Abstract
Background
Vascular α2B-adrenoreceptors have the potential to increase blood pressure by mediating vasoconstriction. A nine-nucleotide deletion in the receptor enhances vasoconstriction and exacerbates hypertension. The aim of this study was to determine the association between insertion/deletion (I/D) polymorphism of the α2B-adrenoceptor and hypertension with and without diabetes.
Methods
The study was carried out in 35 hypertensive patients with diabetes, 35 hypertensive patients without diabetes, and 30 healthy controls. Clinical data, blood lipid profiles, and I/D polymorphism were assessed.
Results
Hypertensive patients were significantly older, with significantly higher systolic/diastolic blood pressures and worse plasma lipid profiles than controls. The frequency of the DD genotype was significantly higher in both hypertensive patients with (77.14%, P < 0.01) and without (71.43%, P < 0.05) diabetes versus controls (40%). Also, the D allele was significantly more common in both hypertensive patients with (84.29%, P < 0.01) and without (80%, P < 0.05) diabetes versus controls (58.33%). Hypertensive patients were more likely to have the D allele with (3.83-fold) and without (2.85-fold) diabetes. The frequencies of the DD genotype and the D allele were not significantly (P > 0.05) different between the patient groups. The DD genotype was associated with significantly lower high-density lipoprotein (P = 0.001) and significantly higher low-density lipoprotein (P = 0.017) levels versus the II and ID genotypes in the hypertensive group without diabetes.
Conclusion
A marked and statistically significant association between DD genotype and D allele of I/D polymorphism in the α2B-adrenoceptor gene may be a risk factor for hypertension ± diabetes. The association between the DD genotype and dyslipidemia may partially explain its role in precipitating hypertension.
Keywords: insertion/deletion polymorphism, α2B adrenoceptor gene, hypertension, type 2 diabetes mellitus
Introduction
Hypertension is a greater burden at the population level in economically developing rather than developed countries.1 It has been identified as the leading risk factor for mortality, and is ranked third as a cause of disability-adjusted life-years.2 Data from the National Health and Nutrition Examination Survey for 2005–2006 found that 29% of US adults aged ≥18 years were hypertensive.3,4 Data on 6671 individuals from the 2008 Egyptian Demographic and Health Survey found that the overall prevalence of prehypertension and hypertension in Egypt was 57.2% and 17.6%, respectively. Only 25.2% of the population had normal blood pressure < 120/80 mmHg.5 The highest prevalence of hypertension was found in the Ismailia, Alexandria, Menya, Menoufia, and Luxor governorates.5
Primary hypertension in humans is likely to be of a complex nature, arising from environmental and genetic factors.6 The alpha2-adrenergic receptor (α2-AR) is widely expressed within the central and peripheral nervous systems. It mediates diverse physiological functions of the sympathetic nervous system, and is also involved in the pathogenesis of cardiovascular disease and modulation of pain.7,8
Three distinct α2-AR subtypes, ie, α2A, α2B, and α2C, that mediate many of the physiological actions of the catecholamines, epinephrine and norepinephrine, have been described.9,10 They belong to the family of G protein-coupled receptors and are linked to the inhibitory G proteins.9,10 The α2-ARs mediate a wide variety of functions, including regulation of blood pressure, sympathetic tone, lipolysis, and insulin secretion.11,12
Studies have suggested that sympathetic outflow from the central nervous system is inhibited by stimulation of α2-AR, thus mediating a hypotensive effect, whereas stimulation of the α2B-AR mediates a hypertensive effect by opposing sympathetic inhibition by α2A-AR in the central nervous system. The α2C-AR does not seem to have any effect in the regulation of blood pressure.6
All three α2-AR subtypes are expressed in both the exocrine and endocrine cells of the human pancreas, including beta cells.13 Insertion/deletion (I/D) polymorphism in the α2B-AR was reported to be associated with impaired beta cell function in a group of Finnish subjects with impaired glucose tolerance. Interestingly, this genetic polymorphism may also predispose its carriers to type 2 diabetes.14
The α2B-AR is critically involved in cardiovascular regulation, because disruption of its genes in mice affects blood pressure responses to α2-AR agonists, eg, clonidine.9,15 The α2B-AR gene is located on chromosome 2 in a region where several genome scans16 have found linkage with blood pressure variation and hypertension.17–19 In the third intracellular loop of the receptor, in an area of importance in downregulation, there is a polymorphism consisting of either an insertion or a deletion of three glutamate amino acids at positions 301–303.6,20
The 301–303 deletion variant is phosphorylated only half as efficiently and fails to undergo homologous desensitization. This indicates that the α2B-AR 301–303 deletion variant might increase long-term receptor signaling (ie, induce vasoconstriction) by preventing normal agonist-mediated desensitization.21 Earlier studies showed a conflicting association of the α2B-AR deletion allele with hypertension. The aim of this study was to determine if I/D α2B-AR polymorphism is a risk factor for hypertension, and if type 2 diabetes mellitus augments this association in a sample of Egyptian patients.
Materials and methods
Subjects
The patients in this study were selected from the Hypertension Clinic, Department of Cardiology, Menoufiya University Hospital. A full history and a general and clinical examination was performed prior to selection. Ethical approval for this investigation was obtained from the research ethics committee at the Faculty of Medicine, Menoufiya University.
The study included a total of 35 hypertensive subjects with type 2 diabetes mellitus, 35 hypertensive subjects without diabetes, and 30 healthy individuals. Exclusion criteria were familial hypercholesterolemia, cancer, renal disease, and any other chronic illness.
Essential hypertension was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg, or current antihypertensive therapy.22 Resting blood pressure was measured in the right arm using a sphygmomanometer.
Type 2 diabetes mellitus was defined as fasting plasma glucose ≥ 126 mg/dL (≥7.0 mmol/L) or symptoms of hyperglycemia and a random plasma glucose ≥ 200 mg/dL (≥11 mmol/L).23 Thirty healthy individuals were selected from volunteers with a negative history of hypertension and diabetes, with a resting systolic blood pressure ≤ 120 mmHg and diastolic blood pressure ≤ 80 mmHg on at least two separate occasions and not receiving any medications. The subjects were divided into group 1 (hypertension with diabetes), group 2 (hypertension without diabetes), and group 3 (control subjects).
Analysis of lipid profiles
A 5 mL sample of venous blood was taken from each patient after an overnight fast for determination of total serum cholesterol, triglycerides, and high-density lipoprotein (HDL) cholesterol levels. Lipid profiles were measured using standard enzymatic colorimetric kits (Spinreact, La Vall D’En Bas, Spain). Serum low-density lipoprotein (LDL) cholesterol was calculated by this formula as triglyceride levels not exceed 400 mg/dL:
| 24 |
DNA analysis
A 5 mL sample of venous blood was collected slowly into an evacuated tube containing ethylenediamine tetra-acetic acid for isolation of peripheral blood mononuclear cells using Lymphoflot solution (Bio Test AG, Dreieich, Germany). Briefly, a 5 mL sample of the patient’s blood was added to an equal volume of saline and mixed carefully. This diluted blood sample was carefully layered onto the Lymphoflot solution so as not to mix the Lymphoflot solution and the diluted blood sample. The mixture was centrifuged at 1500 rpm for 25 minutes at 20°C. The upper plasma layer was drawn off, leaving the lymphocyte layer undisturbed at the interface. The lymphocyte layer was transferred into a clean centrifuge tube containing 4 mL of balanced salt solution and mixed gently, then centrifuged at 1500 rpm for 10 minutes at 4°C. The supernatant was discarded, after which 1 mL of phosphate-buffered saline was added to the lymphocyte pellet, and transferred to a clean CryoTube™ by pipette and stored at −80°C for further DNA extraction and purification.25
Genomic DNA was extracted from peripheral blood mononuclear cells using QIAamp DNA blood mini kits (Qiagen Hilden, Qiagen, Valencia, CA), to yield pure DNA and stored at −20°C for direct amplification. I/D mutation of α2B-AR was detected using the polymerase chain reaction (PCR) method, as previously described.26
DNA was amplified using a forward primer, 5′-AGGGTGTTTGTGGGGCATCT-3′ and a reverse primer, 5′-CAAGCTGAGGCCGGAGACACT-3′ (Midland Certified Reagent Co, Midland, TX). A total volume of 25 μL reaction mixture containing 20 pmol of each primer, 0.4 mmol/L of dNTP, 2 mmol/L of MgCl2, 1 × Taq buffer, 100 mL/L of dimethyl sulfoxide, one unit of Taq DNA polymerase (New England Biolabs, Beverly, MA), and the template DNA was used for amplification of I/D polymorphism in the ADRA2B gene using a 2400 thermal cycler (Perkin Elmer, Boston, MA). The I/D mutation of α2B-AR was shown clearly using a protocol of initial denaturation for 10 minutes at 95°C, denaturation for one minute at 94°C, annealing for 2 minutes at 66°C, extension for one minute at 72°C for 35 cycles, and final extension for 10 minutes at 72°C.
I/D mutation of α2B-AR was detected in the PCR bands in 3% agarose gel electrophoresis and visualized under ultraviolet light. I/D mutation of α2B-AR PCR bands appeared as DD at 103 base pairs, II at 112 base pairs, and ID at both 112 base pairs and 103 base pairs26 (Figure 1).
Figure 1.
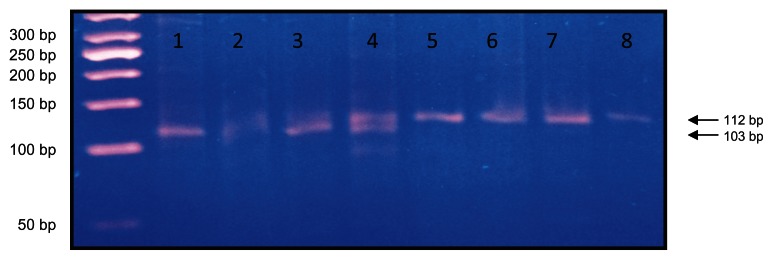
Lane 1 shows DD genotypes at 103 base pairs, lanes 2–4 show ID genotype at 103 and 112 base pairs, and lanes 5–8 show II genotypes at 112 base pairs using a 50-base pair ladder.
Statistical analysis
The results were statistically analyzed using the Statistical Package for the Social Sciences version 14 (SPSS Inc, Chicago, IL). Two types of statistics were included, ie, descriptive statistics (percentage, mean, and standard deviation) and analytic statistics, whereby genotypes and allelic frequencies of I/D polymorphism of the α2B-AR were compared between hypertensive patients with and without diabetes and controls using the Chi-square (χ2), Mann-Whitney, and Kruskal-Wallis tests. All odds ratios involving genotypes and alleles were calculated. A two-tailed Student’s t-test was used to compare quantitative data. Statistical significance was considered to be statistically significant at the P < 0.05 value.
Results
Our results showed significantly higher age (P < 0.05), systolic and diastolic blood pressure (P < 0.001), total cholesterol (P < 0.01), triglycerides (P < 0.01), and LDL cholesterol (P < 0.05), and lower HDL cholesterol (P < 0.01) in hypertensive patients with diabetes versus controls (Table 1). There was no significant difference in gender distribution or smoking status (P > 0.05) between hypertensive patients with diabetes and controls. There was a significant higher age (P < 0.01), systolic and diastolic blood pressure (P < 0.001), proportion of males (P < 0.05), and LDL cholesterol (P < 0.05), and lower HDL cholesterol (P < 0.001) in hypertensive patients without diabetes versus controls, with no statistically significant differences between the two groups for smoking (P > 0.05), total cholesterol (P > 0.05), or triglycerides (P > 0.05, Table 1). Significantly higher triglyceride levels (P < 0.05) were found in hypertensive patients with diabetes than in those without diabetes, but there was no significant difference in any other parameters between these two groups (Table 1).
Table 1.
Demographic and clinical characteristics of hypertensive patients with and without diabetes and controls
| Hypertension with diabetes (n = 35) | Hypertension without diabetes (n = 35) | Controls (n = 30) | P value | |
|---|---|---|---|---|
| Age (years), mean ± SD | 56.80 ± 8.46 | 57.27 ± 5.78 | 47.70 ± 7.61 | <0.05* <0.01* >0.05*** |
| Systolic pressure (mmHg) | 144.67 ± 5.16 | 144.67 ± 5.16 | 118.00 ± 7.88 | <0.001*,** >0.05*** |
| Diastolic pressure (mmHg) | 96.00 ± 8.28 | 96.67 ± 8.16 | 72.50 ± 6.77 | <0.001*,** >0.05*** |
| Gender, n (%) | ||||
| Male | 19 (54.29%) | 25 (71.43%) | 13 (43.33%) | >0.05*,*** |
| Female | 16 (45.71%) | 10 (28.57%) | 17 (56.67%) | <0.05** |
| Smoking, n (%) | ||||
| Positive | 6 (17.14%) | 6 (17.14%) | 5 (16.67%) | >0.05*,**,*** |
| Negative | 17 (48.57%) | 17 (48.57%) | 9 (30.0%) | |
| Exsmoker | 12 (34.29%) | 12 (34.29%) | 16 (53.33%) | |
| Lipid profiles | ||||
| Cholesterol (mg/dL) mean ± SD | 194.21 ± 36.89 | 171.40 ± 32.27 | 147.94 ± 41.2 | <0.01** >0.05**,*** |
| Triglycerides (mg/dL) mean ± SD | 215.72 ± 108.14 | 135.32 ± 104.12 | 95.69 ± 79.83 | <0.01* >0.05** <0.05*** |
| HDL cholesterol (mg/dL) mean ± SD | 33.49 ± 8.37 | 32.10 ± 7.89 | 47.36 ± 9.53 | <0.01* <0.001** >0.05*** |
| LDL cholesterol (mg/dL) mean ± SD | 118.92 ± 36.16 | 112.23 ± 23.53 | 81.48 ± 35.93 | <0.05*,** >0.05*** |
Notes:
Hypertension with diabetes versus controls;
hypertension without diabetes versus controls;
hypertension with and without diabetes.
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation.
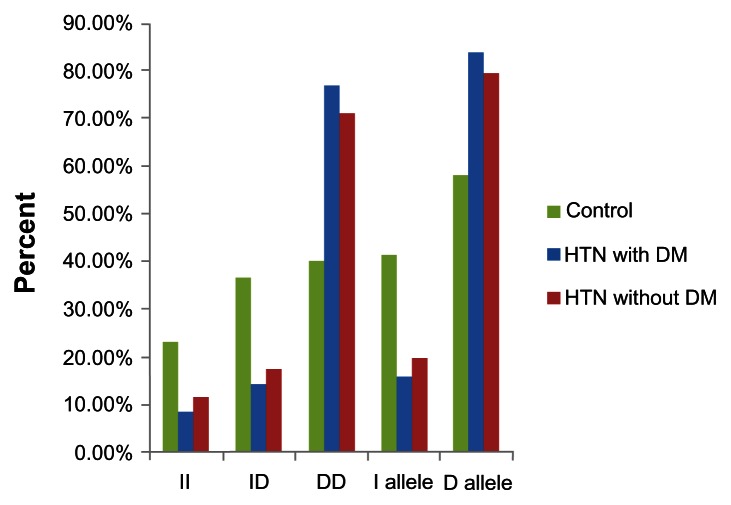
The distribution of I/D genotypes and α2b-AR alleles in patients with hypertensive ± diabetes and controls is shown in Table 2 and Figure 2. The DD genotype was significantly more common in hypertensive patients with (77.14% versus 40.0%, P < 0.01) and without type 2 diabetes (71.43% versus 40.0%, P < 0.05) compared with controls. The D allele was significantly more common in hypertensive patients with (84.29% versus 58.33%, P < 0.01) and without type 2 diabetes (80% versus 58.33%, P < 0.05) compared with controls. There were no statistically significant differences (P > 0.05) in I/D genotypes and alleles between hypertensive patients with and without diabetes. Odds ratios for the DD and ID genotypes and the D allele in hypertensive patients with type 2 diabetes were 5.25 (95% confidence interval [CI] 1.15–23.86, P < 0.01), 1.06 (95% CI 0.19–5.91, P < 0.01), and 3.83 (95% CI 1.68–8.72, P < 0.01), respectively, while odds ratios for the DD and ID genotypes and the D allele in hypertensive patients without type 2 diabetes were 3.64 (95% CI 0.89–14.91, P < 0.05), 0.95 (95% CI 0.19–4.63, P < 0.05), and 2.85 (95% CI 1.31–6.22, P < 0.05, Table 2).
Table 2.
Distribution of I/D genotypes and alleles in hypertensive patients with and without diabetes and controls
| Hypertension with diabetes n = 35 | Hypertension without diabetes n = 35 | Control n = 30 | P value | |
|---|---|---|---|---|
| I/D genotypes, n (%) | ||||
| II | 3 (8.57%) | 4 (11.43%) | 7 (23.33%) | <0.01* |
| ID | 5 (14.29%) | 6 (17.41%) | 11 (36.67%) | <0.05** |
| DD | 27 (77.14%) | 25 (71.43%) | 12 (40.0%) | >0.05*** |
| Odds ratio for DD genotype | 5.25 | 3.64 | ||
| 95% CI | [1.15–23.86] | [0.89–14.91] | ||
| Odds ratios for ID genotype | 1.06 | 0.95 | ||
| 95% CI | [0.19–5.91] | [0.19–4.63] | ||
| I/D allele, n (%) | ||||
| I | 11 (15.71%) | 14 (20%) | 25 (41.67%) | <0.01* |
| D | 59 (84.29%) | 56 (80%) | 35 (58.33%) | <0.05** |
| Odds ratios for D allele | 3.83 | 2.85 | >0.05*** | |
| 95% CI | [1.68–8.72] | [1.31–6.22] | ||
Notes:
Hypertension with diabetes versus control;
hypertension without diabetes versus control;
hypertension with and without diabetes.
Abbreviations: I, insertion; D, deletion; CI, confidence interval.
Figure 2.
Distribution of I/D genotypes and alleles of α2B adrenergic receptor in hypertensive patients with and without diabetes and control.
Abbreviations: DM, diabetes mellitus; HTN, hypertension.
There was no statistically significant difference between the II, ID, and DD phenotypes with regard to age, gender distribution, smoking, diastolic and systolic blood pressure, total cholesterol, or triglycerides. However, patients with the DD genotype had significantly lower HDL (P = 0.001) and higher LDL (P = 0.017) levels than those with the II and ID genotypes in the hypertensive group without type 2 diabetes (Tables 3 and 4).
Table 3.
Clinical parameters in different I/D genotypes in hypertensive patients with and without diabetes and controls
| I/D genotypes | P value | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| II | ID | DD | |||||
|
|
|
|
|||||
| n | % | n | % | n | % | ||
| Control group (n = 30) | |||||||
| Gender | |||||||
| Male | 3 | (42.86) | 5 | (45.45) | 7 | (58.33) | 0.753 |
| Female | 4 | (57.14) | 6 | (54.55) | 5 | (41.67) | |
| Smoking | |||||||
| Positive | 1 | (14.29) | 1 | (9.09) | 3 | (25.0) | 0.782 |
| Negative | 5 | (71.42) | 8 | (72.73) | 6 | (50.0) | |
| Exsmoker | 1 | (14.29) | 2 | (18.18) | 3 | (25.0) | |
| Hypertension with diabetes | |||||||
| Gender | |||||||
| Male | 1 | (33.33) | 3 | (60.0) | 15 | (55.56) | 0.735 |
| Female | 2 | (66.67) | 2 | (40.0) | 12 | (44.44) | |
| Smoking | |||||||
| Positive | 1 | (33.33) | 1 | (20.0) | 4 | (14.82) | 0.924 |
| Negative | 1 | (33.33) | 2 | (40.0) | 14 | (51.85) | |
| Exsmoker | 1 | (33.33) | 2 | (40.0) | 9 | (33.33) | |
| Hypertension without diabetes | |||||||
| Gender | |||||||
| Male | 2 | (50.0) | 4 | (66.67) | 19 | (76.0) | 0.542 |
| Female | 2 | (50.0) | 2 | (33.33) | 6 | (24.0) | |
| Smoking | |||||||
| Positive | 1 | (25.0) | 1 | (16.67) | 4 | (16.0) | 0.896 |
| Negative | 2 | (50.0) | 2 | (33.33) | 13 | (52.0) | |
| Exsmoker | 1 | (25.0) | 3 | (50.0) | 8 | (32.0) | |
Abbreviations: I, insertion; D, deletion.
Table 4.
Clinical parameters and lipid profile in different I/D genotypes in hypertensive patients with and without diabetes and control groups
| I/D genotypes | P value | |||
|---|---|---|---|---|
|
|
||||
| II | ID | DD | ||
| Control group | ||||
| Age (years) | 47.50 ± 3.54 | 50.0 ± 6.22 | 45.50 ± 10.85 | 0.741 |
| Systolic pressure (mmHg) | 115 ± 7.07 | 122.50 ± 9.57 | 115 ± 5.77 | 0.392 |
| Diastolic pressure (mmHg) | 72.50 ± 10.61 | 76.25 ± 7.50 | 68.75 ± 2.50 | 0.426 |
| Total cholesterol (mg/dL) | 131.75 ± 30.62 | 153.53 ± 30.39 | 150.45 ± 60.42 | 0.564 |
| Triglycerides (mg/dL) | 147.45 ± 54.07 | 109.60 ± 79.24 | 155.90 ± 27.79 | 0.441 |
| LDL (mg/dL) | 62.65 ± 4.31 | 79.23 ± 24.96 | 93.15 ± 53.08 | 0.731 |
| HDL (mg/dL) | 39.65 ± 4.45 | 52.40 ± 8.13 | 46.17 ± 11.17 | 0.453 |
| Hypertension with diabetes | ||||
| Age (years) | 56.0 ± 12.12 | 50.00 ± 3.46 | 57.75 ± 8.14 | 0.237 |
| Systolic pressure (mmHg) | 140.0 ± 0.0 | 143.33 ± 5.77 | 145.42 ± 5.09 | 0.195 |
| Diastolic pressure (mmHg) | 90.0 ± 0.0 | 90.0 ± 0.0 | 97.50 ± 8.47 | 0.104 |
| Total cholesterol (mg/dL) | 191.63 ± 49.99 | 174.13 ± 12.75 | 197.03 ± 36.93 | 0.523 |
| Triglycerides (mg/dL) | 148.10 ± 19.05 | 246.20 ± 51.96 | 220.36 ± 115.02 | 0.331 |
| LDL (mg/dL) | 132.27 ± 50.63 | 93.96 ± 3.00 | 120.37 ± 35.52 | 0.273 |
| HDL (mg/dL) | 29.76 ± 4.44 | 30.96 ± 0.63 | 34.27 ± 8.96 | 0.621 |
| Hypertension without diabetes | ||||
| Age (years) | 59.67 ± 0.57 | 56.60 ± 4.22 | 57.09 ± 6.34 | 0.661 |
| Systolic pressure (mmHg) | 146.67 ± 5.77 | 144.0 ± 5.47 | 144.50 ± 5.09 | 0.754 |
| Diastolic pressure (mmHg) | 100.0 ± 0.0 | 96.0 ± 5.47 | 96.36 ± 9.02 | 0.506 |
| Total cholesterol (mg/dL) | 163.30 ± 8.31 | 146.58 ± 10.02 | 178.14 ± 34.05 | 0.128 |
| Triglycerides (mg/dL) | 100.33 ± 43.07 | 77.88 ± 35.90 | 153.15 ± 112.80 | 0.288 |
| LDL (mg/dL) | 104.43 ± 7.56 | 87.30 ± 17.34 | 118.96 ± 21.78 | 0.017 |
| HDL (mg/dL) | 38.83 ± 7.85 | 43.70 ± 2.57 | 28.55 ± 4.93 | 0.001 |
Abbreviations: HDL, high-density lipoprotein; I, insertion; D, deletion; LDL, low-density lipoprotein.
Discussion
The α2B-AR is encoded by the ADRA2B gene located on chromosome 2 and mediates a variety of functions. A polymorphism (12Glu9) resulting in insertion or deletion of three glutamic acid residues from an acidic stretch in the third intracellular loop has been described.27 The deletion allele has been found to be associated with adverse metabolic and vascular effects, including reduced basal metabolic rate,28 obesity,29 impaired insulin secretion,14 earlier onset of diabetes,30 increased risk of acute coronary ischemia,31 and autonomic dysfunction, and increased sympathetic nervous system activity.32 The present study was carried out to clarify the role of the deletion allele of α2B-AR in hypertension and if diabetes augments this association or not.
The present study showed significantly higher age, systolic and diastolic pressure, total cholesterol, triglycerides, and LDL cholesterol, and lower HDL cholesterol in hypertensive patients than in controls. These results are in agreement with other reports showing significant elderly, systolic and diastolic hypertensive,6,26 and lower HDL cholesterol.26 There was no significant difference between the II, ID, and DD genotypes with regard to age, gender, smoking, systolic and diastolic blood pressure, total cholesterol, and triglycerides. These results are consistent with those reported previously;26 however, in our study, the DD genotype was associated with higher LDL cholesterol and lower HDL cholesterol in hypertensive patients without diabetes, indicating that the deletion allele may induce hypertension. Association of deletion allele with dyslipidemia may also attribute to hypertension in addition to receptor desensitization and vasoconstriction so it can be considered as a risk factor for hypertension and coronary artery diseases.
The present study shows a significant association between the DD genotype, the D allele, and hypertension ± diabetes, and this finding is in agreement with other reports.6,26,33,34 However, some studies have reported no significant association between the D allele and hypertension.31,35
The hypertensive effect mediated by the α2B-AR has been shown to be especially important in individuals with salt-sensitive hypertension because salt sensitivity is associated with a positive family history of primary hypertension, and is also a characteristic in a large proportion of patients with primary hypertension.6
There is also evidence that the α2B-AR mediates peripheral vasoconstriction. In vivo studies have shown that the DD genotype of the receptor is associated with reduced dilatation of the brachial artery, reduced coronary blood flow, and increased peripheral resistance in response to adrenaline infusion.6
Our study showed that the DD genotype increased the risk of hypertension ± diabetes as compared with controls (by 5.25-fold and 3.65-fold, respectively) and the D allele increases the risk of hypertension ± diabetes as compared with controls (by 3.83-fold and 2.85-fold, respectively). We also showed a stronger association between the DD genotype, the D allele, and hypertension with diabetes, that may indicate potential use of deletion genotype as a risk factor for diabetes.
Earlier studies reporting a rather weak association between the DD genotype and nondiabetic primary hypertension and a stronger association with early-onset hypertension is by no means surprising, given that the hereditary component of primary hypertension is likely to be the sum of many genes, for which the effect of an individual gene is likely to be small to moderate, and the diabetic phenotype seems to add complexity to the phenotype of primary hypertension.6
Siitonen et al reported that the common I/D polymorphism of the α2B-AR influences receptor function by impairing agonist-promoted receptor phosphorylation and desensitization. Based on this observation, they postulated that impairment of α2B-AR desensitization due to I/D polymorphism causes prolonged inhibition of insulin secretion from pancreatic beta cells. Via this mechanism, the polymorphism may constitute one of the genetic components underlying insulin secretion, and could explain the genetic predisposition of certain individuals to type 2 diabetes.14
A potential explanation for the association between the deletion allele and diabetes is that impairment of α2B-AR desensitization due to the allelic variant causes prolonged inhibition of insulin secretion from pancreatic beta cells. As insulin sensitivity decreases, the requirement for insulin secretion by beta cells increases, so individuals with an impaired capacity to secrete insulin are predisposed to type 2 diabetes.30,36
An alternative explanation for the association between α2-AR I/D polymorphism and early-onset diabetes is altered function of the autonomic nervous system, particularly regulation of vascular tone. Altered regulation of vascular resistance may influence glucose metabolism, either directly through redistribution of blood flow or through reflex modulation of autonomic nervous system activity.37 Redistribution of blood flow away from metabolically active tissues, striated muscle in particular, caused by any mechanism leading to regional alterations in vascular resistance, would be expected to alter glucose metabolism.30,37
Talmud et al reinforced the notion that the α2-AR pathway should be considered as a potential drug target for prevention of type 2 diabetes. However, an α2-AR antagonist, which might promote insulin secretion and lipolysis, could potentially raise blood pressure, so a drug that does not cross the blood–brain barrier might be required.38
In conclusion, our study shows a strong association between the DD genotype, the D allele, and hypertension and that this association is more evident in patients with hypertension and diabetes. We postulate that the deletion allele is associated with diabetes, so α2B-AR polymorphism is one of the genetic factors involved in the prediction of hypertension, and diabetes and may be considered to be a risk factor. Also, association of the DD genotype with higher atherogenic LDL and lower HDL may potentiate its role in precipitating hypertension.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 3.Ostchega Y, Yoon SS, Hughes J, Louis T. Hypertension Awareness, Treatment, and Control – Continued Disparities in Adults: United States, 2005–2006. Hyattsville, MD: National Center for Health Statistics; 2008. NCHS Data Brief 3. [PubMed] [Google Scholar]
- 4.Sorlie D, Stafford RS, Turan TN, et al. Heart Disease and Stroke Statistics – 2011 Update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arafa NAS, Ez-Elarab HS. Epidemiology of prehypertension and hypertension among Egyptian adults. The Egyptian Journal of Community Medicine. 2011;29:1–18. [Google Scholar]
- 6.Von Wowern F, Bengtsson K, Lindblad U, et al. Functional variant in the 2B adrenoceptor gene, a positional candidate on chromosome 2, associates with hypertension. Hypertension. 2004;43:592–597. doi: 10.1161/01.HYP.0000116224.51189.80. [DOI] [PubMed] [Google Scholar]
- 7.Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Chen QJ, Lu L, Jin C, et al. Insertion/insertion genotype of α2B-adrenergic receptor gene polymorphism is associated with silent myocardial ischemia in patients with type 2 diabetes mellitus. Clin Biochem. 2010;43:1201–1204. doi: 10.1016/j.clinbiochem.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen K, Kassimatis T, Lymperopoulos A. Impaired desensitization of a human polymorphic a2B-adrenergic receptor variant enhances its sympatho-inhibitory activity in chromaffin cells. Cell Commun Signal. 2011;9:5. doi: 10.1186/1478-811X-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bylund DB, Eikenberg DC, Hieble JP, et al. IV International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- 11.Ruffolo RR, Nichols AJ, Stadel JM, Hieble JP. Pharmacologic and therapeutic applications of alpha 2-adrenoceptor subtypes. Annu Rev Pharmacol Toxicol. 1993;33:243–279. doi: 10.1146/annurev.pa.33.040193.001331. [DOI] [PubMed] [Google Scholar]
- 12.Philipp M, Brede M, Hein L. Physiological significance of alpha(2)-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol Regul Integr Comp Physiol. 2002;283:R287–R295. doi: 10.1152/ajpregu.00123.2002. [DOI] [PubMed] [Google Scholar]
- 13.Lacey RJ, Chan SL, Cable HC. Expression of alpha 2- and beta-adrenoceptor subtypes in human islets of Langerhans. J Endocrinol. 1996;148:531–543. doi: 10.1677/joe.0.1480531. [DOI] [PubMed] [Google Scholar]
- 14.Siitonen N, Lindstrom J, Eriksson J, et al. Association between a deletion/insertion polymorphism in the alpha2B-adrenergic receptor gene and insulin secretion and type 2 diabetes. The Finnish Diabetes Prevention Study. Diabetologia. 2004;47:1416–1424. doi: 10.1007/s00125-004-1462-z. [DOI] [PubMed] [Google Scholar]
- 15.Philipp M, Hein L. Adrenergic receptor knockout mice: distinct functions of 9 receptor subtypes. Pharmacol Ther. 2004;101:65–74. doi: 10.1016/j.pharmthera.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Von Wowern F, Bengtsson K, Lindgren CM, et al. A genome wide scan for early onset primary hypertension in Scandinavians. Hum Mol Genet. 2003;12:2077–2081. doi: 10.1093/hmg/ddg206. [DOI] [PubMed] [Google Scholar]
- 17.Rice T, Rankinen T, Province MA. Genome-wide linkage analysis of systolic and diastolic blood pressure: the Quebec Family Study. Circulation. 2000;102:1956–1963. doi: 10.1161/01.cir.102.16.1956. [DOI] [PubMed] [Google Scholar]
- 18.Perola M, Kainulainen K, Pajukanta P. Genome-wide scan of predisposing loci for increased diastolic blood pressure in Finnish siblings. J Hypertens. 2000;18:1579–1585. doi: 10.1097/00004872-200018110-00008. [DOI] [PubMed] [Google Scholar]
- 19.Kristjansson K, Manolescu A, Kristinsson A. Linkage of essential hypertension to chromosome 18q. Hypertension. 2002;39:1044–1049. doi: 10.1161/01.hyp.0000018580.24644.18. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki N, Matsunaga T, Nagasumi K. α2b-Adrenergic receptor deletion polymorphism associates with autonomic nervous system activity in young healthy Japanese. J Clin Endocrinol Metab. 2003;88:1184–1187. doi: 10.1210/jc.2002-021190. [DOI] [PubMed] [Google Scholar]
- 21.Dorn GW. Adrenergic signaling polymorphisms and their impact on cardiovascular disease. Physiol Rev. 2010;90:1013–1062. doi: 10.1152/physrev.00001.2010. [DOI] [PubMed] [Google Scholar]
- 22.Duerden MG British Hypertension Society. Guidelines from the British Hypertension Society: BHS is set to bankrupt NHS. BMJ. 2004;329(7465):569–570. doi: 10.1136/bmj.329.7465.569-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein D, Sacks D, Bruns D. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2007;48:436–472. [PubMed] [Google Scholar]
- 24.Wallach J. Interpretation of Diagnostic Tests. 6th ed. Boston, MA: Little Brown and Company; 1996. Metabolic and hereditary disorders. [Google Scholar]
- 25.Sirchia G, Pizzi C, Scalomogna M. A simple procedure for human lymphocyte isolation from peripheral blood. Tissue Antigens. 1972;72:138–139. doi: 10.1111/j.1399-0039.1972.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 26.Vasudevan R, Ismail P, Stanslas J, Shamsudin N, Ali AB. Association of insertion/deletion polymorphism of alpha-adrenoceptor gene in essential hypertension with or without type 2 diabetes mellitus in Malaysian subjects. Int J Biol Sci. 2008;4:362–367. doi: 10.7150/ijbs.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papanas N, Papatheodorou K, Papazoglou D, Kotsiou S, Christakidis D, Maltezos E. An insertion/deletion in the alpha 2B adrenoceptor gene is associated with peripheral neuropathy in patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2007;115:327–330. doi: 10.1055/s-2007-967084. [DOI] [PubMed] [Google Scholar]
- 28.Heinonen P, Koulu M, Pesonen U, Karvonen MK, Rissanen A, Laakso M. Identification of a three-amino acid deletion in the alpha2B adrenergic receptor that is associated with reduced basal metabolic rate in obese subjects. J Clin Endocrinol Metab. 1999;84:2429–2433. doi: 10.1210/jcem.84.7.5818. [DOI] [PubMed] [Google Scholar]
- 29.Sivenius K, Lindi V, Niskanen L, Laakso M, Uusitupa M. Effect of a three amino acid deletion in the alpha2B-adrenergic receptor gene on long term body weight change in Finnish non-diabetic and type 2 diabetic subjects. Int J Obes Relat Metab Disord. 2001;25:1609–1614. doi: 10.1038/sj.ijo.0801798. [DOI] [PubMed] [Google Scholar]
- 30.Papazoglou D, Papanas N, Papatheodorou K, Kotsiou S, Christakidis D, Maltezos E. An insertion/deletion polymorphism in the alpha2B adrenoceptor gene is associated with age at onset of type 2 diabetes mellitus. Exp Clin Endocronol Diabetes. 2006;114:424–427. doi: 10.1055/s-2006-924330. [DOI] [PubMed] [Google Scholar]
- 31.Snapir A, Heinonen P, Tuomalainen TP, Alhopuro P, Karvonen MK, Lakka TA. An insertion/deletion polymorphism in the alpha2B-adrenergic receptor gene is a novel genetic risk factor for acute coronary events. J Am Coll Cardiol. 2001;37:1516–1522. doi: 10.1016/s0735-1097(01)01201-3. [DOI] [PubMed] [Google Scholar]
- 32.Sivenius K, Niskanen L, Laakso M, Uusitupa M. A deletion in the alpha2B-adrenergic receptor gene and autonomic nervous function in central obesity. Obes Res. 2003;11:962–970. doi: 10.1038/oby.2003.133. [DOI] [PubMed] [Google Scholar]
- 33.Lockette W, Ghosh S, Farrow S, et al. Alpha 2-adrenergic receptor gene polymorphism and hypertension in blacks. Am J Hypertens. 1995;8:390–394. doi: 10.1016/0895-7061(95)00024-j. [DOI] [PubMed] [Google Scholar]
- 34.Snapir A, Scheinin M, Groop LC, et al. The insertion/deletion variation in the α2B-adrenoceptor does not seem to modify the risk for acute myocardial infarction, but may modify the risk for hypertension in sib-pairs from families with type 2 diabetes. Cardiovasc Diabetol. 2003;24:2–15. doi: 10.1186/1475-2840-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldwin CT, Schwartz F, Baima J, et al. Identification of a polymorphic glutamic acid stretch in the alpha2B-adrenergic receptor and lack of linkage with essential hypertension. Am J Hypertens. 1999;12:853–857. doi: 10.1016/s0895-7061(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 36.LeRoith D. Beta-cell dysfunction and insulin resistance in type 2 diabetes: role of metabolic and genetic abnormalities. Am J Med. 2002;113:3S–11S. doi: 10.1016/s0002-9343(02)01276-7. [DOI] [PubMed] [Google Scholar]
- 37.Clerk LH, Vincent MA, Lindner JR, Clark MG, Rattigan S, Barrett EJ. The vasodilatory actions of insulin on resistance and terminal arterioles and their impact on muscle glucose uptake. Diabetes Metab Res Rev. 2004;20:3–12. doi: 10.1002/dmrr.414. [DOI] [PubMed] [Google Scholar]
- 38.Talmud PJ, Cooper JA, Gaunt T, et al. Variants of ADRA2A are associated with fasting glucose, blood pressure, body mass index and type 2 diabetes risk: meta-analysis of four prospective studies. Diabetologia. 2011;54:1710–1719. doi: 10.1007/s00125-011-2108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]