Abstract
Background
Cyclopentenone prostaglandins have been identified as potential neurotoxic agents in the setting of hypoxia-ischemia. Cyclooxygenase-2 (COX-2), the upstream enzyme responsible for prostaglandin production is upregulated following hypoxic-ischemic brain injury. However, the temporal production and concentration of cyclopentenone prostaglandins has not been described following global brain ischemia.
Methods
Global brain ischemia was induced in rats by asphyxial cardiac arrest (ACA) followed by resuscitation. Rats were sacrificed between 24 hours and 7 days following resuscitation and their brains removed. Western blot, immunohistochemistry, and mass spectroscopy were performed. A cohort of rats was pretreated with the COX-2 inhibitor SC58125.
Results
COX-2 is induced in hippocampus at 24 hours following ACA. Multiple prostaglandins, including cyclopentenone prostaglandin species, are increased in hippocampus as 24 hours following ACA. Prostaglandin and cyclopentenone prostaglandin concentrations are returned to baseline at 3 and 7 days post-ischemia. The COX-2 inhibitor SC58125 completely abrogates the post-ischemic increase in prostaglandins and cyclopentenone prostaglandins.
Conclusions
Prostaglandins, including cyclopentenone prostaglandins, are increased in ischemic brain, peak at 24 hours and can be attenuated by the COX-2 inhibitor SC58125. These data establish the presence of potentially neurotoxic cyclopentenone prostaglandins in post-ischemic brains, thus identifying a target and therapeutic window for neuroprotective therapies.
1. Introduction
Cyclooxygenase (COX) catalyzes the conversion of arachidonic acid into the intermediate prostaglandin H2 (PGH2), which is subsequently converted via specific prostaglandin synthases into one of the biologically active prostaglandins (PGI2, PGA2, PGD2, PGE2). Cyclooxygenase-2 (COX-2) is an inducible COX isoform and is the most prominent isoform in brain. COX2 expression is increased in CA1 and other brain regions after global ischemia (Nakayama et al., 1998). Neuronal COX-2 has been identified as an important contributor to brain damage following hypoxia-ischemia and COX-2 inhibition is neuroprotective (reviewed in (Candelario-Jalil and Fiebich, 2008)). Unfortunately, COX-2 inhibitors are prothrombotic, limiting their use as neuroprotective agents (Amer et al., 2010). Identification of the specific downstream mediators of COX-2 toxicity may allow development of therapies without prothrombotic side effects (Iadecola and Gorelick, 2005). Potential mediators include specific prostaglandins as well as prostaglandin metabolites (Andreasson, 2010; Hewett et al., 2006). Of particular interest is the cyclopentenone family of prostaglandin metabolites. Cyclopentenone prostaglandins (CyPGs) are highly electrophilic molecules capable of covalently bonding free thiols on proteins. More than fifty protein targets of CyPGs have been identified(Levonen et al., 2004; Sanchez-Gomez et al., 2004) including several proteins that regulate cell death and survival (Garzon et al., 2011; Kondo et al., 2002; Liu et al., 2010; Satoh and Lipton, 2007; Uchida and Shibata, 2008). We have recently shown that CyPGs exacerbate neuronal death in primary neuronal culture exposed to hypoxia(Liu et al., 2010), thus identifying CyPGs as potential mediators of COX-2 dependent post-ischemic neuronal death. To date there is limited evidence that CyPGs are produced in ischemic brain.
In this paper we describe a series of experiments using mass spectroscopy to measure prostaglandin and CyPG content in a rodent model of global brain ischemia (Fink et al., 2004). This model has robust post-ischemic induction of COX-2 in the selectively vulnerable CA1 portion of hippocampus thus facilitating collection and mass spectroscopy measurement of prostaglandins and CyPGs in vivo. The mass spectroscopy methods described herein allow us to report the most comprehensive description of post-ischemic prostaglandins to date. We also demonstrate successful attenuation of both prostaglandin and CyPG production in this model with oral administration of a COX-2 inhibitor. The methods and data within this report represent an initial step towards understanding the relative contributions of several prostaglandin and CyPG species, all downstream of COX-2, towards neuronal death following global brain ischemia.
2. Results
2.1 COX-2 expression and TUNEL staining increase after ACA
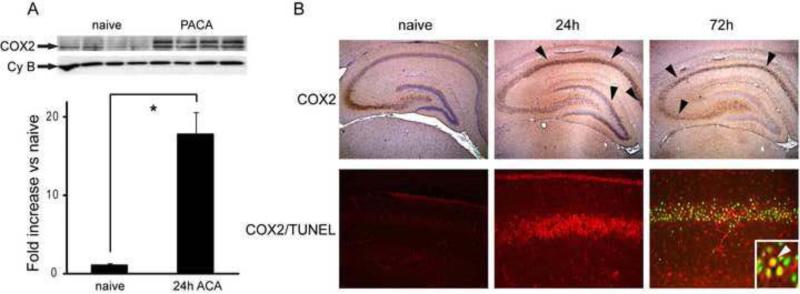
Western blot analysis indicates that COX-2 protein expression is increased following ACA in hippocampus (Figure 1A). Immunohistochemistry reveals that COX2 expression is predominantly in CA1 neurons, peaking at 24 hours; TUNEL staining does not occur until 72 hours after resuscitation.
Figure 1. COX2 induction and TUNEL staining after asphyxial cardiac arrest.
Male PND17 rats underwent asphyxial cardiac arrest (ACA) and were sacrificed at 24 h and 72 h after resuscitation. A. upper: Hippocampal tissue from naive and 24 h ACA rats was immunoblotted with anti-COX2 antibody. As previously described, COX2 forms a doublet at approximately 72 kDa secondary to glycosylation. Anti-cyclophilin B (Cy B) is shown as a loading control. lower: Quantitative densitometric analysis of COX2 bands. n = 4 per group. * P < 0.02 vs naive. Data are means +/- SE. B: upper panel: COX2 immunohistochemical staining (brown, DAB) in rat brain hippocampus 24 h and 72 h after ACA. Photos at 4X taken with a light microscope. Naïve brain shows constitutive expression in CA3. Expression is induced in CA1 and dentate gyrus (arrowheads) at 24 h post ACA. There is thinning of staining (arrowheads) 72 h representing neuronal loss. lower panel: COX2 immunostaining (red) and TUNEL (green) in CA1. COX2/TUNEL colocalization shown at arrow (yellow, inset). Photos at 20X taken with Olympus confocal microscope.
2.2 Prostaglandin and cyclopentenone prostaglandin production are increased 24 h after ACA
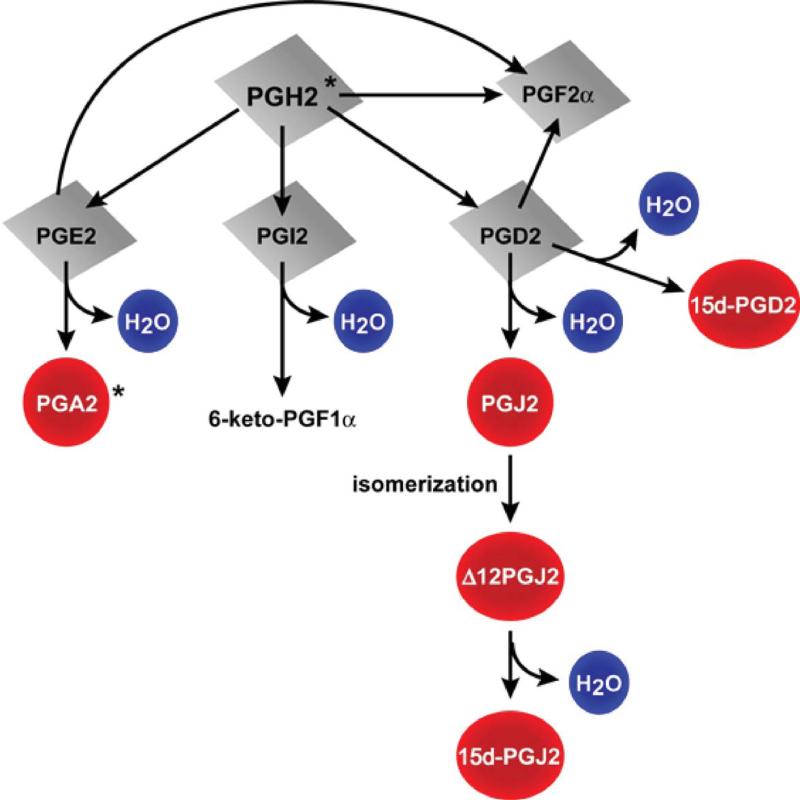
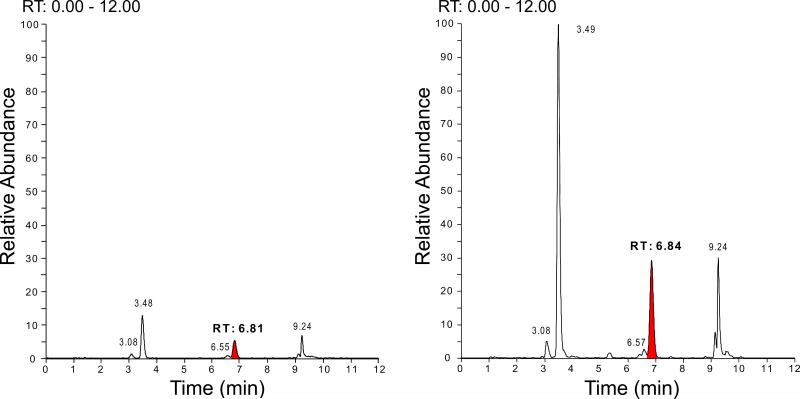
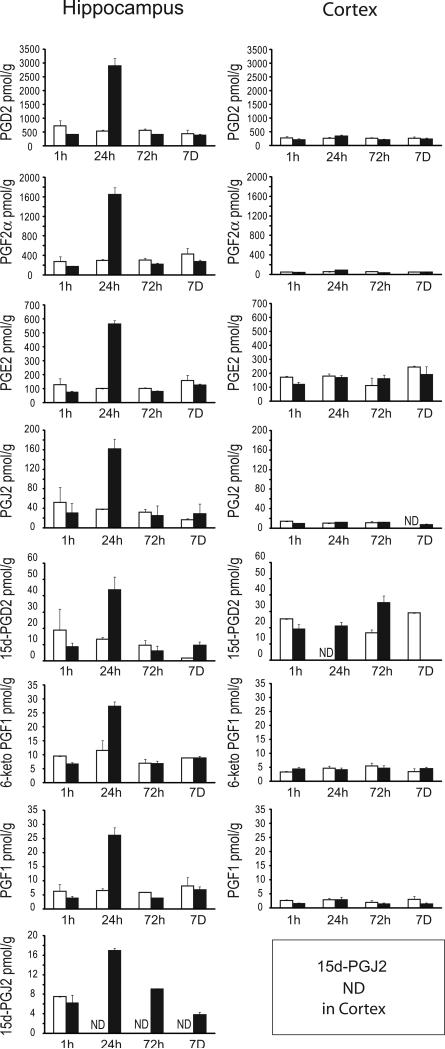
Prostaglandins, including the cyclopentenone prostaglandin species detected in this study are indicated in the schematic diagram (figure 2). Representative mass spectroscopy chromatograms detecting PGJ2 in ACA and naive rat brain hippocampus 24h after resuscitation are shown in figure 3. Figure 4 indicates the temporal pattern for prostaglandin and cyclopentenone prostaglandin species measured from ischemic and sham brain hippocampus and cortex. PGD2, the precursor of most cyclopentenone prostaglandins is the most prominent prostaglandin in both hippocampus and cortex. Ischemic hippocampus shows markedly increased production of all species at 24 hours post-ischemia. In contrast, cortex contains equivalent or lower baseline concentrations of most species with no apparent increase following ischemia. These findings are consistent with the localization of COX-2 expression to hippocampus.
Figure 2. Formation of cyclopentenone prostaglandins from PGH2.
15d:PGJ2: 15-deoxy-Δ12,14 PGJ2; 15d-PGD2: 15-deoxy-Δ12,14 PGD2. Red circles are cyclopentenone prostaglandins. * Not measured in this study.
Figure 3. Detection of PGJ2 in rat brain hippocampus after asphyxial cardiac arrest.
PND17 rats underwent asphyxial cardiac arrest surgery and were sacrificed 24 h after resuscitation. Hippocampal tissue extracts from naive (left) and asphyxial cardiac arrest (right) rat brain were analyzed for PGJ2 (retention time of 6.81) using UPLC MS/MS. Representative chromatograms are shown above. Other peaks represent PGE2 (RT 3.08), PGD2 (RT 3.49), 15d-PGD2 and PGJ2 (RT 9.24)
Figure 4. Temporal prostaglandin production in PND17 rat brain after asphyxial cardiac arrest.
Rats underwent sham (n = 2, white) or asphyxial cardiac arrest (n = 4, black) and were sacrificed at 1 h, 24 h, 72 h, and 7 D after resuscitation. Hippocampus and cortex were removed and analyzed for prostaglandins using UPLC MS/MS. Data are means +/- SE. ND: not detected.
Because PGJ2 and Δ12-PGJ2 are stereoisomers with the same molecular mass, we analyzed a separate cohort of hippocampal ACA and sham samples using a chiral column to differentiate the isomers. Approximately 10% of the PGJ2 signal is Δ12-PGJ2 (99.13 +/-54.02 vs 5.687 +/- 4.87 nM)
This exploratory data was used to confirm the absence of a sham effect and determine optimal timing for the COX-2 inhibitor experiment.
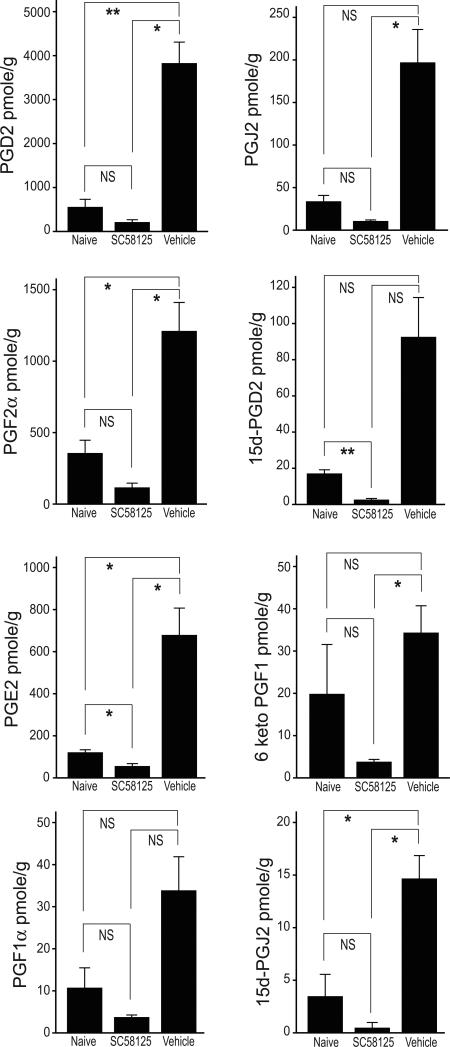
2.3 Pretreatment with a COX-2 inhibitor attenuates increases in prostaglandin and cyclopentenone prostaglandin expression
The effect of pretreatment with the COX-2 inhibitor SC58125 is seen in figure 5. SC58125 completely ablated the increase in all species of prostaglandins at 24 h following ACA. Indeed, SC58125-treated rats with ACA had lower concentrations of all species in comparison to naïve rats.
Figure 5. COX-2 inhibitor abrogates increases in prostaglandin production after asphyxial cardiac arrest.
Rats were administered the COX-2 specific inhibitor SC58125 (30 mg/kg) or vehicle (54% ethanol in saline) 10 min prior to asphyxial cardiac arrest. Animals were sacrificed 24 h after resuscitation and prostaglandins measured in hippocampus using UPLC MS/MS. Naive and ACA + vehicle: n = 4 per group; ACA + SC58125: n = 6 per group. Data are means +/- SE. *p<.05, **p<.01
3. Discussion
This study is the first comprehensive, temporal description of prostaglandin species formation in post-ischemic brain and the second to report detection of CyPG species in ischemic brain. The first report, also by our group, found approximately 20 nM 15d-PGJ2 in rat ipsilateral brain hemisphere 24h after temporary middle cerebral artery occlusion, similar to the concentration reported here from ACA hippocampus (Liu et al., 2010). Prostaglandin species are increased following ischemia with a peak at 24 hours. This increase is consistent with data reported by others examining focal or global brain ischemia using either mass spectroscopy or ELISA (Brose et al., 2011; Candelario Jalil et al., 2007; Iadecola et al., 2001; Nakayama et al., 1998; Nogawa et al., 1997; Nogawa et al., 1998; Taniguchi et al., 2007).
COX-2 inhibition effectively ablates the increase in prostaglandins and CyPGs following ischemia. This is consistent with previous reports demonstrating attenuation of post-ischemic PGE2 with COX-2 inhibitors (Candelario-Jalil et al., 2007; Nakayama et al., 1998; Nogawa et al., 1998). Thus, prostaglandins and CyPGs are potentially manipulable therapeutic targets in ischemic brain. Furthermore, the finding that post-ischemic concentration of prostaglandins peaks at 24 hours with return towards normal at 72 hours suggests that COX-2 directed therapy need not be prolonged.
In vitro experiments demonstrating a neurotoxic effect of CyPGs typically utilize micromolar concentrations. Here, we report in vivo concentrations of CyPGs in the nanomolar range. However, direct comparison of in vitro to in vivo concentrations is problematic. In vitro experiments utilize a one-time addition of cyclopentenone prostaglandins that rapidly react or degrade whereas in vivo conditions are characterized by prolonged and continuous exposure to freshly produced CyPGs secondary to induction of COX-2. Furthermore, cyclopentenones covalently adduct sulfhydral groups on proteins. These reactions are not reversible and therefore not dependent on the equilibrium concentration of the cyclopentenone ligands, rather the binding is cumulative and dependent on the concentration of the ligand in the adjacent the protein and time(Higdon et al., 2012) . A limitation of our methods is that we measured free CyPGs but not CyPGs adducted to proteins. Thus, our detection of average free CyPGs using mass spectroscopy may underestimate the amount of CyPG production within hippocampal neurons and does not measure neurotoxic adducts(Koharudin et al., 2010). Recent studies have shown that circumspect populations of hippocampal neurons contain greatly increased immunoreactively for PgGF2Dα, a PgD2 metabolite(Takei et al., 2012). Additional experiments are necessary to quantify these important adducts in vitro and in vivo.
A strength of this study is the mass spectroscopy method enabling measurement of a broad array of prostaglandins and unbound CyPG species. The ACA model robustly induces COX-2 expression in the selectively vulnerable CA1 hippocampus. Dissection of this COX-2 enriched, post-ischemic hippocampus facilitates measurement of prostaglandin and CyPG species present in the nanomolar range. Although it is debatable whether nanomolar concentrations of CyPGs are sufficient for receptor-mediated physiologic effects(Bell-Parikh et al., 2003; Powell, 2003), the concentration of free CyPGs reported here likely underestimates the total burden of free and adducted CyPGs. Indeed, it is the amount of CyPGs adducted to target proteins that is most likely to mediate neurotoxicity(Liu et al., 2010). In summary, prostaglandins and CyPGs are increased in ischemic brain, peak at 24 hours and can be attenuated by the COX-2 inhibitor SC58125. This groundwork will inform additional research on prostaglandin- and cyclopentenone-dependent pathways of post-ischemic cell death and recovery.
4. Materials and Methods
All animal studies were performed with approval of the University of Pittsburgh Institutional Animal Care and Use Committee.
4.1 Reagents and Antibodies
Anti-COX-2 antibody was purchased from Abcam (Cambridge, MA). The COX-2 inhibitor SC58125 was ordered from Cayman Chemical (Ann Arbor, MI). Diaminobenzidine (DAB) was obtained from Invitrogen (Grand Island, NY) and the TUNEL kit was from Chemicon (Temecula, CA). All other reagents were purchased from Sigma Aldrich unless otherwise noted.
4.2 Asphyxial Cardiac Arrest (ACA)
Rats underwent ACA as described by Fink et al(Fink et al., 2004). In brief, postnatal day 16 –18 male Sprague-Dawley rats were anesthetized with 4% isoflurane/60% N2O/balance oxygen in a Plexiglas chamber until unconscious, then underwent tracheal intubation and were ventilated with 1.5 - 2% isoflurane/60% N2O/balance oxygen for aseptic femoral venous cannulation. Rats were placed on a heating pad and a rectal thermometer was inserted with core temperature maintained at 37.5 +/- 0.5 °C. Ten minutes before asphyxia, 1 mg/kg of vecuronium bromide was administered to the rats while they were still receiving anesthesia. Two minutes before asphyxia, isoflurane was discontinued and rats were exposed to 100% oxygen for 1 min to avoid the confounding cerebroprotective effects of inhaled anesthetics. All rats then received 21% oxygen for the final minute before asphyxia to avoid hyperoxia preinsult. Rats randomized to ACA were disconnected from the ventilator for 8 min. Cardiac arrest, defined as pulseless electrical activity with a mean arterial pressure of 0 mm Hg, occurs within 90 seconds. At the end of 8 minutes asphyxia, cardiopulmonary resuscitation was initiated with chest compressions and by reconnecting the rats to the ventilator (100% oxygen), in addition to IV boluses of 0.005 mg/kg of epinephrine and 1 mEq/kg of sodium bicarbonate. Rats were extubated at 1 hour following return of spontaneous circulation then placed in a recovery chamber with 100% oxygen for 30 min. Sham rats underwent the same anesthesia and surgical procedures without ACA, and were extubated after anesthetic washout. All animals received 1 ml warmed 5 % dextrose/0.45% saline subcutaneously prior to extubation. A separate cohort of animals was treated 10 min prior to ACA with the COX-2 inhibitor 5-(4-fluorophenyl)-1-[4-(methylsulfonyl)phenyl]-3-(trifluoromethyl)-1H-pyrazole (SC58125, 30 mg/kg) or vehicle (3% methyl cellulose in water) via oral gavage. For temporal studies, ACA ( n = 4) and sham (n = 2) rats were sacrificed at 1 h, 24 h, 72 h or 7D. In COX-2 inhibitor studies, vehicle (n = 4) and SC58125 (n = 6) –treated animals were sacrificed 24 h after reperfusion. A cohort of naive (n = 4) rats were included as baseline control. Brain hippocampus and cortex were dissected out and snap frozen in liquid nitrogen. Samples were stored at -80°C until analysis.
4.3 Immunohistochemistry and TUNEL labeling
Rats underwent asphyxial cardiac arrest as described above and were sacrificed at 24 h and 72 h post resuscitation. Brains were blocked in paraffin, cut in 6 μm sections and TUNEL labeled following manufacturer's directions then co-immunostained using anti-COX-2 antibody (Cayman Chemical, Ann Arbor, MI, 1:200,CY3 and 3,3′-diaminobenzidine [DAB]). A cohort of naive rats was used as control (n = 3). COX-2 DAB photos were taken using a light microscope at 4X; TUNEL/COX-2 photos were taken with an Olympus confocal microscope at 20X.
4.4 Western Blot
Western blots were performed as previously described(Hickey et al., 2007). Protein concentrations were measured using the BioRad protein assay (Biorad, Hercules, CA). For COX-2 protein detection, cell lysates were resolved on 12% SDS-PAGE and transferred to PVDF membrane. After blocking with 5% non-fat milk in TBS/Tween-20, membranes were incubated with anti-COX-2 antibody at 4 °C overnight.
Blots were washed and the appropriate secondary antibody applied (anti rabbit 1:5000). Protein signal was visualized with ECL reagents (Pierce).
4.5 Prostaglandin (PG) measurement
15-deoxy-Δ12,14-PGJ2 (15d-PGJ2), PGD2, PGJ2, Δ12 PGJ2, and 15-deoxy Δ12,14-PGD2 (15d-PGD2) were measured by UPLC-MS/MS. . Samples were homogenized in de-ionized water (0.113 mM BHT) and centrifuged. The supernatant was removed and 7.5 ng each of deuterated 15d-PGJ2 and PGD2 were used as the internal standards. Samples were loaded onto Oasis HLB solid phase extraction cartridges (Waters, Oasis, Milford, MA) and washed with three 1 ml volumes of 5% methanol before elution with 100% methanol. Fifteen microliters of 1% acetic acid in methanol were added to eluent prior to dry-down. Extracts were dried under nitrogen and reconstituted in 125 μL of 80:20 methanol: de-ionized water. Analytes were separated via reverse phase UPLC with an Acquity UPLC BEH C18 1.7 μm, 2.1 × 150 mm column. A small set of samples were also analyzed via a Chiralpak AD-RH 5 μm, 2.1 × 150mm chiral column (Chiral Technologies, Inc, West Chester, PA) in order to isolate the stereoisomers PGJ2 and Δ12 PGJ2. MS analysis was performed via a Thermo TSQ Quantum Ultra triple quadrupole mass spectrometer (ThermoFisher Scientific). This method for PG analysis was adapted from a previously published method for quantification of mono-oxygenated arachidonic acid metabolites with some modifications. (Miller et al., 2009) Quantitation by selected reaction monitoring (SRM) analysis on PGD2, PGJ2/Δ12-PGJ2, 15d-PGJ2, and 15d-PGD2 was performed by monitoring their m/z transitions, 351 → 271, 333 → 271, 315 → 271, and 333 → 271, respectively. Parameters were optimized to obtain the highest [M-H]- ion abundance for each analyte. Data was acquired using Xcalibur Software version 2.0.6.
4.6 Statistical analysis
COX-2 western blot band densities were compared using unpaired/independent samples t-test. Concentrations of PGs and CyPGs were compared using ANOVA (one way ANOVA) with appropriate post-hoc analysis (Dunnett T3 or Tukey). All analyses were conducted with SPSS (version 20). A P value < 0.05 was considered statistically significant.
5. Conclusions
Prostaglandins, including cyclopentenone prostaglandins, are increased in ischemic brain, peak at 24 hours and can be attenuated by the COX-2 inhibitor SC58125. These data establish the presence of potentially neurotoxic cyclopentenone prostaglandins in post-ischemic brains, thus identifying a target and therapeutic window for neuroprotective therapies.
COX2 is induced in brain following asphyxial cardiac arrest
Prostaglandins, including cyclopentenone species, are increased in ischemic brain
Concentrations peak at 24 hours after ischemia
Prostaglandin production is completely ablated by a COX-2 inhibitor
Acknowledgement
We would like to thank Li Wang, MS for her help with statistical support.
Supported by: R21HD058846 (RWH) R01NS (SHG)
Statistical support was provided by the Clinical Translational Science Institute of the University of Pittsburgh: Grant Number 5UL1 RR024153-05 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amer M, et al. Use of nonsteroidal anti-inflammatory drugs in patients with cardiovascular disease: a cautionary tale. Cardiol Rev. 2010;18:204–12. doi: 10.1097/CRD.0b013e3181ce1521. [DOI] [PubMed] [Google Scholar]
- Andreasson K. Prostaglandin signalling in cerebral ischaemia. Br J Pharmacol. 2010;160:844–6. doi: 10.1111/j.1476-5381.2010.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell Parikh LC, et al. Biosynthesis of 15-deoxy-delta12,14-PGJ2 and the ligation of PPARgamma. J Clin Invest. 2003;112:945–55. doi: 10.1172/JCI18012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose SA, Thuen BT, Golovko MY. LC/MS/MS method for analysis of E2 series prostaglandins and isoprostanes. J Lipid Res. 2011;52:850–9. doi: 10.1194/jlr.D013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelario-Jalil E, et al. Post-ischaemic treatment with the cyclooxygenase-2 inhibitor nimesulide reduces blood-brain barrier disruption and leukocyte infiltration following transient focal cerebral ischaemia in rats. J Neurochem. 2007;100:1108–20. doi: 10.1111/j.1471-4159.2006.04280.x. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Fiebich BL. Cyclooxygenase inhibition in ischemic brain injury. Curr.Pharm.Des. 2008;14:1401–1418. doi: 10.2174/138161208784480216. [DOI] [PubMed] [Google Scholar]
- Fink EL, et al. Experimental model of pediatric asphyxial cardiopulmonary arrest in rats. Pediatr.Crit Care Med. 2004;5:139–144. doi: 10.1097/01.pcc.0000112376.29903.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon B, et al. Proteomic studies on protein modification by cyclopentenone prostaglandins: expanding our view on electrophile actions. J Proteomics. 2011;74:2243–63. doi: 10.1016/j.jprot.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Hewett SJ, Bell SC, Hewett JA. Contributions of cyclooxygenase-2 to neuroplasticity and neuropathology of the central nervous system. Pharmacol.Ther. 2006 doi: 10.1016/j.pharmthera.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Hickey RW, et al. Cyclooxygenase-2 Activity Following Traumatic Brain Injury in the Developing Rat. Pediatr.Res. 2007:271–276. doi: 10.1203/PDR.0b013e3180db2902. [DOI] [PubMed] [Google Scholar]
- Higdon A, et al. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem J. 2012;442:453–64. doi: 10.1042/BJ20111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, et al. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc.Natl.Acad.Sci.U.S.A. 2001;98:1294–1299. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Gorelick PB. The Janus face of cyclooxygenase-2 in ischemic stroke: shifting toward downstream targets. Stroke. 2005;36:182–185. doi: 10.1161/01.STR.0000153797.33611.d8. [DOI] [PubMed] [Google Scholar]
- Koharudin LM, et al. Cyclopentenone prostaglandin-induced unfolding and aggregation of the Parkinson disease-associated UCH-L1. Proc.Natl.Acad.Sci.U.S.A. 2010;107:6835–6840. doi: 10.1073/pnas.1002295107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, et al. 15-Deoxy-Delta(12,14)-prostaglandin J(2): the endogenous electrophile that induces neuronal apoptosis. Proc.Natl.Acad.Sci.U.S.A. 2002;99:7367–7372. doi: 10.1073/pnas.112212599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levonen AL, et al. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem.J. 2004;378:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, et al. Modification of ubiquitin-C-terminal hydrolase-L1 by cyclopentenone prostaglandins exacerbates hypoxic injury. Neurobiol Dis. 2010 doi: 10.1016/j.nbd.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TM, et al. Rapid, simultaneous quantitation of mono and dioxygenated metabolites of arachidonic acid in human CSF and rat brain. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3991–4000. doi: 10.1016/j.jchromb.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, et al. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci U S A. 1998;95:10954–10959. doi: 10.1073/pnas.95.18.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogawa S, et al. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogawa S, et al. Interaction between inducible nitric oxide synthase and cyclooxygenase-2 after cerebral ischemia. Proc Natl Acad Sci U S A. 1998;95:10966–10971. doi: 10.1073/pnas.95.18.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell WS. 15-Deoxy-delta12,14-PGJ2: endogenous PPARgamma ligand or minor eicosanoid degradation product? J Clin Invest. 2003;112:828–30. doi: 10.1172/JCI19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gomez FJ, et al. Protein thiol modification by 15-deoxy-Delta12,14-prostaglandin J2 addition in mesangial cells: role in the inhibition of pro-inflammatory genes. Mol.Pharmacol. 2004;66:1349–1358. doi: 10.1124/mol.104.002824. [DOI] [PubMed] [Google Scholar]
- Satoh T, Lipton SA. Redox regulation of neuronal survival mediated by electrophilic compounds. Trends Neurosci. 2007;30:37–45. doi: 10.1016/j.tins.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Takei S, et al. Immunohistochemical demonstration of increased prostaglandin F(2)alpha levels in the rat hippocampus following kainic acid-induced seizures. Neuroscience. 2012;218:295–304. doi: 10.1016/j.neuroscience.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, et al. Prostaglandin D2 protects neonatal mouse brain from hypoxic ischemic injury. J.Neurosci. 2007;27:4303–4312. doi: 10.1523/JNEUROSCI.0321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Shibata T. 15-Deoxy-Delta(12,14)-prostaglandin J2: an electrophilic trigger of cellular responses. Chem Res Toxicol. 2008;21:138–44. doi: 10.1021/tx700177j. [DOI] [PubMed] [Google Scholar]