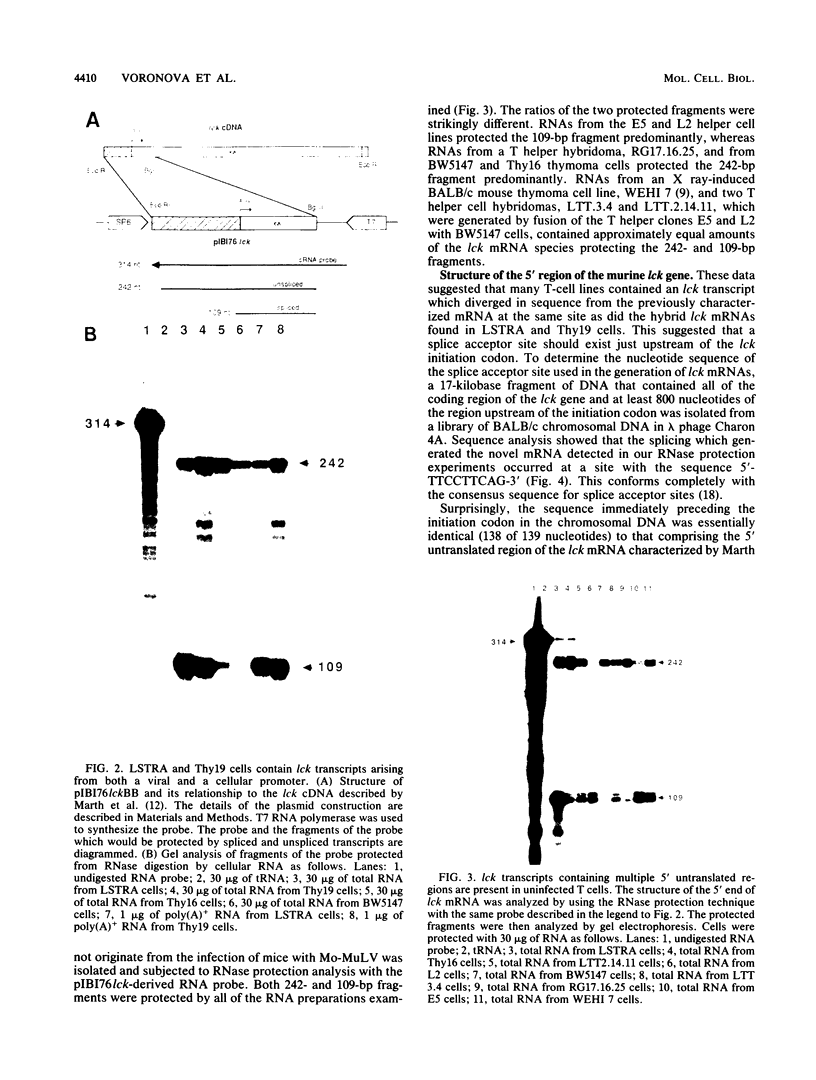
Abstract
p56lck is a new member of the src family of cellular tyrosine protein kinases. It is expressed constitutively at a low level in normal T cells and at an elevated level in the LSTRA and Thy19 Moloney murine leukemia virus-induced thymoma cell lines. It is possible that the expression of p56lck at an elevated level contributes to the transformation of these thymoma cells. The structure of the mRNAs encoding p56lck was examined by using an RNase protection assay. Both a chimeric lck mRNA containing the 5' untranslated region of Moloney virus mRNA and a normal lck mRNA were found in Thy19 and LSTRA cells. The chimeric lck transcript was 4- to 10-fold more abundant than the normal transcript. Transcription arising from a viral promoter is therefore responsible for the elevated levels of lck mRNA in these two cell lines. Surprisingly, uninfected murine T cells were also found to contain lck transcripts with differing 5' untranslated regions. One species of mRNA was colinear with the region of the chromosome just upstream of the initiation codon for p56lck. The other appeared to arise through splicing of an unidentified 5' untranslated exon to a sometimes cryptic splice acceptor just upstream of the region encoding p56lck. These data suggest that lck is expressed through the use of at least two different promoters. The promoters could be subject to different forms of regulation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth R. K., Kim B. S., Lan N. C., Hunkapiller T., Sobieck N., Winoto A., Gershenfeld H., Okada C., Hansburg D., Weissman I. L. The murine T-cell receptor uses a limited repertoire of expressed V beta gene segments. Nature. 1985 Aug 8;316(6028):517–523. doi: 10.1038/316517a0. [DOI] [PubMed] [Google Scholar]
- Benyajati C., Spoerel N., Haymerle H., Ashburner M. The messenger RNA for alcohol dehydrogenase in Drosophila melanogaster differs in its 5' end in different developmental stages. Cell. 1983 May;33(1):125–133. doi: 10.1016/0092-8674(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Casnellie J. E., Harrison M. L., Hellstrom K. E., Krebs E. G. A lymphoma cell line expressing elevated levels of tyrosine protein kinase activity. J Biol Chem. 1983 Sep 10;258(17):10738–10742. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN J. P., BLANCO A. R., GOLDIN A. STUDIES ON INDUCED RESISTANCE AGAINST ISOTRANSPLANTS OF VIRUS-INDUCED LEUKEMIA. Cancer Res. 1964 Apr;24:502–508. [PubMed] [Google Scholar]
- Hagenbüchle O., Tosi M., Schibler U., Bovey R., Wellauer P. K., Young R. A. Mouse liver and salivary gland alpha-amylase mRNAs differ only in 5' non-translated sequences. Nature. 1981 Feb 19;289(5799):643–646. doi: 10.1038/289643a0. [DOI] [PubMed] [Google Scholar]
- Harris A. W., Bankhurst A. D., Mason S., Warner N. L. Differentiated functions expressed by cultured mouse lymphoma cells. II. Theta antigen, surface immunoglobulin and a receptor for antibody on cells of a thymoma cell line. J Immunol. 1973 Feb;110(2):431–438. [PubMed] [Google Scholar]
- Hyman R., Stallings V. Complementation patterns of Thy-1 variants and evidence that antigen loss variants "pre-exist" in the parental population. J Natl Cancer Inst. 1974 Feb;52(2):429–436. doi: 10.1093/jnci/52.2.429. [DOI] [PubMed] [Google Scholar]
- Koga Y., Caccia N., Toyonaga B., Spolski R., Yanagi Y., Yoshikai Y., Mak T. W. A human T cell-specific cDNA clone (YT16) encodes a protein with extensive homology to a family of protein-tyrosine kinases. Eur J Immunol. 1986 Dec;16(12):1643–1646. doi: 10.1002/eji.1830161229. [DOI] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Müller D. Co-transfection of normal NIH/3T3 DNA and retroval LTR sequences: a novel strategy for the detection of potential c-onc genes. EMBO J. 1984 May;3(5):1121–1127. doi: 10.1002/j.1460-2075.1984.tb01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. The arrangement and rearrangement of antibody genes. Nature. 1978 Dec 21;276(5690):790–795. doi: 10.1038/276790a0. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Speculations on RNA splicing. Cell. 1981 Mar;23(3):643–646. doi: 10.1016/0092-8674(81)90425-6. [DOI] [PubMed] [Google Scholar]
- Trevillyan J. M., Lin Y., Chen S. J., Phillips C. A., Canna C., Linna T. J. Human T lymphocytes express a protein-tyrosine kinase homologous to p56LSTRA. Biochim Biophys Acta. 1986 Oct 10;888(3):286–295. doi: 10.1016/0167-4889(86)90228-4. [DOI] [PubMed] [Google Scholar]
- Vogt M. Properties of "mink cell focus-inducing" (MCF) virus isolated from spontaneous lymphoma lines of BALB/c mice carrying Moloney leukemia virus as an endogenous virus. Virology. 1979 Feb;93(1):226–236. doi: 10.1016/0042-6822(79)90290-3. [DOI] [PubMed] [Google Scholar]
- Voronova A. F., Buss J. E., Patschinsky T., Hunter T., Sefton B. M. Characterization of the protein apparently responsible for the elevated tyrosine protein kinase activity in LSTRA cells. Mol Cell Biol. 1984 Dec;4(12):2705–2713. doi: 10.1128/mcb.4.12.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronova A. F., Sefton B. M. Expression of a new tyrosine protein kinase is stimulated by retrovirus promoter insertion. Nature. 1986 Feb 20;319(6055):682–685. doi: 10.1038/319682a0. [DOI] [PubMed] [Google Scholar]