Abstract
We investigated the plasticity effects of diabetes mellitus and diuresis on the non-adrenergic non-cholinergic (NANC) and purinergic (P2X-type) contractile responses in longitudinal rat bladder strips. Female Sprague-Dawley rats received streptozotocin to induce diabetes, or sucrose in water to induce diuresis as a control condition for polyuria. Experiments were carried out at four weeks after treatments, using bladders from not-treated rats as control. Urinary bladder strips were electrically stimulated throughout the experiments to generate neurally evoked contractions (NEC). In all cases, P2X-mediated purinergic contractions were evaluated at the beginning and end of the stimulations with α,β-Methylene Adenosine Triphosphate (α,βMeATP). The NANC responses were assessed by using two independent protocols. First, cholinergic receptors were activated with carbachol (CCh), followed by inhibition of the muscarinic component with atropine. In the second protocol, the application order for CCh and atropine was reversed. The NANC response, unmasked with the application of atropine, and the P2X purinergic contractions were analyzed. NANC contractions in diabetic bladder strips are more resistant to the desensitizing effects caused by activation of cholinergic receptors. In early stages of experimental diabetes, NANC responses in diabetic strips are less sensitive to functional inhibition mediated by the cholinergic activation. However, P2X-mediated purinergic contractions are more sensitive to desensitization in diabetic or diuretic bladders. For instance preventing muscarinic receptor activation with atropine does not counteract the desensitization of purinergic contractions in either diabetic or diuretic strips. We suggest that diabetes may induce a plasticity of the NANC and P2X-mediated bladder contractile responses. The first one may be associated with diabetic neuropathic damage to bladder nerves, while impaired P2X purinergic contractions might be associated with detrusor hypertrophy observed in diabetic and diuretic strips.
Keywords: Diabetic bladder dysfunction, purinergic receptor, cholinergic receptor, non-adrenergic non-cholinergic response, neurally evoked contraction, detrusor muscle
1. Introduction
Neurogenic detrusor contractions are driven by the parasympathetic release of several transmitters, among them acetylcholine acting on cholinergic receptors to initiate the efferent side of the micturition reflex [8, 31]. Furthermore, activation of M3 muscarinic receptors by acetylcholine released from efferent nerve terminals is fundamental for bladder contractions [1]. Experimentally, the application of an electrical field stimulation to urinary bladder strips generates a neurally evoked contraction (NEC) characterized by high levels of acetylcholine acting on detrusor muscarinic receptors [23]. Although nor-epinephrine is also released by electrical stimulation [22], in the rat bladder it activates β3-adrenergic receptors to facilitate detrusor relaxation rather than adrenergic contractions [27, 31].
The pharmacological inhibition of cholinergic receptors using atropine [13] or specific M-type muscarinic antagonists such as 4-Diphenylacetoxy-N-methylpiperidine (4-DAMP), unmasks a remaining NEC known as the non-adrenergic non-cholinergic (NANC) contractile response [2, 12]. In rat detrusor the NANC response is mostly mediated by the activation of P2X-type purinergic receptors by co-released ATP, and can be revealed by pharmacological inhibition of cholinergic transmission [9, 13, 26].
We previously reported that activation of cholinergic receptors with carbachol (CCh) decreases NANC contractions via a desensitization mechanism most probably involving P2X-type purinergic receptors in intact rat bladders [13]. Moreover, the specific inhibition of bladder M-type muscarinic receptors with 4-DAMP prevents the attenuation of the NANC responses, thus suggesting that activation of muscarinic receptors may desensitize a purinergic contractile component mediated by P2X-type receptors [12, 13, 26].
In this report we used detrusor strips from diabetic, diuretic and normal rats to expand our understanding between cholinergic and purinergic signaling in regulating the plasticity of NANC and P2X-mediated bladder contractions in pathological conditions. By following a similar approach as in previous studies [12, 13], we calculated the changes in NEC and NANC by measuring strip contractile changes while preventing muscarinic receptor activation with a high dose of atropine. We also determined the degree of desensitization for the purinergic component by comparing P2X purinergic receptor contractions at the beginning and end of the stimulation protocols.
2. Materials and Methods
Animal procedures were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine, and followed the research standards suggested by the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
2.1. Experimental animal groups
Female Sprague-Dawley rats weighing 250 to 300 g were use during the course of the experiments. Diabetes was induced with a single intravenous tail injection of 50 mg/Kg streptozotocin (STZ; VWR, Radnor, PA), prepared in 100 mM citrate buffer at pH 4.0 followed by a subcutaneous glucose injection (1.0 g/Kg) to prevent severe hypoglycemia. In a second group of rats serving as diuretic control for bladder hypertrophy, diuresis was induced by complementing ad-libitum drinking water with 5% sucrose [15]. Control animals received regular water and i.v. injected with vehicle alone. Blood glucose was weekly monitored with a One Touch Ultra glucometer (LifeScan; Milpitas, CA). STZ-injected rats with persistent blood glucose levels above 250 mg/dl were included in the study.
2.2. Preparation of rat bladder strips for contractile evaluations
Contractile experiments were performed 4 weeks after the induction of diabetes or diuresis. At this time point rats were euthanized and bladders removed into an oxygenated Krebs solution containing (in mM): NaCl (113); KCl (4.7); CaCl2 (1.25); MgSO4 (1.2); NaHCO3 (25); KH2PO4 (1.2); D-glucose (11.5). Solution temperature was set to 37°C and the pH was kept at 7.4 by a continuous bubbling of a 95% O2/5% CO2 gas mixture. After dissecting the whole bladder above the trigone region, two longitudinal strips were obtained from each bladder and mounted in tissue baths filled with oxygenated and warmed Krebs solution. For comparison purposes with our previous studies, special care was taken to preserve urothelial integrity in the bladder strips. Contractile experiments were run in parallel for diabetic/diuretic, diabetic/control or diuretic/control group combinations. A pretension of 10 mN was applied to each strip. After a recovery period of 30 minutes, isometric contractions were determined with a force transducer connected to a 4-channel trans-bridge amplifier (both from WPI; Sarasota, FL). NEC contractions were induced via platinum wire electrodes that generated an electrical field stimulation with square wave impulses (0.25 ms; 20Hz; 200 shocks every 100 s) at 100 V using an S-88 dual channel stimulator (Grass Technologies; West Warwick, RI). This voltage was enough to generate a supra-maximal contractile response from the detrusor strips. Data were collected in real time using the Windaq acquisition software (DataQ; Akron, OH) at a sampling rate of 20 Hz. Chemically induced P2X-type purinergic contractions were determined without electrical stimulation as described below.
2.3. Evaluation of NANC and purinergic contractions
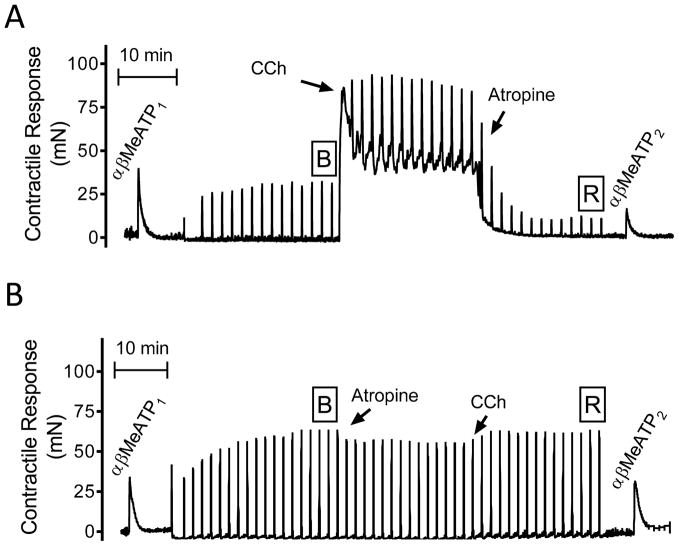
As performed in our previous studies, two stimulation protocols were achieved on each half bladder [12, 13]. In all cases the intact P2X purinergic contractile response was evaluated with the application of the specific P2X agonist α, β-Methylene-Adenosine Triphosphate (αβMeATP) using a single dose of 50 μM at the beginning of each contractile experiment. The stimulation protocols for NEC, NANC and the application of 50 μM carbachol (a cholinergic agonist) or 1 μM atropine (a muscarinic receptor antagonist) are illustrated in figure 1. Briefly, after an initial P2X stimulation (i.e. αβMeATP1) and subsequent wash-out, NECs were induced until the amplitude of these contractions was stable (as indicated with the boxed-B). Then, the NANC bladder contractions were unmasked by adding atropine after either the application of CCh (figure 1A; CCh to atropine group) or by atropine followed by CCh application (figure 1B; atropine to CCh group).
Figure 1.
Stimulation protocols for generation and evaluation of purinergic, neurally evoked (NEC) and non-adrenergic non-cholinergic (NANC) contractions. (A) Representative contractile responses for the protocol where carbachol (CCh) was applied before atropine. (B) Representative contractile responses for the protocol where atropine was applied before CCh. See the corresponding material and methods section for a detailed description of the evaluation protocols.
The percentage of the residual contraction that remained after atropine administration (boxed R in figure 1) was considered as the NANC contractile response. To determine the degree of purinergic contraction desensitization induced by activation of cholinergic receptors, a second application of the same P2X purinergic receptor agonist (i.e. αβMeATP2) was performed without washing-out the CCh/atropine mixture of the organ bath. Purinergic contractions were evaluated approximately 60 minutes apart. Bladder strip viability was verified by raising the extracellular potassium concentration to 140 mM KCl+. For comparison, we calculated the percent of the NANC contraction change as the % of the R/B proportion. The degree of purinergic contractile desensitization evoked by αβMeATP was determined as the percentage of the αβMeATP2/αβMeATP1 quotient.
2.4. Reagents
Krebs salts, αβ-MeATP, sucrose, carbachol and atropine were from Sigma-Aldrich (St. Louis, MO).
2.5. Statistical analysis and figure preparation
Bladder strips from 6 control, 6 diabetic or 6 diuretic rats received were stimulated with the protocols shown in figure 1 and included for analysis (i.e. N=6 per experimental condition). Data are expressed as mean +/− S.E.M. Statistical analysis was performed by one-way ANOVA followed by a Tukey’s post-test to compare NANC and purinergic contractions in control, diabetic and diuretic groups for each of the two stimulation protocols. Contractile traces were imported from the Windaq acquisition software (DataQ; Akron, OH). Figure preparation and statistical analysis were performed with Prism 6.0 (GraphPad Prism; San Diego, CA). Statistical significance was considered at p<0.05.
3. Results
3.1. Diabetes and diuresis effects on body weight, blood glucose and neurally evoked contractions
Four weeks after destruction of β-cells with STZ, the body weight of diabetic rats was 243+/−4 g (*p<0.05 vs control) while no statistical differences were observed between control (302+/−12 g) and sucrose fed (289+/−10 g) animals (figure 2A). Blood glucose levels were significantly elevated in STZ-treated rats (424+/−21 mg/dl; ***p<0.001) in comparison with control (108+/−8 mg/dl) or diuretic (133+/−9 mg/dl) rats (figure 2B). Figure 2C shows the distribution for the NEC amplitude for all the strips receiving the stimulation protocols shown in figure 1, that is, contractions at the time indicated by the boxed-B. The mean value+/−S.E.M. for the magnitude of the initial NEC response in control (1.71+/−0.4 mN/mg), diabetic (0.95+/−0.2 mN/mg) or diuretic strips (2.31+/−0.3 mN/mg) is also illustrated. A statistical difference in the NEC amplitude was observed between diabetic vs diuretic bladder strips (**p<0.01).
Figure 2.
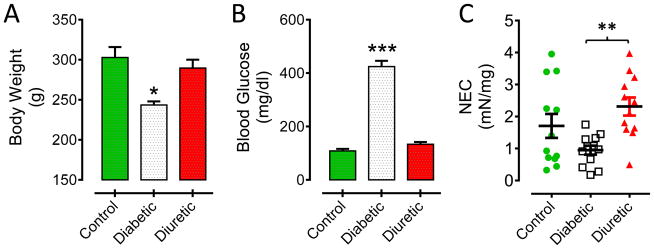
Physiological effects of diabetes and diuresis on: (A) body weight (*p<0.05 vs control and diuretic); (B) blood glucose (***p<0.001 vs control and diuretic) and (C) NEC amplitude (**p<0.01 for diabetic vs diuretic bladder strips).
3.2. Cholinergic receptor activation is more effective in decreasing the NANC response in control and diuretic, but not in diabetic bladder strips
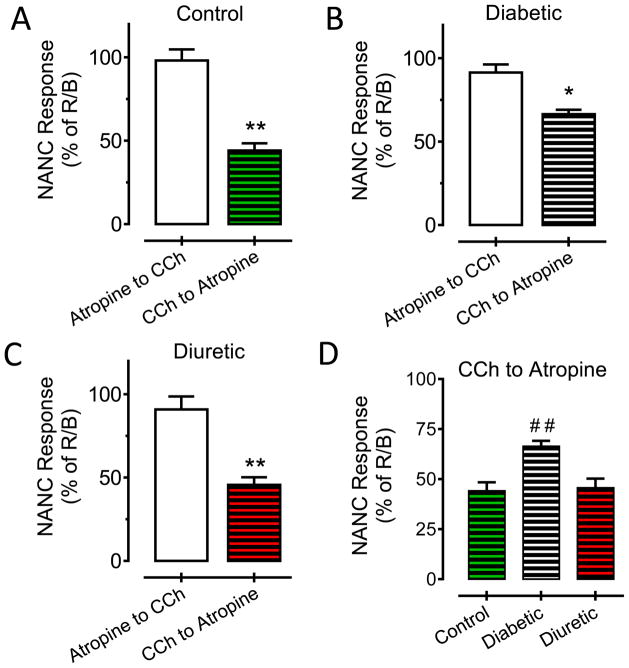
The amplitude of the NANC response in control bladder strips was attenuated 56% when cholinergic receptors were activated with CCh and later inhibited with atropine (figure 3A; **p<0.01 vs atropine to CCh). On the contrary, the NANC responses in diabetic bladder strips were less sensitive to the inhibitory effects caused by cholinergic receptor activation (figure 3B; 34%; *p<0.05 vs atropine to CCh). The degree of NANC inhibition in diuretic strips was the same as in control strips (figure 3C; 55%; **p<0.01). A statistical analysis for the NANC responses when stimulating with CCh before blocking muscarinic receptors with atropine, showed that the NANC contractions in diabetic bladder strips were more resistant to the desensitizing effect produced by CCh (figure 3D; ##p<0.01 vs both control or diuretic CCh to atropine condition). The application of atropine before cholinergic stimulation with CCh significantly prevented the reduction of the NANC contractile amplitude in control (2% decrease), diabetic (8% decrease) or diuretic (9% decrease) rat bladder strips (figure 3A–C, empty bars; p>0.05 vs NEC responses).
Figure 3.
Diabetic effects on non-adrenergic non-cholinergic bladder contractions. (A) NANC responses in bladder strips from control rats (**p<0.01 vs control atropine to CCh condition). (B) NANC responses in bladder strips from diabetic rats (*p<0.05 vs diabetic atropine to CCh condition). (C) NANC responses in bladder strips from diuretic rats (**p<0.01 vs diuretic atropine to CCh condition). (D) Comparisons for the NANC responses during the CCh to atropine protocol in bladder strips from control, diabetic and diuretic rats (# #p<0.01 vs control or diuretic strips).
3.3. Effects of cholinergic receptor activation on P2X-mediated purinergic bladder contractions
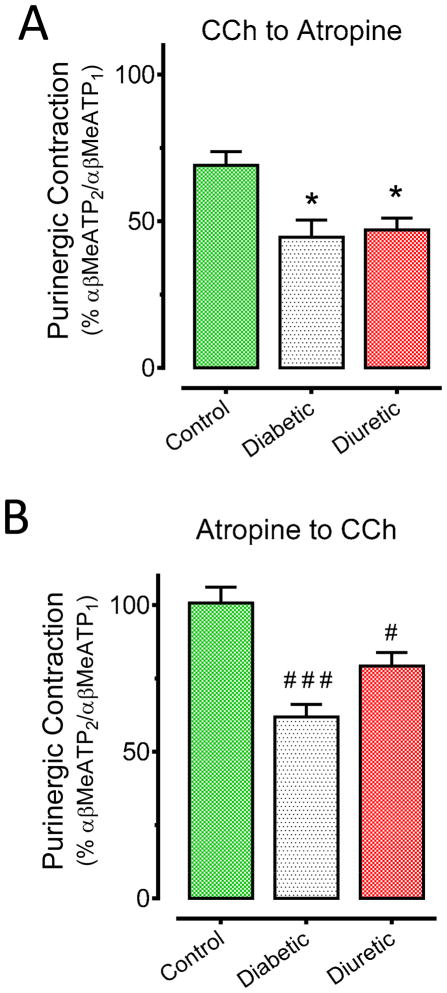
During control contractile experiments we found that a second P2X stimulation (i.e. αβMeATP2) approximately 60 minutes after a first αβMeATP application produced a contraction of identical magnitude only if the first P2X agonist was washed-out and no other electrical or chemical stimulation induced (not shown). The evaluation of the P2X purinergic contractions before (i.e. αβ-MeATP1) and after (i.e. αβ-MeATP2) the stimulation protocol described in figure 1A (CCh to atropine) showed that both diabetic (57%; *p<0.05 vs control CCh to atropine) and diuretic (53%; *p<0.05 vs control CCh to atropine) bladder strips have a higher degree of desensitization than control strips (31%; figure 4A). Following the stimulation protocol described in figure 1B (atropine to CCh), we found that both purinergic contractions had the same contractile magnitude in control strips. However, they were significantly desensitized in diabetic (39%; ###p<0.001 vs control atropine to CCh) and diuretic (21%; #p<0.05 vs control atropine to CCh) bladder strips independently of the cholinergic inhibition (figure 4B).
Figure 4.
Effects of carbachol and atropine on P2X-mediated purinergic contractions. (A) Activation of cholinergic receptors produces a higher purinergic desensitization in diabetic and diuretic bladder strips than in controls (*p<0.05 vs control CCh to atropine condition). (B) Cholinergic inhibition with atropine does not entirely prevent purinergic desensitization in diabetic or diuretic bladder strips after application of CCh (###p<0.001 and #p<0.05 vs control atropine to CCh condition).
4. Discussion
Our results show that NANC contractions in diabetic bladder strips are more resistant to the desensitizing effects caused by activation of cholinergic receptors with CCh. In contrast to normal bladders, the P2X-mediated purinergic contractions are more susceptible to desensitization induced by cholinergic receptor activation in both diabetic and diuretic bladder strips. These observations suggest that preventing cholinergic receptor activation with atropine does not effectively counteract, as observed in normal rats, the desensitization of purinergic and NANC contractions in either diabetic or diuretic bladder strips. Changes in bladder weight and cystometric properties of diabetic rats have demonstrated that these animals develop a specific bladder cystopathy that may involve detrusor and nerve dysfunction [15, 18]. In fact, neuropathy is one of the most common complications of diabetes, affecting the quality of life of patients by decreasing nerve functionality and therefore peripheral sensation [29]. Under hyperglycemic conditions nerve fibers progressively degenerate in diabetic neuropathy affecting autonomic nerve functions [20], for instance the release of different transmitters regulating bladder contractions.
The main origin of NEC and NANC responses is pre-synaptic, as demonstrated by applying tetrodotoxin during electrical field stimulation [7]. We have perform continuous electrical stimulations for periods of 60–90 minutes using the parameters indicated and did not see a significant decrease in NEC responses. Thus, we are confident that the stimulation protocol by itself does not generate a contractile desensitization. Studies in diabetic animal models have found that diabetes can induce either an increase [25] or a decrease [10] in the amplitude of the NEC. We found that NECs have a significant trend towards reduced contractile magnitudes in diabetic strips. This may be explained by reduced efferent nerve activation during electrical stimulation, verified by the application of tetrodotoxin, and associated with a chronic hyperglycemia during diabetes [14]. The specificity of this diabetes-induced damage is supported by the observation that either sucrose-induced diuresis or STZ-provoked diabetes generate a hypertrophy of both detrusor and urothelium, however, only the diabetic bladders show significant increased levels for markers of oxidative stress that may affect nerve function [30]. We hypothesize that impaired NANC and P2X-mediated contractions in diabetic cystopathy are a consequence of a significant reduction in the nerve density at the detrusor layer. This is supported by the observation that diabetic bladders present an important reduction in the expression of neurofilament 200-immunoreactive nerves when normalizing by the detrusor area. This neuropathic diabetic effect was statistically significant at 9 or 20 weeks, with a reduction trend already evident by one week after diabetes induction [16]. The similar magnitudes for the NECs in control and diuretic strips in our study suggests that after 4 weeks of STZ-treatment, contractile mechanisms in diabetic bladders have been already affected, mainly by oxidative stress [30].
Other regulatory players may be affected by diabetes thus contributing to impaired NANC and P2X purinergic contractions. For example, urothelial cells are important contributors of ATP release for the mediation of bladder sensation during filling [3, 11, 17]. One of the main actions for the ATP released from the urothelium is the activation of ionotropic purinergic receptors in nerve terminals to convey sensory transmission, especially through P2X3-type ionotropic purinergic receptors, to the central nervous system [6, 19]. The tissue bath application of αβMeATP can activate either nerve terminal/urothelial P2X3- receptors as well as detrusor P2X1 purinergic receptors. Consequently, with our in vitro preparation an afferent effect of P2X3 activation on the strip contractile purinergic response is not expected, thus it is possible to suggest that the observed desensitizing effect mainly involves desensitization of P2X1-type purinergic receptors expressed in detrusor.
The desensitization of P2X1 purinergic contractions on the diuretic and diabetic detrusor seems to be enhanced by the application of CCh, confirming a specific muscarinic receptor inhibitory interaction taking place in smooth muscle cells [12, 13]. In fact, the IC50 doses of atropine for M2/M3 muscarinic receptor inhibition are in the nM range [4], thus we predict that 1 μM was enough for blocking all muscarinic receptor isoforms, supporting the idea that muscarinic receptor-activation is a key event in mediating the impairment of NANC and P2X-mediated bladder contractions. Nevertheless, in contrast to NANC responses from rats with spinal cord injury [12], the plasticity of the NANC contractions in bladder strips from diabetic rats appear to be less sensitive to muscarinic receptor activation. Because we used the same CCh dose in control or diuretic strips, it is possible to suggest that perhaps a higher expression of muscarinic receptors in diabetic bladders [5, 24] may be supporting the lower desensitizing effect observed in diabetic strips. Additionally, the reduced degree of desensitization for the NANC response in diabetes may not entirely depend on muscarinic receptor activation at the detrusor, but also on reduced neural components affected by diabetes [14, 16]. We believe that the small increase in the contractile tone when applying CCh after atropine is produced by a non-specific activation of other cholinergic receptors that present atropine resistance.
Additional studies have shown that in rats with neurogenic or diabetic bladder overactivity the release of ATP from the urothelial bladder layer is enhanced [18, 21], and might be an important contributor to altering the micturition reflex by generating a high frequency of non-voiding contractions via the activation of P2X1 receptors on the detrusor [6]. Similarly, the inhibition of P2X3-formed purinergic receptors in bladder afferent nerve fibers of rats with spinal cord injury can decrease neural sensory activity and the occurrence of non-voiding contractions [19]. In any case, the inhibition of bladder purinergic receptors, and consequently modulation of the NANC purinergic component, may be an important pharmacological target to ameliorate urinary bladder dysfunction in different human pathological states [28, 31].
5. Conclusions
Our results suggest that diabetes produces a plasticity effect on detrusor contractile components in opposite directions to create a more resistant NANC response but a P2X- mediated contraction with higher degree of desensitization. Targeting cholinergic pathways in bladder dysfunction may alter the responsiveness of non-cholinergic components. However, it is possible that an early intervention targeting NANC and/or P2X-mediated bladder contractile responses in diabetic cystopathy may be helpful to ameliorate bladder dysfunction.
Highlights.
Activation of cholinergic receptors inhibits P2X and NANC bladder contractions
Neurally evoked contractions are smaller in diabetic when compared with diuretic bladder strips
Diabetic NANC responses are less sensitive to inhibition by cholinergic receptor activation
P2X-mediated contractions are more desensitized in diabetic and diuretic bladder strips
Acknowledgments
All research work was performed at the Scott Department of Urology from Baylor College of Medicine, Houston, TX.
We would like to thank Dr. Zaneta Romain for helping during preliminary experiments. This research was supported by the National Institutes of Health (RO1-DK069988 to G.T.S.).
Abbreviations
- NEC
neurally-evoked contraction
- NANC
non-adrenergic non-cholinergic
- 4-DAMP
4-Diphenylacetoxy-N-methylpiperidine
- ATP
Adenosine Triphosphate
- α, βMeATP
α,β-Methylene- Adenosine Triphosphate
- P2X
ionotropic purinergic receptor
- CCh
carbachol
Footnotes
Conflict of interest
Authors do not have any Conflict of Interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abrams P, Andersson KE, Buccafusco JJ, Chapple C, de Groat WC, Fryer AD, Kay G, Laties A, Nathanson NM, Pasricha PJ, Wein AJ. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol, England. 2006:565–578. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambache N, Zar MA. Non-cholinergic transmission by post-ganglionic motor neurones in the mammalian bladder. The Journal of physiology. 1970;210:761–783. doi: 10.1113/jphysiol.1970.sp009240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birder LA. Urinary bladder urothelium: molecular sensors of chemical/thermal/mechanical stimuli. Vascul Pharmacol. 2006;45:221–226. doi: 10.1016/j.vph.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Cawley TA, Jr, Shickley TJ, Ruggieri MR, Luthin GR. Effect of chronic neuroleptic treatment on central and peripheral muscarinic receptors. The Journal of pharmacology and experimental therapeutics. 1993;267:134–139. [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng JT, Yu BC, Tong YC. Changes of M(3)-muscarinic receptor protein and mRNA expressions in the bladder urothelium and muscle layer of streptozotocin-induced diabetic rats. Neuroscience letters. 2007 doi: 10.1016/j.neulet.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 6.Ford AP, Cockayne DA. ATP and P2X purinoceptors in urinary tract disorders. Handb Exp Pharmacol. 2011:485–526. doi: 10.1007/978-3-642-16499-6_22. [DOI] [PubMed] [Google Scholar]
- 7.Fovaeus M, Andersson KE, Andersson PO, Malmgren A, Sjogren C. Tetrodotoxin-resistant contractions induced by electrical stimulation of bladder muscle from man, rabbit and rat. Acta physiologica Scandinavica. 1988;132:233–239. doi: 10.1111/j.1748-1716.1988.tb08322.x. [DOI] [PubMed] [Google Scholar]
- 8.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nature reviews Neuroscience. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoyle CH. Non-adrenergic, non-cholinergic control of the urinary bladder. World J Urol. 1994;12:233–244. doi: 10.1007/BF00191202. [DOI] [PubMed] [Google Scholar]
- 10.Ichiyanagi N, Tsujii T, Masuda H, Kihara K, Goto M, Azuma H. Changed responsiveness of the detrusor in rabbits with alloxan induced hyperglycemia: possible role of 5-hydroxytryptamine for diabetic bladder dysfunction. J Urol. 2002;168:303–307. [PubMed] [Google Scholar]
- 11.Kumar V, Chapple CR, Rosario D, Tophill PR, Chess-Williams R. In vitro release of adenosine triphosphate from the urothelium of human bladders with detrusor overactivity, both neurogenic and idiopathic. European urology. 2010;57:1087–1092. doi: 10.1016/j.eururo.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 12.Lai HH, Munoz A, Smith CP, Boone TB, Somogyi GT. Plasticity of non-adrenergic non-cholinergic bladder contractions in rats after chronic spinal cord injury. Brain Res Bull. 2011;86:91–96. doi: 10.1016/j.brainresbull.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai HH, Smith CP, Munoz A, Boone TB, Szigeti GP, Somogyi GT. Activation of cholinergic receptors blocks non-adrenergic non-cholinergic contractions in the rat urinary bladder. Brain Res Bull. 2008;77:420–426. doi: 10.1016/j.brainresbull.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu G, Daneshgari F. Alterations in neurogenically mediated contractile responses of urinary bladder in rats with diabetes. Am J Physiol Renal Physiol. 2005;288:F1220–1226. doi: 10.1152/ajprenal.00449.2004. [DOI] [PubMed] [Google Scholar]
- 15.Liu G, Daneshgari F. Temporal diabetes- and diuresis-induced remodeling of the urinary bladder in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R837–843. doi: 10.1152/ajpregu.00917.2005. [DOI] [PubMed] [Google Scholar]
- 16.Liu G, Li M, Vasanji A, Daneshgari F. Temporal diabetes and diuresis-induced alteration of nerves and vasculature of the urinary bladder in the rat. BJU International. 2011;107:1988–1993. doi: 10.1111/j.1464-410X.2010.09840.x. [DOI] [PubMed] [Google Scholar]
- 17.Munoz A, Gangitano DA, Smith CP, Boone TB, Somogyi GT. Removal of urothelium affects bladder contractility and release of ATP but not release of NO in rat urinary bladder. BMC urology. 2010;10:10. doi: 10.1186/1471-2490-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munoz A, Smith CP, Boone TB, Somogyi GT. Overactive and underactive bladder dysfunction is reflected by alterations in urothelial ATP and NO release. Neurochem Int. 2011;58:295–300. doi: 10.1016/j.neuint.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz A, Somogyi GT, Boone TB, Ford AP, Smith CP. Modulation of bladder afferent signals in normal and spinal cord-injured rats by purinergic P2X3 and P2X2/3 receptors. BJU Int. 2012 doi: 10.1111/j.1464-410X.2012.11189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Said G, Baudoin D, Toyooka K. Sensory loss, pains, motor deficit and axonal regeneration in length-dependent diabetic polyneuropathy. Journal of neurology. 2008;255:1693–1702. doi: 10.1007/s00415-008-0999-z. [DOI] [PubMed] [Google Scholar]
- 21.Salas NA, Somogyi GT, Gangitano DA, Boone TB, Smith CP. Receptor activated bladder and spinal ATP release in neurally intact and chronic spinal cord injured rats. Neurochem Int. 2007;50:345–350. doi: 10.1016/j.neuint.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somogyi GT, Tanowitz M, Zernova G, de Groat WC. M1 muscarinic receptor-induced facilitation of ACh and noradrenaline release in the rat bladder is mediated by protein kinase C. The Journal of physiology. 1996;496(Pt 1):245–254. doi: 10.1113/jphysiol.1996.sp021681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somogyi GT, Zernova GV, Yoshiyama M, Yamamoto T, de Groat WC. Frequency dependence of muscarinic facilitation of transmitter release in urinary bladder strips from neurally intact or chronic spinal cord transected rats. Br J Pharmacol. 1998;125:241–246. doi: 10.1038/sj.bjp.0702041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens LA, Sellers DJ, McKay NG, Chapple CR, Chess-Williams R. Muscarinic receptor function, density and G-protein coupling in the overactive diabetic rat bladder. Autonomic & autacoid pharmacology, England. 2006:303–309. doi: 10.1111/j.1474-8673.2006.00371.x. [DOI] [PubMed] [Google Scholar]
- 25.Tammela TL, Leggett RE, Levin RM, Longhurst PA. Temporal changes in micturition and bladder contractility after sucrose diuresis and streptozotocin-induced diabetes mellitus in rats. J Urol. 1995;153:2014–2021. [PubMed] [Google Scholar]
- 26.Tong YC, Hung YC, Shinozuka K, Kunitomo M, Cheng JT. Evidence of adenosine 5′-triphosphate release from nerve and P2x-purinoceptor mediated contraction during electrical stimulation of rat urinary bladder smooth muscle. J Urol. 1997;158:1973–1977. doi: 10.1016/s0022-5347(01)64196-x. [DOI] [PubMed] [Google Scholar]
- 27.Tyagi P, Tyagi V. Mirabegron, a beta-adrenoceptor agonist for the potential treatment of urinary frequency, urinary incontinence or urgency associated with overactive bladder. IDrugs : the investigational drugs journal. 2010;13:713–722. [PubMed] [Google Scholar]
- 28.Vesela R, Aronsson P, Andersson M, Wsol V, Tobin G. The potential of non-adrenergic, non-cholinergic targets in the treatment of interstitial cystitis/painful bladder syndrome. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2012;63:209–216. [PubMed] [Google Scholar]
- 29.Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7:573–583. doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- 30.Xiao N, Wang Z, Huang Y, Daneshgari F, Liu G. Roles of Polyuria and Hyperglycemia in Bladder Dysfunction in Diabetes. The Journal of Urology. 2013;189:1130–1136. doi: 10.1016/j.juro.2012.08.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshimura N, Kaiho Y, Miyazato M, Yunoki T, Tai C, Chancellor MB, Tyagi P. Therapeutic receptor targets for lower urinary tract dysfunction. Naunyn-Schmiedeberg’s archives of pharmacology. 2008;377:437–448. doi: 10.1007/s00210-007-0209-z. [DOI] [PubMed] [Google Scholar]