Abstract
Background
Diabetic retinopathy (DR) is classically defined as a microvasculopathy that primarily affects the small blood vessels of the inner retina as a complication of diabetes mellitus. It has been suggested that nitric oxide (NO) and α2β1 integrin (a platelet receptor for collagen) play an important role in the pathogenesis of microvascular complications in DR.
Aim
The aim of this study was to investigate the association of two candidate genes involved in the regulation of retinal vasculature, endothelial nitric oxide synthase (eNOS) and α2β1 integrin (ITGA2) genes, with the development of DR in Egyptian patients with type 2 diabetes mellitus and to investigate whether genetic variants will affect the type of retinopathy (proliferative or nonproliferative).
Methods
In this study, 70 patients were enrolled and categorized into two groups: (1) a DR group consisting of 50 patients with DR, which was further subclassified into 25 patients with nonproliferative DR (NPDR group) and 25 patients with proliferative DR (PDR group) and (2) a diabetes without retinopathy (DWR) group, comprising 20 patients with type 2 diabetes of more than 10 years’ duration who showed no signs of DR. Associations of the genetic polymorphisms of eNOS (G894T) and ITGA2 (BgI II) were studied. Polymerase chain reaction-restriction fragment length polymorphism analysis was performed for all samples to evaluate the genotypes and correlate with the phenotype of the disease.
Results
The allele frequencies of both polymorphisms showed considerable differences between patients with and without DR. The GG genotype of G894T polymorphism of eNOS was associated with a 9.75-fold increased risk of DR (95% confidence interval 1.7–55.4) and the genotype ITGA2 BgI II (+/+) was associated with a 10.1-fold increased risk of DR (95% confidence interval 1.8–57.9), while the α2β1 integrin gene polymorphism of genotype distribution of both eNOS and ITGA2 polymorphisms did not differ significantly between the proliferative and nonproliferative DR groups.
Conclusion
A significant association between the G894T polymorphism of eNOS and BgI II polymorphism of ITGA2 genes and DR was observed, while there was no association between the genetic variants of those two polymorphisms and the type of retinopathy.
Keywords: polymorphism, retinopathy, diabetes, Egypt, retinal vasculature
Introduction
Diabetic retinopathy (DR) is one of the most common microvascular complications of diabetes, affecting 80% of patients with a duration of diabetes of over 20 years.1 Despite remarkable advances in the diagnosis and treatment of DR and its associated complications, DR remains the leading cause of blindness among working-age individuals in developed countries.2
Most diabetic patients, especially those with poor glycemic control, develop DR. The Wisconsin Epidemiologic Study of Diabetic Retinopathy showed that 28.8% of diabetic patients develop retinopathy early, whereas 22.2% with a history of diabetes do not develop retinopathy, irrespective of glycemic exposure.3 The authors suggested that genetic factors could promote the onset of retinopathy in diabetic patients. Over 30 candidate genes involved in different metabolic mechanisms and functional pathways have been reported to be associated with DR.4
eNOS is a constitutively expressed enzyme of 135 kDa in vascular endothelial cells. The eNOS gene, located on chromosome 7q35-36, is composed of 26 exons and spans 21 kb. The eNOS gene has several reported single-nucleotide polymorphisms, one of which is G894T. This polymorphism is a G-to-T transversion at nucleotide position 894, resulting in a GAG to GAT substitution in exon 7 with the replacement of glutamine by aspartate (Glu298Asp). Several studies have observed that this polymorphism has been associated with multiple disease outcomes, including essential hypertension, coronary artery disease, ischemic heart disease, myocardial infarction, and end-stage renal disease.5 Therefore, the potential role of this polymorphism in the etiology of DR with type 2 diabetes (T2D) was investigated in this study.
DR is associated with disorders in the nitric oxide (NO) pathway including impaired NO-mediated vasodilatation, increased oxidative stress, dysregulation at NO synthase isoforms, and NO uncoupling.6 This suggests that eNOS is involved in inflammation and ischemic process in the pathogenesis of DR and that the eNOS gene is a possible candidate gene in the pathophysiology of DR.7
Platelets are known to have a physiological role in maintaining homeostasis and platelet dysfunction associated with diabetes is known to contribute to the development of DR.8 Wide variations in pathopysiological processes involved in the density of a platelet collagen receptor (α2β1 integrin or glycoprotein Ia/IIa) are reportedly associated with polymorphisms in the gene encoding the α subunit at the receptor.9 The aim of the study was to determine the relationship between the G894T polymorphism of the eNOS gene and the BgI II polymorphism of the ITGA2 gene and susceptibility to DR and to determine whether their genetic variants will affect the type of retinopathy.
The gene encoding α2β1 integrin has at least eight polymorphisms and its genetic variations have been shown to affect the density of α2β1 receptors on the platelet surface.10 One of these polymorphisms is the BgI II restriction fragment length polymorphism (BgI II, +/−) within intron 7. It was reported that the platelet α2β1 density and the extent of platelet adhesion to collagen were higher in individuals with BgI II(+) homozygote than in individuals with the BgI II(−) homozygote.9
Subjects and methods
The study included 70 unrelated participants with T2D. All study patients gave informed written consent before blood sampling. Approval was obtained from the Research Ethics Committee of the Menoufiya Faculty of Medicine. The patients were enrolled from the outpatient clinic of the Ophthalmology Department in Menoufiya University Hospital. They were divided into two groups:
DR group: 50 patients suffering from DR, further subclassified into two groups: 25 patients suffering from nonproliferative DR (NPDR group) and 25 patients suffering from proliferative DR (PDR group).
Diabetic without retinopathy (DWR) group: 20 patients with no signs of DR and with known diabetes mellitus (DM) duration ≥10 years (ie, controls).
Diagnosis of T2D was based on criteria established by the American Diabetes Association expert committee as follows: a fasting blood glucose level ≥126 mg/dL (≥7.0 mmol/L) or 2-hour post-load plasma glucose ≥200 mg/dL (≥11.1 mmol/L) or random plasma glucose ≥200 mg/dL, on more than one occasion. Alternatively, diagnosis of T2D was accepted if an individual was on pharmacological treatment and review of medical records indicated justification for treatment. Classification of diabetes was based on clinical features (age of onset) and laboratory data (serum c-peptide).11
A full medical history was taken for each subject, which included information on age, sex, age at onset of diabetes, duration of diabetes, family history, treatment protocols, history of hypertension, and smoking (a smoker was defined as someone who smoked at least five cigarettes daily for more than 1 year).6 All patients underwent full ophthalmological examination, which included intraocular pressure measurement, slit-lamp examination, and fundus examination. Fundus fluorescein angiography was performed in patients with DR to differentiate between proliferative and nonproliferative retinopathy, then DR patients were classified into either the NPDR or PDR subtype according to Early Treatment Diabetic Retinopathy Study (ETDRS) criteria.12 Finally, laboratory investigation was performed, including: fasting glucose level;13 HbA1c;14 lipid profile, including serum cholesterol;15 triglycerides;16 high-density lipoprotein (HDL)-cholesterol17 and low-density lipoprotein (LDL)-cholesterol18 (by enzymatic colorimetric test); and genotyping for the G894T polymorphism of the eNOS gene and the BgI II polymorphism of the ITGA2 gene.
Blood sampling
A total of 10 mL of fasting venous blood was withdrawn from the cubital vein of every subject: 4 mL of this was transferred slowly into a vacuum ethylenediaminetetraacetic acid (EDTA) tube for isolation of white blood cells for genotyping; 2 mL was transferred slowly into a vacuum EDTA tube for measuring hemoglobin A1c (HbA1c); and 2 mL was transferred slowly into a vacuum EDTA tube and centrifuged for 5 minutes at 4000 revolutions per minute. The plasma obtained for determination of plasma glucose was frozen at −20°C till analysis. The remaining 2 mL was transferred slowly into a plain tube for determination of serum total cholesterol, triglycerides, and HDL-cholesterol; left for 30 minutes for clotting; and centrifuged for 10 minutes at 4000 revolutions per minute. The serum obtained was frozen at −20°C till analysis. LDL-cholesterol was estimated by Friedewald’s formula.18
Peripheral blood mononuclear cells isolation
For separation of peripheral blood mononuclear cells, Lymphoflot solution (Biotest AG, Dreieich, Germany) was used.19
DNA extraction
This was performed using QIAamp® DNA Blood Mini kits (QIAGEN, Hilden, Germany).
Genotyping of ITGA2 (BgI II) polymorphism20
The amplification reaction was done in 25 uL final volume (10 uL DNA template + 15 uL master mix containing 2.5 uL 10× polymerase chain reaction [PCR] buffer, 1.5 uL MgCl 25 mM, 0.5 uL deoxyribonucleotide [dNTPs] 10 mM, 0.5 uL Taq polymerase 5 U/uL, 1.0 uL forward primer 50 mM {F 5′-GATTTAACTTTCCCGACTGCCTTCC-3′}, 1.0 uL reverse primer 50 mM {R 5′-CATAGGTTTTTGGGAACAGGTGG-3′}, and 8 uL distilled water). PCR amplification was done using a PerkinElmer GeneAmp thermal cycler 2400 (Waltham, MA). PCR conditions were 34 cycles, with the first two cycles at 94°C for 1 minute, 69°C for 1 minute, and 72°C for 1 minute; the second two cycles at 94°C for 1 minute, 67°C for 1 minute, and 72°C for 1 minute; the remaining 30 cycles at 94°C for 1 minute, 65°C for 1 minute, and 72°C for 1 minute; and the final extension step of 72°C for 1 minute.
Recognition site BgI II
5′ ... A▼GATCT ... 3′
3′ ... TCTAG▲A ... 5′
Amplified DNA was digested with BgI II restriction enzyme (BgI II) at 37°C for 3 hours (2 uL 10× buffer, 1.0 uL BgI II, 7 uL distilled water, and 10 uL PCR product).
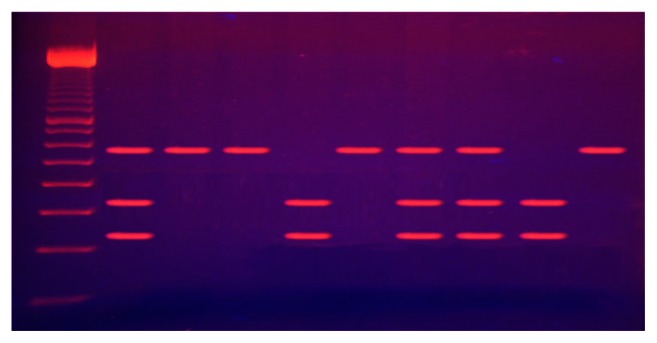
The BgI II digestive products were run by 3% agarose gel electrophoresis for 30 minutes and stained with ethidium bromide. The bands were visualized under ultraviolet light. Digested PCR products yielded visible fragments of 240 and 340 bp of the PCR product containing BgI II(+), whereas those containing BgI II(-) would not cut with a single band of 580 bp (Figure 1).
Figure 1.
Electrophoresis of digested ITGA2 (BgI II) polymerase chain reaction products.
Notes: Lane 1: 100 bp ladder; Lanes 2, 7, 8: BgI II +/− genotype; Lanes 3, 4, 6, 10: BgI II −/− genotype; Lanes 5, 9: BgI II +/+ genotype.
Genotyping of G894T polymorphism of eNOS gene21
The amplification reaction was done in 25 uL final volume (10 uL DNA template + 15 uL master mix containing 2.5 uL 10× PCR buffer, 2.0 uL Mgcl 25 mM, 1.0 uL dNTPs 10 mM, 0.5 uL Taq polymerase 5 U/uL, 1.0 uL forward primer 20 mM {F 5′-AAGGCAGGAGACAGTGGATGGA-3′}, 1.0 uL reverse primer 20 mM {R 5′-CCCAGTCAATCCCTTTGGTGCTCA-3′}, and 7 uL distilled water).
PCR amplification was done under the following conditions: one cycle at 94°C for 5 minutes; five cycles at 95°C for 20 seconds, 65°C for 20 seconds, and 74°C for 20 seconds; five cycles at 95°C for 20 seconds, 60°C for 20 seconds, and 74°C for 20 seconds; five cycles at 95°C for 20 seconds, 55°C for 20 seconds, and 74°C for 20 seconds; five cycles at 95°C for 20 seconds, 50°C for 20 seconds, and 74°C for 20 seconds; and a final extension step of 74°C for 7 minutes.
Recognition site Ban II
5′ ... GRGCY▼C ... 3′
3′ ... C▲YCGRG ... 5′
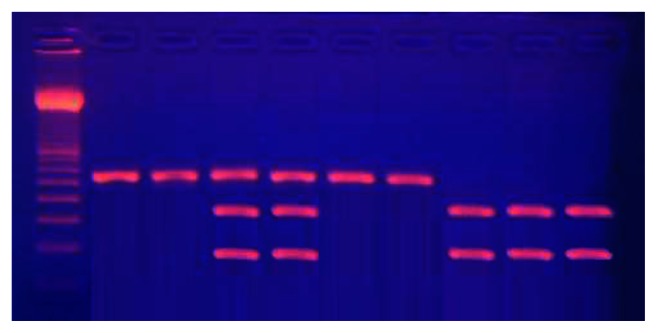
The G894T polymorphism was genotyped using PCR-RFLP using the Ban II digestive enzyme (New England Biolabs, Ipswich, MA). A guanine at nucleotide position 894 results in a glutamic acid at amino acid position 298, while a thymine at nucleotide position 894 results in aspartic acid at amino acid position 298. The PCR product was incubated with the restriction enzyme at 37°C for 4 hours (2 uL 10× buffer, 2.5 uL Ban II, 10 uL PCR product, and 5.5 uL DW). Ban II restriction enzyme produces two fragments of 163 bp and 85 bp in length (Figure 2).
Figure 2.
Electrophoresis of digested G894T polymorphism of eNOS gene poly-merase chain reaction products.
Notes: Lane 1: 50 bp ladder; Lanes 2, 3, 6, 7: GG genotype; Lanes 8, 9, 10: TT genotype; Lanes 4, 5: CT genotype.
Statistical analysis
Statistical analysis was made by IBM computer with the aid of Microsoft® Excel (Redmond, WA) and other statistical software programs including SPSS (v 11.5; IBM Corp, Armonk, NY), EpiCalc 2000 (Brixton Health, UK), and Epi Info™ 2000 (Centers for Disease Control and Prevention, Druid Hills, GA).
Qualitative data were expressed in the form of number and percentage and quantitative data were expressed as mean and standard deviation. The following statistical tests were used: chi-square test, a two-way and multiway test of significance to determine the association between two or more qualitative variables; the t-test, which assesses whether the means of two groups are statistically different from each other; and multivariate logistic regression analysis for the association of ITGA2 genotype and eNOS genotype with DR and the type of DR.
Results
In this study, there was no significant statistical difference between the DR group and the DWR group regarding age and sex distribution (P > 0.05) (Table 1).
Table 1.
Differences in the sex distribution, age of patients at the onset of diabetes mellitus (DM), and duration of DM between diabetic retinopathy (DR) and diabetic without retinopathy (DWR) patients
| Parameters | Studied diabetic patients (n = 70) | Test of significance | P value | |||
|---|---|---|---|---|---|---|
|
|
||||||
| DR group (n = 50) | DWR group (n = 20) | |||||
|
|
|
|||||
| n | % | n | % | |||
| Sex | ||||||
| Male | 32 | 64 | 13 | 65 | χ2 = 0.006 OR (95% CI) = 0.96 (0.32–2.83) | >0.05 |
| Female | 18 | 36 | 7 | 35 | ||
| Age of onset of DM in years (SD) | 57.0 ± 6.52 | 59.2 ± 4.28 | t = 1.37 | >0.05 | ||
| Duration of DM (SD) | 15.4 ± 7.23 | 14.4 ± 3.02 | t = 0.61 | >0.05 | ||
Abbreviations: χ2, chi-squared test; CI, confidence interval; OR, odds ratio; SD, standard deviation; t, Student’s t-test.
The age of patients ranged from 50 to 64 in the DR group and 54 to 64 in the DWR group. The mean value for DM duration was 15.4 ± 7.23 in the DR group and 14.4 ± 3.02 in the DWR group, so there was no significant statistical difference between the two groups (P > 0.05) (Table 1).
Concerning the medical history of the patients, there was no statistical difference between the DR group and DWR group in terms of family history of DM, the presence of hypertension, or smoking (P > 0.05), but there was significant statistical difference between the two groups regarding insulin therapy (P < 0.001) (Table 2).
Table 2.
Distribution of present, family, and medical histories between diabetic retinopathy (DR) and diabetic without retinopathy (DWR) patients
| Parameters | Studied diabetic patients (n = 70) | χ2 | OR (95% CI) | P value | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| DR group (n = 50) | DWR group (n = 20) | ||||||
|
|
|
||||||
| n | % | n | % | ||||
| Family history of DM | |||||||
| Present | 18 | 36 | 6 | 30 | 0.23 | 1.31 (0.43–4.01) | >0.05 |
| Absent | 32 | 64 | 14 | 70 | |||
| Hypertension | |||||||
| Present | 27 | 54 | 8 | 40 | 1.12 | 1.76 (0.61–5.05) | >0.05 |
| Absent | 23 | 46 | 12 | 60 | |||
| Smoking | |||||||
| Smoker | 18 | 36 | 4 | 20 | 1.70 | 2.25 (0.65–7.76) | >0.05 |
| Nonsmoker | 32 | 64 | 16 | 80 | |||
| Insulin therapy | |||||||
| Present | 31 | 62 | 4 | 20 | 10.08 | 6.53 (1.90–22.45) | <0.001 |
| Absent | 19 | 38 | 16 | 80 | |||
Abbreviations: χ2, chi-squared test; CI, confidence interval; OR, odds ratio; SD, standard deviation.
The biochemical laboratory profile showed that fasting blood sugar and HbA1c levels were significantly higher in the DR group compared with the DWR group (P < 0.0001). Concerning the lipid profile of the patients, only serum cholesterol and serum triglyceride levels were significantly higher in the DR group compared with the DWR group, while there was no significant statistical difference between the two groups regarding HDL and LDL levels (P > 0.05) (Table 3).
Table 3.
Differences in the biochemical laboratory profile between diabetic retinopathy (DR) and diabetic without retinopathy (DWR) groups
| Parameters | Studied diabetic patients (n = 70) | Student’s t-test | P value | |
|---|---|---|---|---|
|
|
||||
| DR group (n = 50) (SD) | DWR group (n = 20) (SD) | |||
| Fasting blood sugar level (mg/dL) | 156.9 ± 16.42 | 124.4 ± 7.09 | 8.52 | <0.0001 |
| HbA1c (%) | 7.8 ± 1.15 | 6.3 ± 0.79 | 5.32 | <0.0001 |
| Serum cholesterol (mg/dL) | 242.7 ± 40.8 | 215.8 ± 20.6 | 2.80 | <0.01 |
| Serum triglycerides (mg/dL) | 169.6 ± 17.89 | 147.8 ± 23.31 | 4.21 | <0.0001 |
| Serum HDL (mg/dL) | 33.7 ± 2.05 | 33.0 ± 1.00 | 1.56 | >0.05 |
| Serum LDL (mg/dL) | 174.9 ± 37.91 | 158.3 ± 26.27 | 1.80 | >0.05 |
Abbreviations: HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation.
For the BgI Π polymorphism of the ITGA2 gene, in the DR group 18 patients (36%) were +/+ genotype, 24 patients (48%) were +/− genotype, and eight patients (16%) were −/− genotype; in the DWR group, two patients (10%) were +/+ genotype, nine patients (45%) were +/− genotype, and nine patients (45%) were −/− genotype. There was significant statistical difference between the two groups concerning the distribution of genetic alleles, with a significantly higher frequency of the +/+ genotype in patients with DR. Increased risk for DR was observed in the +/+ genotype (odds ratio [OR] −10.1, 95% confidence interval [CI] 1.8–57.9) (Table 4).
Table 4.
Association of ITGA2 genotype and eNos genotype with diabetic retinopathy
| Parameters | Studied diabetic patients (n = 70) | χ2 | OR (95% CI) | P value | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| DR (n = 50) | DWR (n = 20) | ||||||
|
|
|
||||||
| n | % | n | % | ||||
| ITGA2 genotype | |||||||
| +/+ | 18 | 36 | 2 | 10 | 8.35 | 10.1 (1.8–57.9) | <0.01 |
| +/− | 24 | 48 | 9 | 45 | 3.0 (0.9–10.2) | ||
| −/− | 8 | 16 | 9 | 45 | – | ||
| eNOS genotype | |||||||
| GG | 13 | 26 | 2 | 10 | 13.63 | 9.75 (1.7–55.4) | <0.001 |
| GI | 29 | 58 | 6 | 30 | 7.25 (2.1–25.4) | ||
| TT | 8 | 16 | 12 | 60 | – | ||
Abbreviations: DWR, diabetics without retinopathy; OR, odds ratio; SD, standard deviation.
For the G894T polymorphism of the eNOS gene, in the DR group 13 patients (26%) were GG genotype, 29 patients (58%) were GT genotype, and eight patients (16%) were TT genotype; in the DWR group, two patients (10%) were GG genotype, six patients (30%) were GT genotype, and twelve patients (60%) were TT genotype. There was significant statistical difference between the two groups regarding allele distribution, with a predominance of the G-allele carried in the DR group compared with the DWR group (P < 0.001). Increased risk for DR was observed in the GG genotype (OR 9.75, 95% CI 1.7–55.4) (Table 4).
Multivariable logistic regression analysis for the relationship between DR and ITGA2 polymorphism and eNOS polymorphism (with adjustment for factors such as age at onset of diabetes, sex, duration of DM, presence of hypertension, family history of DM, and insulin therapy) showed that ITGA2 and eNOS polymorphisms have independent effects on the development of DR even after adjustment for all the previously mentioned factors (Table 5).
Table 5.
Multivariate logistic regression analysis for the association of ITGA2 genotype and eNOS genotype with dia-betic retinopathy (DR)
| Parameters | B-coefficient | SE | Odds ratio | P value |
|---|---|---|---|---|
| Age of onset of diabetes | 0.08 | 0.06 | 1.08 | 0.22 |
| Sex | −0.04 | 0.69 | 0.96 | 0.95 |
| Duration of DM | −0.04 | 0.06 | 0.96 | 0.51 |
| Hypertension | 0.88 | 0.68 | 2.41 | 0.20 |
| Family history of DM | 0.04 | 0.71 | 1.04 | 0.96 |
| Insulin therapy | 1.18 | 0.73 | 3.26 | 0.001 |
| (ITGA2) genotype | 0.77 | 0.51 | 2.16 | 0.004 |
| eNOS genotype | 0.66 | 0.54 | 1.93 | 0.001 |
| Constant | −11.24 | 4.58 | – | – |
Abbreviations: DM, diabetes mellitus; SE, standard error.
Based on the type of retinopathy, the DR group was subclassified into an NPDR group and a PDR group. Concerning patient characteristics, there was significant statistical difference regarding the age of onset of DM, with a younger age of onset in the PDR group (P < 0.005), while there was no significant statistical difference between the two groups regarding sex distribution. There were no significant statistical difference regarding duration of DM or the interval between the onset of DM and development of retinopathy (P > 0.05) (Table 6).
Table 6.
Differences in the sex distribution, age of patients at onset of diabetes mellitus (DM), duration of DM, and the interval between onset of DM and onset of retinopathy, between patients with proliferative and nonproliferative diabetic retinopathy (PDR and NPDR, respectively) groups
| Parameters | DR patients (n = 50) | Test of significance | P value | |||
|---|---|---|---|---|---|---|
|
|
||||||
| NPDR group (n = 25) (SD) | PDR group (n = 25) (SD) | |||||
|
|
|
|||||
| n | % | n | % | |||
| Sex | ||||||
| Male | 15 | 60 | 17 | 68 | χ2 = 0.35 | <0.005 |
| Female | 10 | 40 | 8 | 32 | OR (95% CI) = 0.71 (0.22–2.25) | |
| Age at onset of DM in years | 59.7 ± 5.41 | 54.2 ± 6.48 | t = 3.25 | >0.05 | ||
| Duration of DM | 15.5 ± 8.31 | 15.4 ± 6.14 | t = 0.06 | >0.05 | ||
| Interval between onset of DM and onset of retinopathy | 5.9 ± 2.42 | 5.5 ± 1.92 | t = 0.58 | >0.05 | ||
Abbreviations: χ2, chi-squared test; CI, confidence interval; OR, odds ratio; SD, standard deviation; t, Student’s t-test.
There was also no significant statistical difference between the NPDR group and the PDR group in terms of family history of DM, presence of hypertension, smoking, and insulin therapy (Table 7).
Table 7.
Distribution of present, family, and medical histories between patients with proliferative and nonproliferative diabetic retinopathy (PDR and NPDR, respectively)
| Parameters | DR patients (n = 50) | χ2 | OR (95% CI) | P value | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| NPDR (n = 25) | PDR (n = 25) | ||||||
|
|
|
||||||
| n | % | n | % | ||||
| Family history of DM | |||||||
| Present | 10 | 40 | 8 | 32 | 0.35 | 1.42 (0.44–4.52) | >0.05 |
| Absent | 15 | 60 | 17 | 68 | |||
| Hypertension | |||||||
| Present | 15 | 60 | 12 | 48 | 0.73 | 1.63 (0.53–4.98) | >0.05 |
| Absent | 10 | 40 | 13 | 52 | |||
| Smoking | |||||||
| Smoker | 7 | 28 | 11 | 44 | 1.39 | 0.49 (0.15–1.61) | >0.05 |
| Nonsmoker | 18 | 72 | 14 | 56 | |||
| Insulin therapy | |||||||
| Present | 13 | 52 | 18 | 72 | 2.12 | 0.42 (0.13–1.36) | >0.05 |
| Absent | 12 | 48 | 7 | 28 | |||
| Macular edema | |||||||
| Present | 12 | 48 | 12 | 48 | 0.08 | 1.00 (0.33–3.03) | >0.05 |
| Absent | 13 | 52 | 13 | 52 | |||
Abbreviations: χ2, chi-squared test; CI, confidence interval; DM, diabetes mellitus; DR, diabetic retinopathy; OR, odds ratio; SD, standard deviation.
The biochemical laboratory profile showed that fasting blood sugar and HbA1c levels did not differ significantly between the two groups (P > 0.05), while the lipid profile – including serum cholesterol, serum triglycerides, serum HDL-cholesterol, and serum LDL-cholesterol – was significantly higher in the PDR group (P < 0.0001) (Table 8).
Table 8.
Differences in the biochemical laboratory profile between patients with proliferative and nonproliferative diabetic retinopathy (PDR and NPDR, respectively)
| Parameters | DR patients (n = 50) | Student’s t-test | P value | |
|---|---|---|---|---|
|
|
||||
| NPDR (n = 25) (SD) | PDR (n = 25) (SD) | |||
| Fasting blood sugar level (mg/dL) | 161.2 ± 16.38 | 152.6 ± 15.62 | 1.89 | >0.05 |
| HbA1c (%) | 7.9 ± 1.39 | 7.6 ± 0.82 | 1.23 | >0.05 |
| Serum cholesterol (mg/dL) | 213.4 ± 18.85 | 272.0 ± 35.47 | 7.30 | <0.0001 |
| Serum triglycerides (mg/dL) | 158.7 ± 15.86 | 180.5 ± 12.41 | 5.41 | <0.0001 |
| Serum HDL (mg/dL) | 34.6 ± 2.16 | 32.8 ± 1.45 | 3.61 | <0.001 |
| Serum LDL (mg/dL) | 149.9 ± 22.63 | 199.9 ± 33.46 | 6.19 | <0.0001 |
Abbreviations: DM, diabetes mellitus; DR, diabetic retinopathy; HbA1c, hemoglobin A1c; SD, standard deviation.
For the BgI II polymorphism of the ITGA2 gene, in the NPDR group nine patients (36%) were +/+ genotype, eleven patients (44%) were +/− genotype, and five patients (20%) were −/− genotype. In the PDR group, nine patients (36%) were +/+ genotype, 13 patients (52%) were +/+ genotype, and three patients (12%) were −/− genotype. There was no significant statistical difference for BgI II polymorphism between the two groups (Table 9).
Table 9.
Association of integrin α2β1 genotype (ITGA2) and eNOS genotype with the type of diabetic retinopathy (DR)
| Parameters | DR patients (n = 50) | χ2 | OR (95% CI) | P value | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| NPDR (n = 25) | PDR (n = 25) | ||||||
|
|
|
||||||
| n | % | n | % | ||||
| ITGA2 genotype | |||||||
| +/+ | 9 | 36.0 | 9 | 36.0 | 0.67 | 0.60 (0.11–3.30) | >0.05 |
| +/− | 11 | 44.0 | 13 | 52.0 | 0.51 (0.10–2.62) | ||
| −/− | 5 | 20.0 | 3 | 12.0 | – | ||
| eNOS genotype | |||||||
| GG | 5 | 20.0 | 8 | 32.0 | 1.23 | 0.38 (0.06–2.30) | >0.05 |
| GT | 15 | 60.0 | 14 | 56.0 | 0.64 (0.13–3.20) | ||
| TT | 5 | 20.0 | 3 | 12.0 | – | ||
Abbreviations: χ2, chi-squared test; CI, confidence interval; NPDR, nonproliferative diabetic retinopathy; OR, odds ratio; PDR, proliferative diabetic retinopathy.
For the G894T polymorphism of the eNOS gene, in the NPDR group five patients (20%) were GG genotype, 15 patients (60%) were GT genotype, and five patients (20%) were TT genotype. In the PDR group, eight patients (32%) were GG genotype, 14 patients (56%) were GT genotype, and three patients (12%) were TT genotype. There was no statistical difference concerning G894T polymorphism between the two groups (Table 9).
Multivariable logistic regression analysis for the relationship between the type of retinopathy, whether proliferative or nonproliferative, and ITGA2 and eNOS polymorphism after adjustment for age at onset of DM, sex, duration of DM, gap between onset of DM and development at retinopathy, the presence of hypertension, family history of DM, and insulin therapy, showed that ITGA2 and eNOS polymorphisms still have no effect on the type of retinopathy (Table 10).
Table 10.
Multivariate logistic regression analysis for the association of ITGA2 genotype and eNOS genotype with the type of diabetic retinopathy
| Parameters | B-coefficient | SE | Odds ratio | P value |
|---|---|---|---|---|
| Age of onset of diabetes | −0.16 | 0.06 | 0.86 | 0.01 |
| Sex | −0.33 | 0.74 | 0.72 | 0.66 |
| Duration of DM | 0.01 | 0.07 | 1.01 | 0.93 |
| Gap between onset of DM and retinopathy | −0.08 | 0.21 | 0.92 | 0.70 |
| Hypertension | 0.56 | 0.83 | 1.75 | 0.50 |
| Family history of DM | −0.07 | 0.74 | 0.94 | 0.93 |
| Insulin therapy | −0.27 | 0.75 | 0.77 | 0.72 |
| (ITGA2) genotype | −0.09 | 0.53 | 0.92 | 0.87 |
| eNOS genotype | −0.11 | 0.56 | 0.90 | 0.85 |
Abbreviations: DM, diabetes mellitus; SE, standard error.
Discussion
DM is a worldwide medical problem and is a significant cause of morbidity. Microvascular and macrovascular complications are highly prevalent among diabetic patients.22
DR, one of the most significant complications of diabetes, has become the leading cause of vision loss and blindness in both developed and developing countries.23 Visual loss results mainly from central macular edema and less frequently from PDR. The development of these pathological changes is strongly related to hyperglycemia.24
Although the Diabetes Control and Complications Trial showed that tight control of hyperglycemia can reduce the incidence of retinopathy, it is clear that hyperglycemia alone does not explain the development of this complication, as it may be absent in some patients with poor glycemic control even over a long period, while others may develop retinopathy in a relatively short period despite good glycemic control.25 These observations suggest the presence of hereditary factors. The strongest evidence for the genetic predisposition towards DR has been derived from twin, family, and transracial studies.26
The search for DR genes has predominantly been undertaken using the candidate gene approach. Several pathways and processes have been strongly implicated, including the renin-angiotensin system, the polyol pathway, nonenzymatic glycation, endothelial dysfunction, vascular tone maintenance, extracellular matrix remodeling, and angiogenesis, which is dysregulated in diabetes leading to proliferation of new fragile capillaries that culminate in PDR.27
Correspondingly, host genes involved in these pathway processes have been treated as potential candidate genes. These genes include the angiotensin-converting enzyme, vascular endothelial growth factor (VEGF), aldose reductase, the receptor for advanced glycation end products, glucose transporter-1, inducible and constitutive NO synthases (NOS2A, NOSB), transforming growth factor beta, and endothelin isoforms and their cellular receptors.26
NO, synthesized continuously in the endothelium from L-arginine by eNOS, plays an important role in maintaining basal vascular tone through its effect on the soluble guanylate cyclase signaling pathway.7 It also inhibits platelet as well as leukocyte adhesion to vascular endothelium and inhibits proliferation of smooth muscle cells via guanylate cyclase-independent mechanisms.27 It has been demonstrated, in vivo and in vitro, that overproduction of NO may induce oxidative stress in retinal, endothelial, and glomerular cells.7 Also, it has been experimentally shown that NO contributes to angiogenic properties of VEGF, a molecule implicated in the development of proliferative retinopathy.3
Since platelets are essential for hemostasis, and abnormalities of platelet function may cause vascular abnormalities, platelets are thought to be involved in the pathogenesis of microvascular complications in diabetes.8 Platelets from diabetic patients are hyperreactive to aggregating agents such as collagen, thrombin, and adenosine diphasphate. The platelet membrane glycoprotein Ia/IIa, α2β1 integrin, serves as a platelet receptor for collagen.20 It mediates platelet primary adhesion to subendothelial tissues, which is an essential step in thrombus formation.
To examine the hypothesis that genetic variation of α2β1 integrin is associated with the development of diabetic microangiopathy, the association between the BgI II polymorphism and development of DR among T2D patients was also investigated in this study. There was no significant statistical difference between the DR group and the DWR group regarding the duration of DM and that duration did not statistically affect the type of retinopathy. These findings are in contrast to Roy’s28 and Longo-Mbenza et al’s,29 who found that the frequency and severity of DR were significantly associated with longer duration of diabetes.
Results of the present study showed that there is significant statistical difference between the NPDR and PDR groups concerning the age of onset of DM, with younger age of onset (54.52 ± 6.48 years) for PDR compared with NPDR (59.7 ± 5.41 years). This is in agreement with Li et al,6 who showed that subjects who were younger at DM onset had a higher risk of PDR than subjects with later onset of DM.
Further, the present study showed that fasting blood sugar and HbA1c levels were significantly higher in the DR group compared with the DWR group; however, those levels did not differ between NPDR group PDR group, which is in agreement with the findings of Lecaire et al,30 Axer-Siegel et al,31 Higgins et al,32 and Longo-Mbenza et al,29 who all observed higher levels of blood glucose and HbA1c in diabetic patients with DR compared with those without DR.
The results of this study also showed that only serum cholesterol and triglyceride levels were significantly higher in the DR group compared with the DWR group; there were no significant statistical differences between the groups regarding HDL and LDL levels. Regarding type of retinopathy, all elements of the lipid profile, including serum cholesterol, triglycerides, HDL, and LDL, were significantly higher in the PDR group. Lyons et al33 found that there was no significant association between plasma triglyceride and cholesterol levels between their DR group and patients without retinopathy, while there was a significant positive association with LDL and significant negative association with HDL. Rianta et al34 found significant association between lipid profile and DR but no significant association between lipid profile and the severity of the disease.
In the present study, for the G894T polymorphism of the eNOS gene, there was a significant statistical difference between the DR and DWR groups regarding the genotype distribution, with a predominance of G-allele carriers in the DR group. Increased risk for DR was observed in patients with GG genotype (OR 9.75, 95% CI 1.7–55.4). However, there was no statistical difference concerning the genotypes of the polymorphism between the PDR and NPDR groups. This is in agreement with Chen et al,7 who found that the GG genotype is associated with increased risk for DR. Suganthalakshmi et al3 found that the genotypes of eNOS did not differ significantly between their DR and DWR study groups. However, it was significantly associated with the severity of DR in Caucasian populations. Awata et al10 found that the eNOS polymorphism was not associated with the presence of DR nor the type of DR, whether PDR or NPDR. Rippin et al35 found no association between the eNOS polymorphism and the development of diabetic microvascular complications in UK Caucasian populations. Li et al6 showed no association between the eNOS polymorphism and the occurrence or the type of DR. Miyamoto et al21 found limited information about whether this missense mutation gives rise to functional alteration of eNOS enzymatic activity or a genetic marker associated with some causal loci. Thus, they investigated the Glu298Asp variants and did not find them to be located in any functional consensus sequence. However, computer analysis revealed that the Glu298Asp mutation results in a conformational change in the eNOS protein from helix to tight turn.
The eNOS protein is a member of cytochrome P450 reductase and is composed of a heme-binding region, a Ca/calmodulin binding region, and nicotinamide adenine dinucleotide phosphate-cytochrome P450 oxidoreductase. The Glu298Asp variant in exon 7 is located in the intermediate portion of the heme-binding and Ca/calmodulin binding sites, thus alters the activity of the enzyme.36
Du et al37 have demonstrated that in patients with DR, the levels of NO in plasma, vitreal fluid, and aqueous fluid are increased. However, although eNOS was localized in Müller cells and in the vascular endothelium of the retina, both in vivo and in vitro studies revealed that the level of the constitutively expressed eNOS was reduced in retinal vascular endothelial cells when diabetes was present.22 It was suggested that both high glucose levels and osmotic stress in the retinal endothelial cells of diabetics may increase inducible NOS activity and generate an uncoupled eNOS, resulting in the overproduction of NO and, subsequently, its derivatives as peroxynitrite.7 Excess amounts of peroxynitrite result in oxidative stress and generate cytotoxic effects by increasing DNA damage, stimulating lipid peroxidation, and depleting glutathione levels.38 Furthermore, NO is known to increase vascular permeability and angiogenesis, a major feature of advanced DR.39 Thus, it was thought that in diabetics, high glucose levels and osmotic stress may directly cause downregulated expression of eNOS in subjects with the GG genotype, which in turn activates an uncoupled overproduction of NO and its high reactive oxidants. However, in subjects with the AA genotype, NO is produced mainly from the constitutively expressed eNOS with uncoupled expression relatively suppressed.7
Several pathological pathways could contribute to the development of DR. Decreased activity of eNOS will lead to deficiency of endothelial-derived NO, coupled with concomitant activation of inducible NOS, which can produce a large amount of NO. This NO, when encountering reactive oxygen species, can generate highly reactive oxygen species, leading to oxidative stress in retinal tissues.40 A second pathway is that decreased activity of eNOS increases expression of glial fibrillary acidic protein in Müller cells in diabetic retina. Such upregulation of glial fibrillary acidic protein is an early event in the pathogenesis of retinopathy and precedes the onset of vascular changes in the disease.41 A third pathway involves the observation that the G894T polymorphism is associated with the development of essential hypertension, an important independent risk factor for both initial development and subsequent progression of DR.42 Lastly, hypoxia-induced angiogenesis is an important pathology in DR. A number of factors are expressed in hypoxic conditions and have been shown to trigger the hypoxia response elements of the VEGF gene, bringing about overexpression of VEGF that leads to neovascularization. eNOS is one such important part of hypoxia events. Knockout animal models of eNOS have shown poor angiogenesis, suggesting the contribution of eNOS to DR pathology.43
In this study, regarding the BgI II polymorphism of the ITGA2 gene, there was a significant statistical difference between the DR and DWR groups in the distribution of genetic alleles, with a significantly higher frequency of the genotype +/+ in patients with DR. Increased risk of DR was observed in BgI II+ allele carriers (OR 10.1, 95% CI 1.8–57.9), while in terms of the type of retinopathy, there was no significant statistical difference between the NPDR and PDR groups regarding genotype distribution. Matsubara et al44 stated that the BgI II polymorphism is associated with the prevalence of retinopathy among patients with T2D and suggested that BgI II(+)-containing platelets can more easily interact with nonenzymatically glycosylated collagen, which is higher in diabetic patients, enhancing microthrombus formation and small vessel occlusion and thus accelerating the occurrence of retinopathy. Therefore, BgI II(+) allele carriers might benefit more from antiplatelet therapies. Petrovic et al20 also provided evidence for the association of BgI II(+/+) genotype and risk for DR in a group of Caucasians with T2D; this was in contrast to Li et al,6 who found no significant difference between the DR and DWR groups regarding genotype or allele distribution of ITGA2 polymorphism.
Conclusion
This study observed a significant association between the G894T polymorphism of eNOS gene and the BgI II polymorphism of ITGA2 gene and DR, while there was no association between the genetic variants of those two polymorphisms and the type of retinopathy.
Identification of genetic variations contributing to DR disease pathogenesis would have tremendous implications in terms of clinical intervention and management of the disease. A number of inhibitors to key molecules that are involved in detrimental pathways of DR, such as anti-VEGF, antiplatelet, anti-protein kinase C, and anti-insulin-like growth factor, are under research, so specific management of the disease will depend upon each patient’s genetic and environmental profile rather than generalized treatment of laser photocoagulation.38
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Abhary S, Burdank, Gupta A, Petrovsky N, Graig J. Diabetic retinopathy is not associated with carbonic anhydrase gene polymorphism. Mol Vis. 2009;15:1179–1184. [PMC free article] [PubMed] [Google Scholar]
- 2.Hietala K, Forsblom C, Summanen P, Groop PH FinnDiane Study Group. Heritability of proliferative diabetic retinopathy. Diabetes. 2008;57(8):2176–2180. doi: 10.2337/db07-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suganthalakshmi B, Anand R, Kim R, et al. Association of VEGF and eNOS gene polymorphisms in type 2 diabetic retinopathy. Mol Vis. 2006;12:336–341. [PubMed] [Google Scholar]
- 4.Balasubbu S, Sundaresan P, Rayendran A, et al. Association analysis of nine candidate gene polymorphism in Indian patients with type 2 diabetic retinopathy. BMC Med Genet. 2010;11:158. doi: 10.1186/1471-2350-11-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warpeha KM, Chakravarthy U. Molecular genetics of microvascular disease in diabetic retinopathy. Eye (Lond) 2003;17(3):305–311. doi: 10.1038/sj.eye.6700348. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Louey JW, Choy KW, et al. EDN1 Lys198Asn is associated with diabetic retinopathy in type 2 diabetes. Mol Vis. 2008;14:1698–1704. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Huang H, Zhou J, et al. Polymorphism of the endothelial nitric oxide synthase gene is associated with diabetic retinopathy in a cohort of West Africans. Mol Vis. 2007;13:2142–2147. [PubMed] [Google Scholar]
- 8.Stratmann B, Tschoepe D. Pathobiology and cell interactions of platelets in diabetes. Diab Vasc Dis Res. 2005;2(1):16–23. doi: 10.3132/dvdr.2005.001. [DOI] [PubMed] [Google Scholar]
- 9.Pavkovic M, Petlichkovski A, Stojanovic A, Trajkov D, Spiroski M. BgI II polymorphism of the α2β1 integrin gene in Macedonian population. Maced J Med Sci. 2010;3(2):119–122. [Google Scholar]
- 10.Awata T, Neda T, Iizuka H, et al. Endothelial nitric oxide synthases gene is associated with diabetic macular edema in type 2 diabetes. Diabetes Care. 2004;27(9):2184–2190. doi: 10.2337/diacare.27.9.2184. [DOI] [PubMed] [Google Scholar]
- 11.Szabó GV, Kunstár A, Acsády G. Methylentetrahydrofolate reductase and nitric oxide synthase polymorphism in patients with atherosclerosis and diabetes. Pathol Oncol Res. 2009;15(4):631–637. doi: 10.1007/s12253-009-9163-z. [DOI] [PubMed] [Google Scholar]
- 12.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs: an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98(Suppl 5):786–806. [PubMed] [Google Scholar]
- 13.Burtis E, Santos-Rosa M, Bienvenu J, Whicher J. Role of the clinical laboratory in diabetes mellitus. In: Bruit C, Ashwood E, editors. Tietz Textbook of Clinical Chemistry. 4th ed. St Louis, MO: Mosby; 2006. pp. 837–903. [Google Scholar]
- 14.Gonen B, Rubenstein AH. Determination of glycohemoglobin. Diabetologia. 1978;15:1–5. doi: 10.1007/BF01219319. [DOI] [PubMed] [Google Scholar]
- 15.Meiattini F, Prencipe L, Bardelli F, Giannini G, Tarli P. The 4- hydroxybenzoate/4-aminophenazone chromogenic system used in the enzymic determination of serum cholesterol. Clin Chem. 1978;24(12):2161–2165. [PubMed] [Google Scholar]
- 16.Fossati P, Lorenzo P. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28:2077–2080. [PubMed] [Google Scholar]
- 17.Rifai N, Warnick R. Lipids, lipoproteins apolipoproteins and other cardiovascular risk factors. In: Carl AB, Edward RA, David EB, editors. Tietz Textbook of Clinical Chemistry and Molecular Diagnosis. 4th edition. Ch 26. Saunders; 2006. pp. 918–922. [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 19.Sirchia G, Pizzi C, Scalamogna M. A simple procedure for human lymphocyte isolation for peripheral blood. Tissue Antigens. 1972;2(2):139–140. doi: 10.1111/j.1399-0039.1972.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 20.Petrovic MG, Hawlina M, Peterlin B, Petrovic D. Bg III gene polymorphism of the alpha2beta1 integrin gene is a risk factor for diabetic retinopathy in Caucasians with type 2 diabetes. J Hum Genet. 2003;48(9):457–460. doi: 10.1007/s10038-003-0060-0. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto Y, Saito Y, Kajiyama N, et al. Endothelial nitric oxide synthase gene is positively associated with essential hypertension. Hypertension. 1998;32(1):3–8. doi: 10.1161/01.hyp.32.1.3. [DOI] [PubMed] [Google Scholar]
- 22.Juan Li, Yong-Hua Hu. Susceptibility genes for diabetic retinopathy. Int J Opthalmol. 2009;2(1):1–6. [Google Scholar]
- 23.Keech AC, Mitchell P, Summanen PA, et al. FIELD study investigators. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370(9600):1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 24.Raman R, Gupta A. Absence of diabetic retinopathy in patient who has had diabetes mellitus for 69 years and inadequate glycemic control: case presentation: response. Diabetol Metab Syndr. 2010;2:20. doi: 10.1186/1758-5996-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Diabetes Control and Complications Trial (DCCT) The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes. 1996;45:1289–1298. [PubMed] [Google Scholar]
- 26.Ng DP. Human genetics of diabetic retinopathy: current perspectives. J Ophthalmol. 2010;2010 doi: 10.1155/2010/172593. pii:172593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dias RG, Alves M, Pereira A, et al. Glu298Asp eNOS gene polymorphism causes attenuation in nonexercising muscle vasodilatation. Physiol Genomics. 2009;37(2):99–107. doi: 10.1152/physiolgenomics.90368.2008. [DOI] [PubMed] [Google Scholar]
- 28.Roy MS. Diabetic retinopathy in African Americans with type 1 diabetes: the New Jersey 715: II. Risk factors. Arch Opthalmol. 2000;118(1):105–119. doi: 10.1001/archopht.118.1.105. [DOI] [PubMed] [Google Scholar]
- 29.Longo-Mbenza B, Muaka M, Mbenza G, et al. Risk factors of poor control of HbA1c and diabetic retinopathy: paradox with insulin therapy and high values of HDL in African diabetic patients. Int J Diabetes and Metabolism. 2008;16:69–78. [Google Scholar]
- 30.Lecaire T, Palta M, Zhang H, Allen C, Klein R, D’Alessio D. Lower-than-expected prevalence and severity of retinopathy in an incident cohort followed during the first 4–14 years of type 1 diabetes: the Wisconsin Diabetes Registry Study. Am J Epidemiol. 2006;164(2):143–150. doi: 10.1093/aje/kwj166. [DOI] [PubMed] [Google Scholar]
- 31.Axer-Siegel R, Herscovici Z, Gabbay M, Mimouni K, Weinberger D, Gabbay U. The relationship between diabetic retinopathy, glycemic control, risk factors indicators and patient education. Isr Med Assoc J. 2006;8(8):523–526. [PubMed] [Google Scholar]
- 32.Higgins GT, Khan J, Pearce IA. Glycaemic control and control of risk factors in diabetes patients in an ophthalmology clinic: what lessons have we learned from the UKPDS and DCCT studies? Acta Ophthalmol Scand. 2007;85(7):772–776. doi: 10.1111/j.1600-0420.2007.00944.x. [DOI] [PubMed] [Google Scholar]
- 33.Lyons TJ, Jenkins AJ, Zheng D, et al. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest Ophthalmol Vis Sci. 2004;45(3):910–918. doi: 10.1167/iovs.02-0648. [DOI] [PubMed] [Google Scholar]
- 34.Rianta R, Bardosono S, Victor A. Relationship between plasma lipid profile and the severity of diabetic retinopathy in type 2 diabetic patients. Medical Journal of Indonesia. 2008;17(4):221–225. [Google Scholar]
- 35.Rippin JD, Patel A, Belyaev ND, Gill GV, Barnett AH, Bain SC. Nitric oxide synthase gene polymorphisms and diabetic nephropathy. Diabetologia. 2003;46(3):426–428. doi: 10.1007/s00125-003-1046-3. [DOI] [PubMed] [Google Scholar]
- 36.Ohtoshi K, Yamasaki Y, Gorogawa S, et al. Association of (-)786T-C mutation of endothelial nitric oxide synthase gene with insulin resistance. Diabetologia. 2008;45(11):1594–1601. doi: 10.1007/s00125-002-0922-6. [DOI] [PubMed] [Google Scholar]
- 37.Du Y, Smith M, Miller CM, Kern TS. Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J Neurochem. 2002;80(5):771–779. doi: 10.1046/j.0022-3042.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 38.Serrano NC, Díaz LA, Casas JP, Hingorani AD, Moreno-De-Lucca D, Páez MS. Frequency of eNOS polymorphisms in the Colombian general population. BMC Genet. 2010;11:54–61. doi: 10.1186/1471-2156-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izumi N, Nagaoka T, Mori F, Sato E, Takahashi A, Yoshida A. Relation between plasma nitric oxide levels and diabetic retinopathy. Jpn J Ophthalmol. 2006;50(5):465–468. doi: 10.1007/s10384-006-0344-y. [DOI] [PubMed] [Google Scholar]
- 40.Liu VW, Huang PL. Cardiovascular roles of nitric oxide: a review of insights of nitric oxide synthase gene disrupted mice. Cardiovasc Res. 2008;77(1):19–29. doi: 10.1016/j.cardiores.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Q, Verma A, Han PY, et al. Diabetic eNOS-knockout mice develop accelerated retinopathy. Invest Ophthalmol Vis Sci. 2010;51(10):5240–5246. doi: 10.1167/iovs.09-5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007;369(9559):425–435. doi: 10.1016/S0140-6736(07)60198-6. [DOI] [PubMed] [Google Scholar]
- 43.Uthra S, Raman R, Mukesh BN, et al. Genetics of diabetic retinopathy. Int J Hum Genet. 2008;8(1–2):155–159. [Google Scholar]
- 44.Matsubara Y, Murata M, Maruyama T, et al. Association between diabetic retinopathy and genetic variations in alpha2beta1 integrin, a platelet receptor for collagen. Blood. 2000;95(5):1560–1564. [PubMed] [Google Scholar]
- 45.Pulkkinen A, Viitanen L, Kareinen A, Lehto S, Vauhkonen I, Laakso M. Intron 4 polymorphism of the endothelial nitric oxide synthase gene is associated with elevated blood pressure in type 2 diabetic patients with coronary heart disease. J Mol Med (Berl) 2000;78(7):372–379. doi: 10.1007/s001090000124. [DOI] [PubMed] [Google Scholar]
- 46.Buraczynska M, Ksiazek P, Zaluska W, Nowicka T, Ksiazek A. Endothelial nitric oxide synthase gene intron 4 polymorphism in patients with end-stage renal disease. Nephrol Dial Transplant. 2004;19:2302–2306. doi: 10.1093/ndt/gfh077. [DOI] [PubMed] [Google Scholar]
- 47.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]