglutamate synthetase (NAGS) deficiency: diagnosis, clinical observations and treatment. Adv Exp Med Biol. 1982;153:39–45. doi: 10.1007/978-1-4757-6903-6_6. [DOI] [PubMed] [Google Scholar]
- 12.Tuchman M, Caldovic L, Daikhin Y, et al. N-carbamylglutamate markedly enhances ureagenesis in N-acetylglutamate deficiency and propionic acidemia as measured by isotopic incorporation and blood biomarkers. Pediatr Res. 2008;64(2):213–217. doi: 10.1203/PDR.0b013e318179454b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belanger-Quintana A, Martinez-Pardo M, Garcia MJ, et al. Hyperam-monaemia as a cause of psychosis in an adolescent. Eur J Pediatr. 2003;162(11):773–775. doi: 10.1007/s00431-002-1126-2. [DOI] [PubMed] [Google Scholar]
- 14.Caldovic L, Morizono H, Tuchman M. Mutations and polymorphisms in the human N-acetylglutamate synthase (NAGS) gene. Hum Mutat. 2007;28(8):754–759. doi: 10.1002/humu.20518. [DOI] [PubMed] [Google Scholar]
- 15.Grody WW, Chang RJ, Panagiotis NM, Matz D, Cederbaum SD. Menstrual cycle and gonadal steroid effects on symptomatic hyperam-monaemia of urea cycle-based and idiopathic aetiologies. J Inherit Metab Dis. 1994;17(5):566–574. doi: 10.1007/BF00711592. [DOI] [PubMed] [Google Scholar]
- 16.Caldovic L, Morizono H, Panglao MG, et al. Late onset N- acetylglutamate synthase deficiency caused by hypomorphic alleles. Hum Mutat. 2005;25(3):293–298. doi: 10.1002/humu.20146. [DOI] [PubMed] [Google Scholar]
- 17.Bachmann C, Brandis M, Weissenbarth-Riedel E, Burghard R, Colombo JP. N-acetylglutamate synthetase deficiency, a second patient. J Inherit Metab Dis. 1988;11(2):191–193. doi: 10.1007/BF01799871. [DOI] [PubMed] [Google Scholar]
- 18.Burlina AB, Bachmann C, Wermuth B, et al. Partial N-acetylglutamate synthetase deficiency: a new case with uncontrollable movement disorders. J Inherit Metab Dis. 1992;15(3):395–398. doi: 10.1007/BF02435986. [DOI] [PubMed] [Google Scholar]
- 19.Caldovic L, Morizono H, Daikhin Y, et al. Restoration of ureagenesis in N-acetylglutamate synthase deficiency by N-carbamylglutamate. J Pediatr. 2004;145(4):552–554. doi: 10.1016/j.jpeds.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 20.Caldovic L, Morizono H, Panglao MG, Cheng SF, Packman S, Tuchman M. Null mutations in the N-acetylglutamate synthase gene associated with acute neonatal disease and hyperammonemia. Hum Genet. 2003;112(4):364–368. doi: 10.1007/s00439-003-0909-5. [DOI] [PubMed] [Google Scholar]
- 21.Elpeleg O, Shaag A, Ben-Shalom E, Schmid T, Bachmann C. N- acetylglutamate synthase deficiency and the treatment of hyperam-monemic encephalopathy. Ann Neurol. 2002;52(6):845–849. doi: 10.1002/ana.10406. [DOI] [PubMed] [Google Scholar]
- 22.Elpeleg ON, Colombo JP, Amir N, Bachmann C, Hurvitz H. Late-onset form of partial N-acetylglutamate synthetase deficiency. Eur J Pediatr. 1990;149(9):634–636. doi: 10.1007/BF02034751. [DOI] [PubMed] [Google Scholar]
- 23.Guffon N, Schiff M, Cheillan D, Wermuth B, Haberle J, Vianey-Saban C. Neonatal hyperammonemia: the N-carbamoyl-L-glutamic acid test. J Pediatr. 2005;147(2):260–262. doi: 10.1016/j.jpeds.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 24.Guffon N, Vianey-Saban C, Bourgeois J, Rabier D, Colombo JP, Guibaud P. A new neonatal case of N-acetylglutamate synthase deficiency treated by carbamylglutamate. J Inherit Metab Dis. 1995;18(1):61–65. doi: 10.1007/BF00711374. [DOI] [PubMed] [Google Scholar]
- 25.Hinnie J, Colombo JP, Wermuth B, Dryburgh FJ. N-Acetylglutamate synthetase deficiency responding to carbamylglutamate. J Inherit Metab Dis. 1997;20(6):839–840. doi: 10.1023/a:1005344507536. [DOI] [PubMed] [Google Scholar]
- 26.Morris AA, Richmond SW, Oddie SJ, Pourfarzam M, Worthington V, Leonard JV. N-acetylglutamate synthetase deficiency: favourable experience with carbamylglutamate. J Inherit Metab Dis. 1998;21(8):867–868. doi: 10.1023/a:1005478904186. [DOI] [PubMed] [Google Scholar]
- 27.Pandya AL, Koch R, Hommes FA, Williams JC. N-acetylglutamate synthetase deficiency: clinical and laboratory observations. J Inherit Metab Dis. 1991;14(5):685–690. doi: 10.1007/BF01799936. [DOI] [PubMed] [Google Scholar]
- 28.Plecko B, Erwa W, Wermuth B. Partial N-acetylglutamate synthetase deficiency in a 13-year-old girl: diagnosis and response to treatment with N-carbamylglutamate. Eur J Pediatr. 1998;157(12):996–998. doi: 10.1007/s004310050985. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt E, Nuoffer JM, Haberle J, et al. Identification of novel mutations of the human N-acetylglutamate synthase gene and their functional investigation by expression studies. Biochim Biophys Acta. 2005;1740(1):54–59. doi: 10.1016/j.bbadis.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Batshaw ML. Hyperammonemia. Curr Probl Pediatr. 1984;14(11):1–69. doi: 10.1016/0045-9380(84)90047-1. [DOI] [PubMed] [Google Scholar]
- 31.Bachmann C, Krahenbuhl S, Colombo JP. Purification and properties of acetyl-CoA:L-glutamate N-acetyltransferase from human liver. Biochem J. 1982;205(1):123–127. doi: 10.1042/bj2050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heckmann M, Wermuth B, Haberle J, Koch HG, Gortner L, Kreuder JG. Misleading diagnosis of partial N-acetylglutamate synthase deficiency based on enzyme measurement corrected by mutation analysis. Acta Paediatr. 2005;94(1):121–124. doi: 10.1111/j.1651-2227.2005.tb01799.x. [DOI] [PubMed] [Google Scholar]
- 33.Hwu WL, Chien YH, Tang NL, Law LK, Lin CY, Lee NC. Deficiency of the carnitine transporter (OCTN2) with partial N-acetylglutamate synthase (NAGS) deficiency. J Inherit Metab Dis. 2007;30(5):816. doi: 10.1007/s10545-007-0594-y. [DOI] [PubMed] [Google Scholar]
- 34.Vockley J, Vockley CM, Lin SP, et al. Normal N-acetylglutamate concentration measured in liver from a new patient with N-acetylglutamate synthetase deficiency: physiologic and biochemical implications. Biochem Med Metab Biol. 1992;47(1):38–46. doi: 10.1016/0885-4505(92)90006-k. [DOI] [PubMed] [Google Scholar]
- 35.Broere D, van Gemert WG, Kneepkens CM, et al. A 6-year-old boy with hyperammonaemia: partial N-acetylglutamate synthase deficiency or portosystemic encephalopathy? Eur J Pediatr. 2000;159(12):905–907. doi: 10.1007/pl00008367. [DOI] [PubMed] [Google Scholar]
- 36.Caldovic L, Morizono H, Gracia Panglao M, et al. Cloning and expression of the human N-acetylglutamate synthase gene. Biochem Biophys Res Commun. 2002;299(4):581–586. doi: 10.1016/s0006-291x(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 37.Haskins N, Panglao M, Qu Q, et al. Inversion of allosteric effect of arginine on N-acetylglutamate synthase, a molecular marker for evolution of tetrapods. BMC Biochem. 2008;9:24. doi: 10.1186/1471-2091-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morizono H, Caldovic L, Shi D, Tuchman M. Mammalian N-acetylglutamate synthase. Mol Genet Metab. 2004;81(Suppl 1):S4–S11. doi: 10.1016/j.ymgme.2003.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caldovic L, Lopez GY, Haskins N, et al. Biochemical properties of recombinant human and mouse N-acetylglutamate synthase. Mol Genet Metab. 2006;87(3):226–232. doi: 10.1016/j.ymgme.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Shi D, Sagar V, Jin Z, et al. The crystal structure of N-acetyl-L-glutamate synthase from Neisseria gonorrhoeae provides insights into mechanisms of catalysis and regulation. J Biol Chem. 2008;283(11):7176–7184. doi: 10.1074/jbc.M707678200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gessler P, Buchal P, Schwenk HU, Wermuth B. Favourable long-term outcome after immediate treatment of neonatal hyperammonemia due to N-acetylglutamate synthase deficiency. Eur J Pediatr. 2010;169(2):197–199. doi: 10.1007/s00431-009-1006-0. [DOI] [PubMed] [Google Scholar]
- 42.Haberle J, Schmidt E, Pauli S, et al. Mutation analysis in patients with N-acetylglutamate synthase deficiency. Hum Mutat. 2003;21(6):593–597. doi: 10.1002/humu.10216. [DOI] [PubMed] [Google Scholar]
- 43.Chang DK, Cheng SF, Trivedi VD, Lin KL. Proline affects oligomerization of a coiled coil by inducing a kink in a long helix. J Struct Biol. 1999;128(3):270–279. doi: 10.1006/jsbi.1999.4182. [DOI] [PubMed] [Google Scholar]
- 44.MacArthur MW, Thornton JM. Influence of proline residues on protein conformation. J Mol Biol. 1991;218(2):397–412. doi: 10.1016/0022-2836(91)90721-h. [DOI] [PubMed] [Google Scholar]
- 45.Viguera AR, Serrano L. Stable proline box motif at the N-terminal end of alpha-helices. Protein Sci. 1999;8(9):1733–1742. doi: 10.1110/ps.8.9.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell S, Ellingson C, Coyne T, et al. Genetic variation in the urea cycle: a model resource for investigating key candidate genes for common diseases. Hum Mutat. 2009;30(1):56–60. doi: 10.1002/humu.20813. [DOI] [PubMed] [Google Scholar]
- 47.Wakabayashi Y, Iwashima A, Yamada E, Yamada R. Enzymological evidence for the indispensability of small intestine in the synthesis of arginine from glutamate. II. N-acetylglutamate synthase. Arch Biochem Biophys. 1991;291(1):9–14. doi: 10.1016/0003-9861(91)90098-4. [DOI] [PubMed] [Google Scholar]
- 48.Wakabayashi Y, Yamada E, Hasegawa T, Yamada R. Enzymological evidence for the indispensability of small intestine in the synthesis of arginine from glutamate. I. Pyrroline-5-carboxylate synthase. Arch Biochem Biophys. 1991;291(1):1–8. doi: 10.1016/0003-9861(91)90097-3. [DOI] [PubMed] [Google Scholar]
- 49.Schubiger G, Bachmann C, Barben P, Colombo JP, Tonz O, Schupbach D. N-acetylglutamate synthetase deficiency: diagnosis, management and follow-up of a rare disorder of ammonia detoxication. Eur J Pediatr. 1991 Mar;150(5):353–356. doi: 10.1007/BF01955939. [DOI] [PubMed] [Google Scholar]
- 50.Forget PP, van Oosterhout M, Bakker JA, Wermuth B, Vles JS, Spaapen LJ. Partial N-acetyl-glutamate synthetase deficiency masquerading as a valproic acid-induced Reye-like syndrome. Acta Paediatr. 1999;88(12):1409–1411. doi: 10.1080/080352599750030194. [DOI] [PubMed] [Google Scholar]
- 51.Bachmann C, Krahenbuhl S, Colombo JP, Schubiger G, Jaggi KH, Tonz O. N-acetylglutamate synthetase deficiency: a disorder of ammonia detoxication. N Engl J Med. 1981;304(9):543. doi: 10.1056/NEJM198102263040918. [DOI] [PubMed] [Google Scholar]
- 52.Nordenstrom A, Halldin M, Hallberg B, Alm J. A trial with N- carbamylglutamate may not detect all patients with NAGS deficiency and neonatal onset. J Inherit Metab Dis. 2007;30(3):400. doi: 10.1007/s10545-007-0454-9. [DOI] [PubMed] [Google Scholar]
- 53.Corne C, Fouilhoux A, Aquaviva C, Besson G. First French case of NAGS deficiency. 20 years of follow up [abstract] Mol Genet Metab. 2011;102(3):275. [Google Scholar]
- 54.Labedan B, Boyen A, Baetens M, et al. The evolutionary history of carbamoyltransferases: A complex set of paralogous genes was already present in the last universal common ancestor. J Mol Evol. 1999;49(4):461–473. doi: 10.1007/pl00006569. [DOI] [PubMed] [Google Scholar]
- 55.Lawson FS, Charlebois RL, Dillon JA. Phylogenetic analysis of carbamylphosphate synthetase genes: complex evolutionary history includes an internal duplication within a gene which can root the tree of life. Mol Biol Evol. 1996;13(7):970–977. doi: 10.1093/oxfordjournals.molbev.a025665. [DOI] [PubMed] [Google Scholar]
- 56.Ouzounis CA, Kyrpides NC. On the evolution of arginases and related enzymes. J Mol Evol. 1994;39(1):101–104. doi: 10.1007/BF00178255. [DOI] [PubMed] [Google Scholar]
- 57.Qu Q, Morizono H, Shi D, Tuchman M, Caldovic L. A novel bifunctional N-acetylglutamate synthase-kinase from Xanthomonas campestris that is closely related to mammalian N-acetylglutamate synthase. BMC Biochem. 2007;8:4. doi: 10.1186/1471-2091-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caldovic L, Morizono H, Yu X, et al. Identification, cloning and expression of the mouse N-acetylglutamate synthase gene. Biochem J. 2002;364(Pt 3):825–831. doi: 10.1042/BJ20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grisolia S, Cohen PP. The catalytic role of carbamyl glutamate in citrulline biosynthesis. J Biol Chem. 1952;198(2):561–571. [PubMed] [Google Scholar]
- 60.Reglero A, Rivas J, Mendelson J, Wallace R, Grisolia S. Deacylation and transacetylation of acetyl glutamate and acetyl ornithine in rat liver. FEBS Lett. 1977;81(1):13–17. doi: 10.1016/0014-5793(77)80917-4. [DOI] [PubMed] [Google Scholar]
- 61.Kim S, Paik WK, Cohen PP. Ammonia intoxication in rats: protection by N-carbamoyl-L-glutamate plus L-arginine. Proc Natl Acad Sci USA. 1972;69(12):3530–3533. doi: 10.1073/pnas.69.12.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coude FX, Sweetman L, Nyhan WL. Inhibition by propionyl-coenzyme A of N-acetylglutamate synthetase in rat liver mitochondria. A possible explanation for hyperammonemia in propionic and methylmalonic acidemia. J Clin Invest. 1979;64(6):1544–1551. doi: 10.1172/JCI109614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stewart PM, Walser M. Failure of the normal ureagenic response to amino acids in organic acid-loaded rats. Proposed mechanism for the hyperammonemia of propionic and methylmalonic acidemia. J Clin Invest. 1980;66(3):484–492. doi: 10.1172/JCI109879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coude FX, Grimber G, Parvy P, Rabier D. Role of N-acetylglutamate and acetyl-CoA in the inhibition of ureagenesis by isovaleric acid in isolated rat hepatocytes. Biochim Biophys Acta. 1983;761(1):13–16. doi: 10.1016/0304-4165(83)90356-2. [DOI] [PubMed] [Google Scholar]
- 65.De Lonlay P, Benelli C, Fouque F, et al. Hyperinsulinism and hyperam-monemia syndrome: report of twelve unrelated patients. Pediatr Res. 2001;50(3):353–357. doi: 10.1203/00006450-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 66.Coude FX, Rabier D, Cathelineau L, Grimber G, Parvy P, Kamoun P. A mechanism for valproate-induced hyperammonemia. Adv Exp Med Biol. 1982;153:153–161. doi: 10.1007/978-1-4757-6903-6_21. [DOI] [PubMed] [Google Scholar]
- 67.Williams CA, Tiefenbach S, McReynolds JW. Valproic acid-induced hyperammonemia in mentally retarded adults. Neurology. 1984;34(4):550–553. doi: 10.1212/wnl.34.4.550. [DOI] [PubMed] [Google Scholar]
- 68.O’Connor JE, Costell M, Grisolia S. Carbamyl glutamate prevents the potentiation of ammonia toxicity by sodium benzoate. Eur J Pediatr. 1989;148(6):540–542. doi: 10.1007/BF00441553. [DOI] [PubMed] [Google Scholar]
- 69.Kasapkara CS, Ezgu FS, Okur I, Tumer L, Biberoglu G, Hasanoglu A. N-carbamylglutamate treatment for acute neonatal hyperammonemia in isovaleric acidemia. Eur J Pediatr. 2011;170(6):799–801. doi: 10.1007/s00431-010-1362-9. [DOI] [PubMed] [Google Scholar]
- 70.Ah Mew N, McCarter R, Daikhin Y, Nissim I, Yudkoff M, Tuchman M. N-carbamylglutamate augments ureagenesis and reduces ammonia and glutamine in propionic acidemia. Pediatrics. 2010;126(1):e208–e214. doi: 10.1542/peds.2010-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soyucen E, Demirci E, Aydin A. Outpatient treatment of propionic acidemia-associated hyperammonemia with N-carbamoyl-L-glutamate in an infant. Clin Ther. 2010;32(4):710–713. doi: 10.1016/j.clinthera.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 72.Filippi L, Gozzini E, Fiorini P, Malvagia S, la Marca G, Donati MA. N-carbamylglutamate in emergency management of hyperammonemia in neonatal acute onset propionic and methylmalonic aciduria. Neonatolog y. 2009;97(3):286–290. doi: 10.1159/000255168. [DOI] [PubMed] [Google Scholar]
- 73.Schwahn BC, Pieterse L, Bisset WM, Galloway PG, Robinson PH. Biochemical efficacy of N-carbamylglutamate in neonatal severe hyperammonaemia due to propionic acidaemia. Eur J Pediatr. 2010;169(1):133–134. doi: 10.1007/s00431-009-1036-7. [DOI] [PubMed] [Google Scholar]
- 74.Gebhardt B, Dittrich S, Parbel S, Vlaho S, Matsika O, Bohles H. N-carbamylglutamate protects patients with decompensated propionic aciduria from hyperammonaemia. J Inherit Metab Dis. 2005;28(2):241–244. doi: 10.1007/s10545-005-5260-7. [DOI] [PubMed] [Google Scholar]
- 75.Gebhardt B, Vlaho S, Fischer D, Sewell A, Bohles H. N-carbamylglutamate enhances ammonia detoxification in a patient with decompensated methylmalonic aciduria. Mol Genet Metab. 2003;79(4):303–304. doi: 10.1016/s1096-7192(03)00095-7. [DOI] [PubMed] [Google Scholar]