Abstract
Coccidioides posadasii spherules stimulate macrophages to make cytokines via TLR-2 and Dectin-1. We used formalin-killed spherules and 1,3-β-glucan purified from spherules to stimulate elicited peritoneal macrophages and myeloid dendritic cells (mDCs) from susceptible (C57BL/6) and resistant (DBA/2) mouse strains. DBA/2 macrophages produced more TNF-α and IL-6 than macrophages from C57BL/6 mice, and the amount of TNF-α made was dependent on both TLR2 and Dectin-1. DCs from C57BL/6 mice made more IL-10 and less IL-23p19 and IL-12p70 than did DBA/2 DC. These responses were inhibited by a monoclonal antibody to Dectin-1. DBA/2 mice expressed full-length Dectin-1, whereas C57BL/6 mice spliced out exon 3, which encodes most of the stalk. RAW cells transduced to express the full-length Dectin-1 responded better to FKS than cells expressing truncated Dectin-1. We compared the isoform of Dectin-1 expressed by 34 C57BL/6 X DBA/2 recombinant inbred (BXD RI) lines with their susceptibility to Coccidioides immitis. In 25 of 34 RI lines susceptibility or resistance corresponded to short or full-length isoforms, respectively. These results suggest that alternative splicing of the Dectin-1 gene contributes to susceptibility of C57BL/6 mice to coccidioidomycosis, and affects the cytokine responses of macrophages and mDCs to spherules.
Keywords: cytokines, fungal, inflammation, monocytes/macrophages, dendritic cells
Introduction
Coccidioidomycosis is an airborne fungal infection that is endemic in the arid regions of the lower Sonoran life zone in the New World. Coccidioidomycosis is caused by the dimorphic fungus Coccidioides, which is now believed to include two species, Coccidioides immitis and Coccidioides posadasii.1 Infection is acquired by inhalation of arthroconidia. Once inside the body, arthroconidia become spherules, the pathognomonic parasitic form of this fungus. Spherules enlarge, endosporulate and eventually rupture, releasing thousands of endospores that can mature into spherules, thereby repeating the parasitic phase cycle. Thus, during infection the infected host is exposed to immature, mature and rupturing spherules, and newly released endospores, which differ quantitatively in their cell wall and cytoplasmic composition.2 Approximately 60% of the dry weight of C. posadasii spherules is glucan,3 and Kellner et al.,4 showed that the β-1,3 glucan synthase gene that encodes the enzyme responsible for β-glucan synthesis in C. posadasii is an essential gene. Thus, 1, 3-β-glucan is a major and essential component of the spherule cell wall.
In the majority of people, the infection is asymptomatic and self-limited, and they develop delayed hypersensitivity to the fungus manifested by a positive skin test.5 However, 5−10% of infections are not self-limited, and in many of those patients, infections disseminate to extra-pulmonary sites.6 African-Americans and Filipinos are at least 10 times more likely to develop disseminated coccidioidomycosis than are Caucasians, even if the primary pneumonia is self-limited.7 This suggests that there is a genetic basis for the extra-pulmonary dissemination of this infection in humans.
Due to the difficulty of studying the genetics of resistance to a non-communicable infection in people, we have used inbred mice to study the genetics of resistance. Inbred mice vary by several orders of magnitude in their susceptibility to coccidioidomycosis.8,9 Resistance is the dominant phenotype8,9 C57BL/6 (B6) is one of the most susceptible strains and DBA/2 is the most resistant inbred strain, whether the mice are infected intraperitoneally or intranasally.8,10 Using BXD recombinant inbred (RI) lines, we identified genetic loci on chromosomes 6 and 4 that are involved in resistance to C. immitis peritonitis in mice11 and called them Cms1 and Cms2. Subsequently Dectin-1 was discovered to be the 1,3-β- glucan receptor on myeloid cells and it is encoded on mouse chromosome 6, close to where we mapped a resistance locus.12 This led us to begin to study the role of Dectin-1 in resistance to coccidioidomycosis.
There is increasing evidence that Dectin-1 plays a major role in innate resistance to several opportunistic fungi,13 but there are almost no data on the role of Dectin-1 in resistance to any of the primary pathogenic fungi. Since 1,3-β-glucan is a prominent component of spherule wall, we tested the ability of formalin-killed spherules (FKS) of C. posadasii to release proinflammatory cytokines. We previously showed that stimulation of peritoneal macrophages by FKS and live spherules was Toll-like receptor (TLR)-2 and Dectin-1-dependent.3 This confluence of genetic mapping and in vitro evidence that macrophages recognize spherules via Dectin-1 prompted us to determine if there is a Dectin-1 polymorphism between B6 and DBA/2 strains, which could contribute to genetic susceptibility of B6 mice to Coccidioides spp.
In this paper, we compared the responses of elicited peritoneal macrophages and bone marrow-derived dendritic cells (myeloid dendritic cells (mDCs)) from B6 and DBA/2 mice to FKS and 1,3-β glucan from spherules. We showed that FKS-stimulated macrophages from B6 mice formed less tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), and their mDCs made more IL-10. We found the DBA/2 cells expressed a full-length Dectin-1, whereas B6 cells expressed a truncated stalkless isoform due to splicing out of exon 3. RAW cells transduced to express the truncated isoform of Dectin-1 also formed less TNF-α and macrophage inflammatory protein (MIP)-2 than cells expressing the full-length isoform. Furthermore, 17 of 20 C57BL/6 X DBA/2 recombinant inbred (BXD RI) lines expressing the truncated isoform were susceptible to experimental coccidioidomycosis.
Results
DBA/2J and C57BL/6 mice express different isoforms of Dectin-1
We first compared the responses of elicited peritoneal macrophages from B6 and DBA/2 mice to FKS and to 1,3-β-glucan that was isolated from spherules. As shown in Figure 1, macrophages from DBA/2 mice that were incubated overnight with FKS or 1,3-β-glucan produced twice as much TNF-α, MIP-2 (not shown) and IL-6 as did elicited peritoneal macrophages from B6 mice. Furthermore, those responses were substantially reduced by 2A11, a blocking monoclonal antibody against Dectin-1. B6 and DBA/2 macrophages responded similarly to lipopolysaccharide (LPS) and that was unaffected by 2A11 (not shown). DBA/2 macrophages also made more IL-6 in response to heat-killed Staphylococcus aureus, a TLR-2 ligand, but not to LPS, a TLR-4 ligand (Figure 1b). As we have previously shown,3 when stimulated with FKS, elicited peritoneal macrophages from TLR-2−/− mice make much less TNF-α than do control B6 macrophages (Figure 2). We found that the same was true when the cells were stimulated with β glucan from spherules. Interestingly, sDectin substantially lowered the amount of TNF-α made by stimulated B6 cells, to a level comparable to that made by TLR-2−/− cells. However, sDectin had no significant effect on the response of TLR-2−/− macrophages. Although TLR-2−/− macrophages made only 10% as much TNF-α as did B6 macrophages, the residual TNF-α production was not further reduced by pretreatment with sDectin. Thus, maximal production of TNF-α by B6 peritoneal macrophages exposed to FKS or β-glucan requires collaboration of both Dectin-1 and TLR-2.
Figure 1.
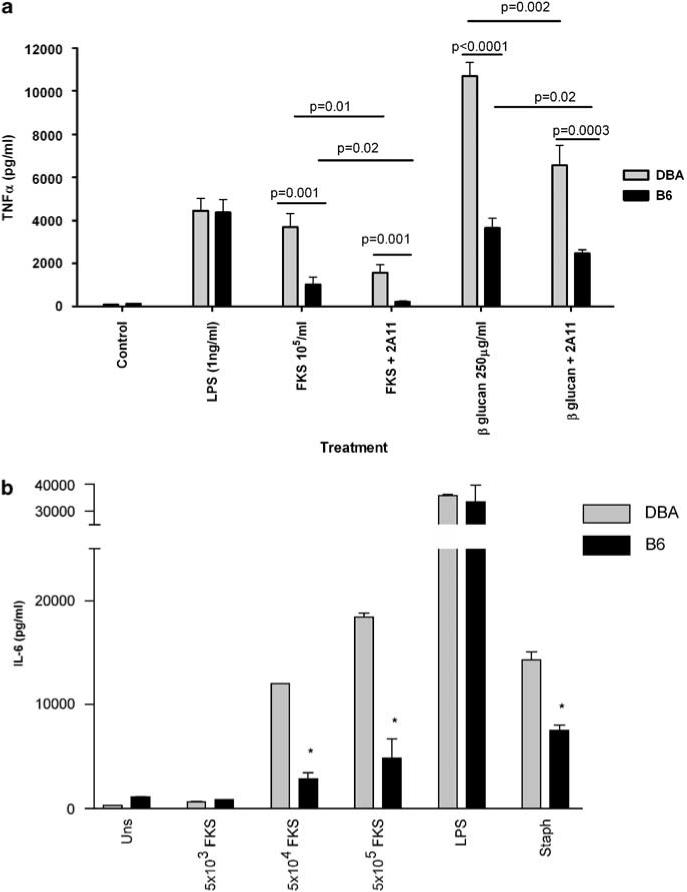
Comparison of TNF-α and IL-6 secretion by DBA/2 and C57BL/6 NaIO4-elicited peritoneal macrophages stimulated by LPS, FKS or 1,3-β-glucan from Coccidioides posadasii. Cells were adhered to plastic wells for 2 h and then non-adherent cells were washed away. The adherent cells were treated with the monoclonal antibody 2A11 or an isotype control at 4 °C and then stimulated overnight with LPS, FKS or 1,3-β-glucan from spherules as described under Materials and methods. (a) TNF-α. There was no significant difference in the amount of TNF-α produced by macrophages from both mouse strains in response to LPS. DBA/2 macrophages stimulated by FKS and β-glucan produced significantly more TNF-α. Anti-Dectin-1 antibodies (2A11) significantly lowered response to FKS and β-glucan but not LPS (not shown). Each bar represents the mean of triplicate determinations and three independent experiments. Error bars indicate the s.e.m. (b) IL-6. DBA/2- and B6-elicited peritoneal macrophages were stimulated with different numbers of FKS, LPS or heat-killed S. aureus. Mouse peritoneal macrophages of DBA/2 (DBA) mice and C57BLl/6 (B6) mice were left unstimulated (Uns) or stimulated with 5 × 103, 5 × 104 or 5 × 105 FKS, 1 ng ml−1 LPS, or 106 particles per ml of heat-killed S. aureus (Staph). Bars represent mean ± s.e.m. of duplicate assays performed in triplicate. *P <0.05. FKS, formalin-killed spherules; IL, interleukin; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α.
Figure 2.
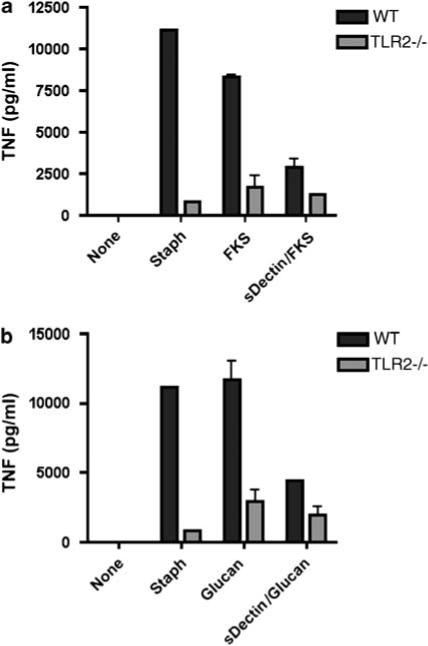
Soluble Dectin-1 inhibits TNF-α production by elicited peritoneal macrophages from B6 mice, but not from TLR-2−/− mice. The adherent elicited peritoneal macrophages were preincubated with sDectin at 4 °C for 30 min before adding FKS (a) or β-glucan (b). After overnight incubation at 37 °C, we removed the supernatants and assayed them for TNF-α. All assays were performed in triplicate and bars show the means ± s.e.m. The differences between the responses of to B6 and TLR-2−/− cells to S. aureus (Staph), FKS and β-glucan stimulation were highly significant (P >0.01). Incubation of B6 cells with sDectin significantly lowered the amount of TNF-α secreted by B6 cells stimulated with FKS or β-glucan. There was no effect on the response to Staph (not shown). sDectin did not significantly lower the amount of TNF-α secreted by TLR-2−/− macrophages stimulated with FKS or β glucan. FKS, formalin-killed spherule; TLR, Toll-like receptor; TNF-α, tumor necrosis factor-α.
In trying to explain the different between the responses of DBA/2 and B6 macrophages to β-glucan ligands, we compared the expression of Dectin-1 mRNA and protein in macrophages from the two strains of mice. Using an fluorescein isothiocyanate (FITC)-labeled goat anti-rat IgG to detect surface-bound 2A11 we found that B6 cells bound slightly more 2A11; the mean fluorescence for DBA/2 cells was 36.9 and that for B6 was 53.4 (Figure 3). We also used quantitative real-time PCR to measure mRNA for Dectin-1 B6 and DBA/2-elicited peritoneal macrophages and normalized those result to the expression levels of glycerladehyde-3-phosphate dehydrogenase; there was no significant difference in the levels of Dectin-1 mRNA (exons 1 and 6) from the two strained mice (not shown).
Figure 3.
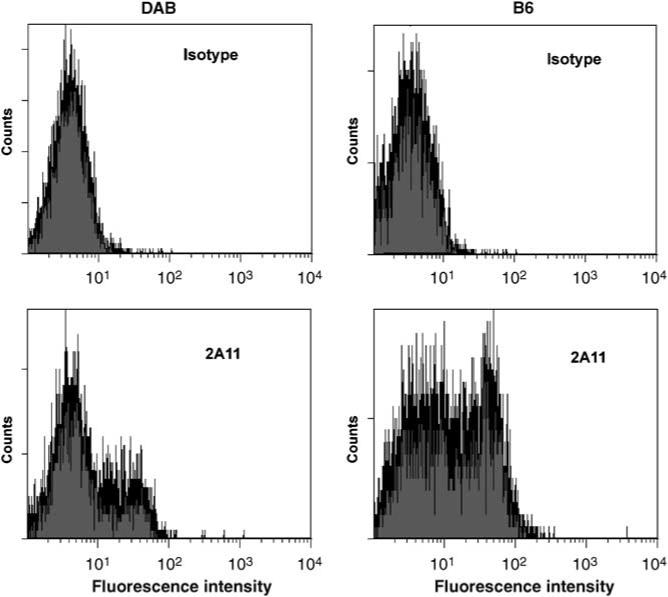
DBA/2- and B6-elicited peritoneal macrophages express similar amounts of Dectin-1 on their surface. Elicited peritoneal macrophages were incubated with rat anti-Dectin-1 (2A11) or an isotype control antibody, followed by incubation with FITC-labeled goat anti-rat Ig antibody. The upper row shows the results with the isotype control antibody added to elicited peritoneal macrophages from B6 and DBA/2 mice. The bottom row shows the results with 2A11 labeling of the cells. The mean fluorescent intensity for 2A11 + B6 cells was 53 and for DBA/2 it was 37 units. FITC, fluorescein isothiocyanate.
We then used reverse transcriptase-PCR to amplify the Dectin-1 mRNA from both DBA/2 and B6 macrophages, with primers that spanned from the third to fifth exon, and we did not obtain a product from the B6 cells. When we used primers designed to amplify the full-length Dectin-1 cDNA the predominant band from B6 cells was approximately 100 bp smaller than the cDNA from DBA/2 cells (Figure 4). We sequenced both bands and found that the shorter sequence from B6 cells did not include exon 3, as was reported shortly after we did the sequencing,14 whereas the DBA/2 sequence was identical to the published sequence for the full-length Balb/c cDNA.15 In addition, there were two non-conservative base-pair changes in the B6 that were the same as those that were recently reported.14 Because it has been reported that alternative splicing of the human Dectin-1 gene may be age- and organ-specific,16 we amplified Dectin-1 cDNA from uninfected lungs, thymus and spleens of adult B6 mice, and found the same splice variant; additionally, the splice variant was expressed in thymus and spleen from 1-day-old B6 mice (Figure 5, and data not shown). Cells in B6 lungs from mice infected with C. immitis also expressed the truncated form of Dectin-1. Adult DBA/2 mice expressed un-spliced Dectin-1 in their lungs and spleens, regardless of infection (not shown).
Figure 4.
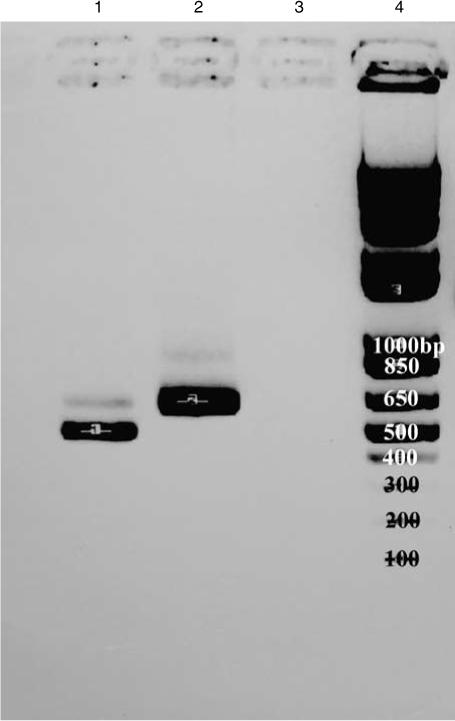
The isoforms of Dectin-1 expressed by macrophages from C57BL/6 and DBA/2 mice are of different sizes. mRNA from NaIO4-elicited peritoneal macrophages was reversed-transcribed and the products were run on 1.5% agarose gel. Lane 1 contains cDNA from C57BL/6 peritoneal macrophages (∼600 bp). Lane 2 contains cDNA from DBA/2 peritoneal macrophages (∼735 bp). Lane 3 contains a negative control. Lane 4 contains size markers. Note that the DBA/2 cells express primarily the full-length gene, whereas the C57BL/6 cells express primarily the truncated isoform of the gene.
Figure 5.
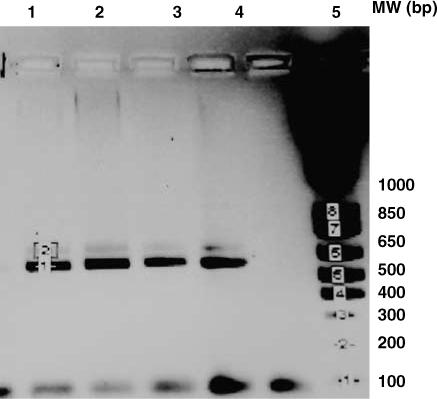
Expression of the truncated Dectin-1 isoform by C57BL/6 mice. Lanes 1−3 contains cDNA from the thymuses of three 1-day-old C57BL/6 mice. Lanes 4 contains the PCR product from the thymus of a C57BL/6 adult mouse.
Overexpression of Dectin-1 isoforms in RAW cells resulted in differential cytokine production in response to FKS
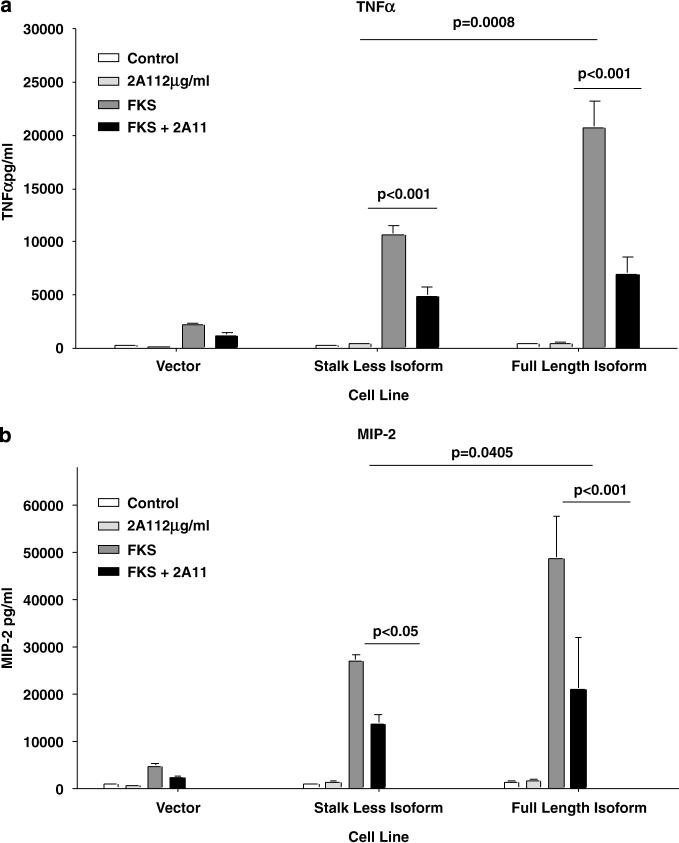
Having established that the two mouse strains differentially spliced Dectin-1 mRNA, and that their cells respond differently to 1,3-β-glucan and FKS, we wanted to determine whether the isoform of Dectin-1 affected the responsiveness to FKS. We transduced RAW 264.7 cells with plasmids encoding different isoforms of Dectin-1.14 We found that RAW cells overexpressing the full length of Dectin-1 isoform produced about twice as much TNF-α (Figure 6a) and MIP-2 (Figure 6b) in response to stimulation with FKS and 1,3-β- glucan from Coccidioides spp.; the difference in the concentrations of both cytokines was statistically significant (P = 0.0008 and P = 0.04, respectively). TNF-α secretion by both in response to FKS was inhibited by preincubation with 2A11. However, as with peritoneal macrophages, 2A11 did not totally inhibit production of these inflammatory cytokines, even when we used twice the concentration (not shown). That suggests that there may be other receptors involved in the recognition and subsequent cell activation triggered by FKS.
Figure 6.
Cytokine production in response to Coccidioides spp. FKS by transduced RAW cells expressing two different isoforms of Dectin-1. The RAW cells were transduced with either full-length or truncated isoforms of Dectin-1, or with only the vector. They were incubated with FKS, with or without 2A11 addition, as described under Materials and methods. (a) TNF-α and (b) MIP-2 concentrations in the supernatant fluids, respectively. All experiments were performed with triplicate wells and bars show the means ± s.d. The production of both cytokines was inhibited by 75% by the anti-Dectin-1 monoclonal antibody, 2A11. The P-values for inhibition by 2A11 are shown on the graphs. FKS, formalin-killed spherule; TNF-α, tumor necrosis factor-α.
The differences in responses of the two cell lines to FKS could not be attributed to differences in expression of the two forms of Dectin-1, since 2A11 binding to the two transduced cell lines was similar; mean fluorescent intensity was 54 for the RAW cells transduced with full-length cDNA and 50 for cells transduced with the truncated B6 cDNA (the fluorescent index for controls incubated with the secondary FITC-labeled antibody alone was <5).
Comparison of cytokines produced by mDCs from DBA/2 and B6 mice after stimulation with FKS
Since DCs are the primary antigen-presenting cells and highly express Dectin-1, we tested bone marrow-derived mDCs from B6 and DBA/2. In these experiments, we used smaller, less mature spherules in order to allow the mDCs to ingest the particles. As shown in Figure 7, B6 mDCs stimulated with FKS produced only about half as much IL-23 and IL-12p40, and 10% as much IL-12p70 as DBA/2 mDC. Both strains produced the same amount of IL-6. In contrast, B6 mDCs produced three times as much IL-10. Interestingly, the amounts of IL-12p40, IL-12p70, IL-6 and IL-23 produced by FKS-stimulated DBA/2 cells were significantly, but not fully, reduced by 2A11, whereas that antibody had little effect on the amount of those cytokines produced by B6 mDC. Pretreatment of B6 mDCs with 2A11 reduced IL-10 levels by only ∼13%, which was a statistically significant reduction, but still did not account for most of the response. FKS stimulation increased the expression of CD80 by 1.83-fold on DBA/2 cells, and by 1.50-fold on B6 cells. There were similarly small increases in CD86 that were not significantly different on the DC from the two strains.
Figure 7.
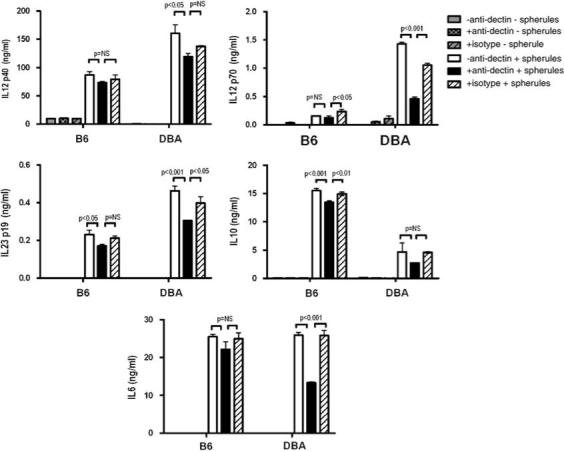
Cytokine production by mDCs from B6 and DBA/2 stimulated by FKS. Bone marrow derived mDCs were incubated with FKS, with or without preincubation with anti-dectin-1, and the concentrations of the IL-10, IL-12p40, IL-12 p70, IL-23 and IL-6 in the supernatant fluids were measured. All experiments were performed with triplicate wells and bars show the means ± s.d. One of two independent experiments with similar results is shown. FKS, formalin-killed spherule; IL, interleukin; mDC, myeloid dendritic cell.
Dectin-1 splicing by recombinant inbred BXD mice is correlated with their resistance to coccidioidomycosis
To determine whether there was an association between resistance to coccidioidomycosis and expression of the full-length Dectin-1 gene in inbred mice, we extracted RNA from stored, frozen lung tissue from 34 C. immitis-infected BXD lines. All the BXD lines that we predicted to be B6 at Dectin-1 based on two adjacent simple sequence polymorphism (SSLP) markers, D6MIT61 and D6MIT194, expressed the stalkless isoform of Dectin-1, and nearly all the BXD lines predicted to be DBA/2 expressed the full-length isoform. There were only three exceptions; although BXD 5, 11 and 40 were predicted to be DBA/2 at the Dectin-1 locus based on the SSLP markers, they expressed equal amounts of both the isoforms. To confirm that they were genetically DBA/2 at that locus, we took advantage of the fact that the G-to-A change at position 110 in the B6 gene (ccgg to ccag) abolishes an MspI restriction site (New England Biolabs, Ipswich, MA, USA). Since cDNA from these strains was cut by MspI, we concluded that all three of the ambiguous BXD strains were genetically DBA/2 at the Dectin-1 locus, and that control of mRNA splicing may lie outside that genetic region or be due to another unknown point mutation in that gene. We then compared the expression of the 2 isoforms of Dectin-1 with our previous categorization of BXD lines as susceptible or resistant to C. immitis.11 Our criteria for susceptibility of a BXD line were that the geometric mean count of organisms isolate from either lung or spleen was >10-fold greater than counts from DBA/2 mice that were included as controls in the same experiment. There was a 75% correlation between the isoform of Dectin-1 and our prior categorization of the lines as susceptible or resistant based either on colony-forming units (CFUs) in the lung or in the spleen (Fisher's exact test for correlation between the CFUs and Dectin-1 genotype for both lung and spleen was statistically significant; P = 0.023 and P = 0.0356, respectively) (Table 1). Seventeen of the 20 BXD lines expressing the truncated form of Dectin-1 were susceptible as judged by lung CFU, and 16 of 20 were susceptible as judged by spleen CFUs.
Table 1.
Susceptibility to intraperitoneal infection with C. immitis is correlated with expression of the truncated Dectin-1 isoform in BXD RI mice
|
Lungb
Dectin-1 (cDNA) |
Spleenc
Dectin-1 (cDNA) |
|||
|---|---|---|---|---|
| Classificationa | Full lengthd | Stalk lessd | Full lengthd | Stalk lessd |
| R | 24 | 9 | 24 | 11.8 |
| S | 18 | 50 | 18 | 47.1 |
| Total | 41 | 59 | 42 | 58.9 |
Abbreviations: BXD RI, C57BL/6 × DBA/2 recombinant inbred; R, resistant; S, susceptible.
Mice were classified as resistant or susceptible based on comparison with the number of CFUs isolated from resistant DBA/2 mice that were included in each experiment as controls.
Fisher's exact test, P = 0.023.
Fisher's exact test, P = 0.036.
The values correspond to percentages of mice having the two respective characteristics in each case.
Discussion
Dectin-1 is a recently described C-type lectin-like receptor for 1,3-β-linked soluble glucans (inhibitory) and particulate 1,3-β-glucan (stimulatory).17 Dectin-1 is expressed on polymorphonuclear leukocytes, macrophages and DC, and on the latter two cell types it is both a signaling and a non-opsonic phagocytic receptor, although phagocytosis is not necessary for cytokine secretion.18 This receptor has been shown to be required for resistance to opportunistic unicellular fungi,19,20 and it is important for resistance to at least one opportunistic mold,21 but there is no information about its function in innate immunity to primary pathogenic fungi.
In this study, we focused on the role of Dectin-1 in resistance to coccidioidomycosis because we have previously shown that Dectin-1 plays a role in recognition of live and formalin-fixed spherules,3 the tissue invasive form of the dimorphic fungi that cause this disease. We, and others, have also shown that different strains of inbred mice vary in their susceptibility to coccidioidomycosis; DBA/2 mice are resistant to experimental coccidioidomycosis and B6 mice are susceptible to experimental coccidioidomycosis.8,22 Because we have previously mapped a resistance locus in BXD RI mice to chromosome 6,11 not far from where the Clec7a (Dectin-1) gene is located, we postulated that polymorphisms in Dectin-1 might be one of the explanations for the difference in susceptibility to this fungal infection. We first compared the responses of macrophages from DBA/2 and B6 mice to FKS and to 1,3-β-glucan purified from spherules. We found that DBA/2 macrophages produced more TNF-α and IL-6 than B6 macrophages, and these responses were inhibited by 2A11, showing that they were mediated at least in part by Dectin-1. The cells responded equally well to LPS (a TLR-4 agonist), although they also differed in their response to heat-killed S. aureus, a TLR-2 agonist. These results suggest that there may be differences between the strains in their TLR pathways and additional work needs to be performed to compare TLR-2 pathways in these mice.
We next compared the expression of Dectin-1 by the macrophages from the two inbred strains. Heinsbroek et al.14 found a higher level of expression of Dectin-1 on thioglycolate-elicited macrophages from B6 mice than Balb/c, and we found that B6 macrophages expressed ∼30% more Dectin-1 than DBA/2 macrophages, as assessed by fluorescence-activated cell sorting analysis (Figure 3), but they had equivalent amount of Dectin-1 mRNA. These results exclude overexpression of Dectin-1 as the explanation for why DBA/2 macrophages responded better to stimulation by Dectin-1 agonists. We sequenced the mRNA for Dectin-1 from the two strains and discovered that the B6 gene product lacked exon 3 (Figure 4). This exon encodes most of the stalk for this receptor and it is predicted that the protein will be truncated. The DBA/2 sequence was identical to the published full-length sequence from the Balb/c strain. The B6 sequence also had two amino-acid substitutions in the remaining stalk region, outside the lectin-binding site.23 Neither of these two polymorphisms was predicted to affect glycosylation sites.24 It is unknown if those polymorphisms in the B6 gene affect the function of the receptor.
DBA/2 mice are unusual among inbred strains in that they primarily express the full-length Dectin-1, whereas other mouse strains primarily express either the stalkless isoform that lacks the third exon, or they express approximately equal amounts of the two isoforms.14 We found that expression of stalkless Dectin-1 by B6 mice was not age- or organ-specific, nor was it affected by the fungal infection. This splice variant is identical to one of the splice variants found in human peripheral blood cells.25,26 Although relatively few people have been studied and the ethnic make-up of the volunteers was not described, the truncated isoform is so far the predominant form in people.16,25,26
Having demonstrated that B6 and DBA/2 mice express different isoforms of Dectin-1, we wanted to determine whether the different isoforms of Dectin-1 responded differently to stimulation by FKS. Therefore, we stimulated RAW macrophage-like cells that were transduced to express the two isoforms of Dectin-1.14 We found that the RAW cells expressing the full-length Dectin-1 responded better to stimulation with FKS and responses of both cell lines were inhibited by 2A11, confirming association between the isoform of Dectin-1 and the response to stimulation by FKS. The differences in the levels of TNF-α and MIP-2 that we found could not be explained by different levels of protein expression, as the two cell lines expressed nearly equal amounts of Dectin-1 as assessed by mean fluorescence of 2A11-labeled cells.
We, and others, have shown that susceptible and resistant mice show different immunological responses to experimental coccidioidomycosis.2,27 While macrophages are important effector cells in the innate and acquired immune response to C. immitis, DCs are the most important antigen-presenting cells and different pathogens can modulate the way they affect the immune response.28 Since Dectin-1 is also expressed on DCs, we compared the responses of DBA/2 and B6 bone marrow-derived mDCs to FKS. We previously reported that B6 mice produce more IL-10 than DBA/2 mice with coccidioidomycosis, and that IL-10-deficient B6 mice are more resistant to this infection.29 Furthermore, hIL-10 transgenic mice overexpressing functional IL-10 are more susceptible,30 establishing that high levels of IL-10 are both necessary and sufficient for making mice susceptible to coccidioidomycosis. It is therefore of greater interest that mDCs from B6 mice produced three times as much IL-10 as did mDCs from DBA/2 mice. Others have shown that zymosan can stimulate IL-10 secretion through Dectin-1-independent pathways,19 but curdlan, a purified β-glucan, can also stimulate mDCs to produce IL-10 in a Syk and CARD-dependent manner, which is almost certainly the Dectin-1-signaling pathway.31 Although B6 mDCs produced three times as much IL-10 as DBA/2 mDC, and pretreatment with 2A11 significantly reduced production of IL-10 by B6 cells, blocking Dectin-1 did not reduce secretion to the DBA/2 level, suggesting that there are other pathways in B6 cells that are involved in this response to FKS. Recently Dennehy et al.32 showed that there was collaboration between the TLR/nuclear factor-kB and Dectin-1 Syk/CARD pathways so that more TNF-α and MIP-2 are produced by B6 macrophages stimulated with glucan phosphate and TLR agonists, than by the those stimulated with TLR agonists alone. This is consistent with our findings and strongly suggests that FKS stimulate both pathways.
In addition to the difference in IL-10 production, we found that mDCs from DBA/2 mice produced more IL-12p70 and IL-23, but there was no difference in the amount of IL-6 produced by DCs from the two strains. IL-23 is necessary for maintenance of IL-17T cells,33 and IL-17 has been shown to be necessary for resistance to the opportunistic fungal pathogen Candida albicans.34 Others have shown that curdlan stimulates B6 mDCs to produce IL-23, IL-12p40 and IL-6 in a Syk and CARD-dependent manner, and that curdlan can skew the immune response in vitro toward a TH-IL-17 response.31 These studies were conducted with mice on a B6 genetic background. Our results suggest that B6 mice produce sub-optimal IL-23 response, which could limit their IL-17 response. In support of that, we found that the infected lungs of DBA/2 mice have more IL-17 than do B6 lungs (J Fierer, unpublished data).
Having established a correlation between the isoform of Dectin-1 and the way mDCs and elicited peritoneal macrophages responded in vitro to FKS stimulation, we then asked whether there was a correlation between the isoform of Dectin-1 and resistance to coccidioidomycosis. To help address that question, we took advantage of a prior experiment that we performed to map resistance loci in BXD RI mice.11 We had classified the 34 BXD RI lines as either susceptible or resistant to coccidioidomycosis on the basis of the ratio between CFUs in the lungs and spleens and the results in control DBA/2 and B6 mice.11 We used stored lung tissue from 34 lines to extract mRNA and to determine the isoform of Dectin-1 that they expressed. There was a 75% concordance between the isotype of Dectin-1 and our prior classification of the line as resistant or susceptible to C. immitis. In nearly all cases, the BXD lines expressing the truncated form of Dectin-1 were susceptible to infection. We did not expect a 100% correlation because our mapping studies had revealed that resistance is a polygenic trait. Nevertheless, this result provided additional evidence that Dectin-1 or a nearby gene is the gene on chromosome 6 that we named Cms2. Interestingly, the same Dmit markers on chromosome 6 that localized Cms2 was recently identified as a QTL for resistance to another pathogenic fungus, Histoplasma capsulatum.35
In summary, we compared the ability of elicited peritoneal macrophages and mDCs from DBA/2 (resistant) and B6 (susceptible) mice to respond to stimulation with FKS. We found that DBA/2 cells produced more proinflammatory cytokines and they expressed full-length Dectin-1, whereas B6 cells splice out exon 3 of the gene and make a stalkless, truncated isoform of Dectin-1. In addition, we showed that transduction of the full-length cDNA made RAW cells more responsive to FKS than did the truncated cDNA. Finally, we established a correlation between the isoform of the Dectin-1 gene expressed and susceptibility to coccidioidomycosis in BXD RI lines. These studies provide evidence that Dectin-1 is involved in innate immunity to coccidioidomycosis and alternative splicing of the Dectin-1 mRNA has functional significance.
Materials and methods
Animals
C57BL/6J, DBA/2J and BXD RI mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). All mice were females 8−12 weeks of age. They were kept under specific, pathogen-free conditions at the Animal Facility of the Veterans Medical Center in La Jolla, CA.
Fungus
C. immitis and C. posadasii were grown in vitro in a BSL3 laboratory under conditions described previously.29,36 Arthroconidia (RS) were obtained from mycelia grown on Mycosel agar plus gentamicin and were harvested by scraping the normal saline-wetted mycelial mat whenever the levels of sporulation were high. The mycelia were washed twice, disrupted by shaking with glass beads and then washed again. The suspension of mycelia and arthroconidia was then passed over a nylon wool column to remove debris and large mycelial fragments. The arthroconidial suspension was kept in normal saline (0.9% NaCl) at 4 °C until use. Before each experiment an aliquot was cultured to confirm viability.
To produce spherules, C. posadasii (isolate C735A) was grown in the mycelial phase on a slant of 3.6% Mycosel agar, 0.5% yeast extract and 50 μg ml−1 of gentamicin at room temperature. Once mycelia covered most of the surface of the slant, arthroconidia were isolated in normal saline and washed twice by centrifugation at 2200 g at 4 °C for 15 min. The pellet was used to inoculate 125 ml of modified Converse medium.37 The culture was incubated in a 180-r.p.m. shaker incubator at 39 °C in the presence of 11% CO2. After 10 days, when spherules had matured, the culture was harvested by centrifugation at 2200 g at 4 °C for 10 min. The FKS were prepared by resuspending the spherules in 5% formaldehyde in 0.9% NaCl for overnight and then the pellet was washed three times in normal saline. We then passed the FKS suspension through a 95% Percoll gradient in 0.25 m sucrose to separate the spherules from arthroconidia and mycelial fragments.38 All preparations were made using pyrogen-free reagents and solutions. All lots of FKS and β-glucan contained less than 4.3 pg of endotoxin per 106 spherules, measured with QCL-1000 Chromogenic LAL from BioWhittaker (Cambrex Co., Walkersville, MD, USA).
Reagents
Lipopolysaccharide from Salmonella minnesota was purchased from List Biological Laboratories (Campbell, CA, USA) and heat-killed S. aureus was purchased from Molecular Probes/Invitrogen (Carlsbad, CA, USA). We purchased the following antibodies to stain the cells and perform functional studies: 2A11 (rat anti-mouse dectin-1, purified IgG conjugated to R Phycoerythrin) and unconjugated 2A11 (endotoxin low) from Serotec (Oxford, UK); functional-grade purified mouse IgG2b isotype antibody and purified rat anti-mouse CD16/CD32 monoclonal antibody (mouse Fc-block) from eBiosciences (San Diego, CA); rat IgG2b isotype control conjugated to R Phycoerythrin from BD Pharmingen (San Jose, CA, USA); rabbit anti-rat Ig FITC-labeled polyclonal antibody (multiple adsorption) from BD Biosciences (San Jose, CA, USA). All reagents were tested and were free of LPS contamination. β-Glucan was extracted from C. posadasii spherules as previously described.3
Soluble Dectin-1
To generate a DNA template suitable for cloning of the extracellular portion of human Dectin-1, we made a neutral point mutation in human Dectin-1 gene, hBGRA (a gift from Gordon D Brown)26 to remove the NcoI restriction site. DNA fragments coding for amino acids 130−247 and 119−247 of human Dectin-1 were generated by PCR using the DNA template. The PCR fragments were cloned into the NcoI/NheI site of the vector pBAC11 (Novagen, Madison, WI, USA) to generate a baculovirus vector expressing the C-terminal 128 amino acids of human Dectin-1 with C-terminal 6His-tag (sDectin128). The recombinant baculoviruses expressing soluble Dectin-1 were produced in the insect Sf9 cells by transfection with the each of the two plasmids, using the BacVector-3000 DNA kit (Novagen). The recombinant proteins were expressed in Hi-5 cells infected with the two recombinant baculoviruses. The proteins were purified from the harvested media using Ni-NTA Superflow (Qiagen, Gaithersburg, MD, USA) as described previously.39
Infection
The BXD mice were infected intraperitoneally in a safety hood in a BSL3 laboratory with RS arthroconidia as we have previously described.11 Our protocol was approved by the local Biosafety Committee and the Institutional Animal Care and Use Committee of the Veterans Medical Center, San Diego, CA.
PCR amplification of mRNA
RNA was isolated from cells and tissues using UltraSpec (Biotecx Laboratories Inc., Houston, TX, USA) and reverse-transcribed to cDNA using Superscript 2 kit (Invitrogen, San Diego, CA, USA), as previously described.29 To amplify full-length Dectin-1 cDNA, we used the following primers: forward, ATGAAATATCACTCT CATATAGAGAATCTGG (nucleotides 88−118) and reverse, TGGCCAGACAGCATAAGGAAAC (nucleotides 1801−1779). The PCR reaction conditions were as follows: 94 °C for 1 min, annealing at 63 °C for 1 min, 40 cycles at 72 °C for 1 min and 72 °C for 6 min.
Quantitative real-time PCR analysis
The expression level of Dectin-1 mRNA from peritoneal macrophages of C57BL/6 and DBA/2J mice was quantified by Syber Green real-time PCR (Bio-Rad, Hercules, CA, USA) with the ABI Prism 7000 SDS v1.1. detection system (Applied Biosystems, Foster City, CA, USA), and fold expression of Dectin-1 mRNA was normalized against the expression of GAPDH mRNA. The following primers were used for real-time PCR reactions for exon 1 of Dectin-1: forward, TAGTAG TGGTTGCTGCAGTGCTG and reverse, GATAGGAAG TTGTCTTTCTCCTCTGG. The primers for exon 6 of Dectin-1 were as follows: forward, ATCAGCATTCTT CCCCAACTCG and reverse, CAGTTCCTTCTCACAG ATACTGTATGA. The sequences of the glycerladehyde-3-phosphate dehydrogenase primers used were as follows: forward, TGCAGTGGCAAAGTGGAGATT and reverse, TGGAACATGTAGACCATGTAGTTGAG.
Cloning and sequencing of Dectin-1 cDNA
The Dectin-1 cDNA transcripts were amplified from lung tissue of infected B6 and DBA/2 mice by PCR using Platinum Taq DNA Polymerase High Fidelity (Invitrogen), and using the following two primers: forward, TAGTAGTGGTTGCTGCAGTGCTG and reverse, TGGC CAGACAGCATAAGGAAAC. The PCR conditions were the same as described above. The PCR products were purified using Purelink PCR Purification kit (Invitrogen), cloned into TOPO vector (pcDNA6.2/V5/GW/D-TOPO) (Invitrogen) and sequenced. Sequencing was performed by the Cancer Center, University of California San Diego.
In vitro macrophage assays
The elicited peritoneal macrophages were harvested 4 days after an intraperitoneal injection of 5 mm NaIO4.40 For functional studies, the elicited peritoneal macrophages were cultured in 48-flat-well plates (Corning Glass, Corning, NY, USA) at 2 × 105 cells per well in 0.2 ml of RPMI with 10% fetal calf serum, 2 mm l-glutamine, 10 mm HEPES and 50 μg ml−1 of gentamicin (RPMI-supplemented media). The cells were allowed to attach for 2 h at 37 °C and then washed with phosphate-buffered saline to remove non-adherent cells. The adherent cells were cultured in 100 μl of RPMI-supplemented media with 2 μg ml−1 2A11 or 2 μg ml−1 IgG2b isotype control antibodies (both low endotoxin). Thereafter, either 1 ng ml−1 LPS, 1 × 106 particles per ml of heat-killed S. aureus, 1 × 105 FKS per ml, or 250 μg ml−1 1,3-β-glucan to a final volume of 0.2 ml, was added. After 18−20 h of culture at 37 °C in a 5% CO2 atmosphere, the supernatants were collected and frozen at −70 °C until analysis. We measured the concentration of TNF-α, IL-6 and MIP-2 by enzyme-linked immunosorbent assays and used kits from BD Biosciences and R&D Systems (Minneapolis, MN, USA) according to the manufacturer's instructions.
RAW 264.7 cells, stably transduced with the different isoforms of Dectin-1, full-length and the truncated form without exon 3 (Dectin-1 full-length and Dectin-1 stalk-less, respectively), and the vector control, were constructed as described previously (Heinsbroek et al.14). The cells were grown in RPMI with 10% fetal calf serum, 2mm l-glutamine, 10 mm HEPES, 50 μg ml−1 of gentamicin and 600 μg ml−1 of G418. Cells were subcultured 1 day before assay. The transduced cells were washed, resuspended in medium without antibiotics and plated at 2 × 105 cells per well in 48-well plates (Corning Glass). After cells had adhered, the medium was removed and replaced with fresh medium and cells were incubated for 30 min at 4 °C with 2 μg ml−1 2A11 or 2 μg ml−1 isotype IgG2b in a final volume of 100 μl per well. Then 1 ng ml−1 LPS, 105 ml−1 FKS or 250 μg ml−1 1,3-β-glucan from spherules were added in the respective wells for a final volume of 200 μl per well. Supernatants were harvested after 18 h of incubation at 37 °C under 5% CO2 and assayed by ELISA for TNF-α and MIP-2 cytokines. Fluorescence-activated cell sorting analysis was performed to confirm Dectin-1 expression on these cells.
Dectin-1 on the cell surface of peritoneal macrophages
Single-cell suspensions obtained above were washed in 1 × phosphate-buffered saline with 1% fetal bovine serum. The cells were incubated with CD16/CD32 for 30 min at 4 °C to prevent non-specific binding of antibodies to Fc receptors. Then, cells were labeled for 30 min at 4 °C with 2A11 and stained with rabbit FITC anti-rat IgG. Finally, cells were washed and fixed with 1% formaldehyde in 1 × phosphate-buffered saline. Cell acquisition was performed with dual-laser flow cytometer (FACSCalibur; BD Biosciences, Mountain View, CA, USA). The data were analyzed using Cell Quest Software (BD Biosciences).
In vitro bone marrow-derived DCs
Mouse bone marrow-derived DCs were cultured as previously described.41 In brief, bone marrow from B6 or DBA mouse femurs and tibia was plated on day 0 into bacterial Petri dishes (Fisher Scientific, Pittsburgh, PA, USA) at 5 × 105 cells ml−1 in DC medium, which consisted of RPMI (Irvine Scientific, Irvine, CA) supplemented with 10% heat-inactivated fetal calf serum (Life Technologies, Gaithersburg, MD), 2 mm l-glutamine (Cellgro, Natham, VA), 100 U ml−1 penicillin and 100 mg ml−1 streptomycin (Cellgro), and 5 ng ml−1 recombinant murine granulocyte–macrophage colony-stimulating factor (BD Pharmingen, San Diego, CA, USA). On day 3, we added an equal volume of DC medium with granulocyte–macrophage colony-stimulating factor. The non-adherent cells were harvested on day 6, plated at 5 × 106 cells ml−1 and incubated in the absence or presence of 96-h (young) spherules42 (1:1 ratio of DC:spherule), 2A11 antibody (2 μg ml−1) or isotype control antibody (2 μg ml−1). After 24 h, supernatants were collected and analyzed by ELISA for IL12 p40, IL12 p70, IL10 (BD PharMingen) or IL23 p19 (eBiosciences) according to the manufacturer's instructions. Expression of CD80 and CD86 was measured as described previously.41
Statistical analysis
All experiments with peritoneal macrophages and transduced RAW cell lines were performed in triplicate and repeated at least three times. The results were presented as the mean and s.e.m. from one representative experiment or pooled experiments. The software program GraphPad Prism version 4.0 (San Diego, CA, USA) was used for all statistical tests of significance (to a P-value of ≤0.05). Two-way analysis of variance with Bonferroni post-test analysis was used to compare different groups of data. To analyze BXD recombinant mice data, Fisher's exact test and χ2-test were used.
Acknowledgements
This work was supported in part by Merit Review Grants from the Veterans Administration (JF and TK). Dr Jiménez-A was supported by Convenio Especial de Cooperación No. 067-2002 Suscrito entre Colciencias-Icetex, Colombia. This work was performed as part to fulfill her requirements as a PhD student of the Medical Sciences PhD program of UPB-CIB-CES, Medellin, Colombia. We thank Dr John Galgiani (University of Arizona) for the generous gift of 96 h (young) spherules.
References
- 1.Fisher MC, Koenig GL, White TJ, Taylor JW. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia. 2002;94:73–84. [PubMed] [Google Scholar]
- 2.Cox RA, Magee DM. Coccidioidomycosis: host response and vaccine development. Clin Microbiol Rev. 2004;17:804–839. doi: 10.1128/CMR.17.4.804-839.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viriyakosol S, Fierer J, Brown GD, Kirkland TN. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on toll-like receptor 2 and dectin 1. Infect Immun. 2005;73:1553–1560. doi: 10.1128/IAI.73.3.1553-1560.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kellner EM, Orsborn KI, Siegel EM, Mandel MA, Orbach MJ, Galgiani JN. Coccidioides posadasii contains a single 1,3-B-glucan synthase gene that appears to be essential for growth. Eukaryotic Cell. 2005;4:111–120. doi: 10.1128/EC.4.1.111-120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens DA, Levine HB, TenEyck DR. Dermal sensitivity to different doses of spherulin and coccidioidin. Chest. 1974;65:530–533. doi: 10.1378/chest.65.5.530. [DOI] [PubMed] [Google Scholar]
- 6.Drutz DJ, Catanzaro A. Coccidioidomycosis. Part I. Am Rev Respir Dis. 1978;117:559–583. doi: 10.1164/arrd.1978.117.3.559. [DOI] [PubMed] [Google Scholar]
- 7.Pappagianis D. Epidemiology of coccidioidomycosis. In: McGinnis MR, editor. Current Topics in Medical Mycology. Springer-Verlag; New York: 1988. pp. 199–238. [DOI] [PubMed] [Google Scholar]
- 8.Kirkland TN, Fierer J. Inbred mouse strains differ in resistance to lethal Coccidioides immitis infection. Infect Immun. 1983;40:912–916. doi: 10.1128/iai.40.3.912-916.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkland TN, Fierer J. Genetic control of resistance to Coccidioides immitis: a single gene that is expressed in spleen cells determines resistance. J Immunol. 1985;135:548–552. [PubMed] [Google Scholar]
- 10.Cox RA, Kennell W, Boncyk L, Murphy JW. Induction and expression of cell-mediated immune responses in inbred mice infected with Coccidioides immitis. Infect Immun. 1988;56:13–17. doi: 10.1128/iai.56.1.13-17.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fierer J, Walls L, Wright F, Kirkland TN. Genes influencing resistance to Coccidioides immitis and the interleukin-10 response map to chromosomes 4 and 6 in mice. Infect Immun. 1999;67:2916–2919. doi: 10.1128/iai.67.6.2916-2919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown GD, Gordon S. A new receptor for β-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 13.Dennehy KM, Brown GD. The role of the β-glucan receptor Dectin-1 in control of fungal infection. J Leukoc Biol. 2007;82:253–258. doi: 10.1189/jlb.1206753. [DOI] [PubMed] [Google Scholar]
- 14.Heinsbroek SEM, Taylor PR, Rosas M, Willment JA, Williams DL, Gordon S, et al. Expression of functionally different Dectin-1 isoforms by murine macrophages. J Immunol. 2006;176:5513–5518. doi: 10.4049/jimmunol.176.9.5513. [DOI] [PubMed] [Google Scholar]
- 15.Ariizumi K, Shen G-L, Shikano S, Xu S, Ritter RI, Kumamoto T, et al. Identification of a novel, dendritic cell-associated molecule, Dectin-1, by subtractive cDNA cloning. J Biol Chem. 2000;275:20157–20167. doi: 10.1074/jbc.M909512199. [DOI] [PubMed] [Google Scholar]
- 16.Willment JA, Marshall ASJ, Reid DM, Williams DL, Wong SYC, Gorder S, et al. The human beta-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur J Immunol. 2005;35:1539–1547. doi: 10.1002/eji.200425725. [DOI] [PubMed] [Google Scholar]
- 17.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 18.McCann F, Carmona E, Puri V, Pagano RE, Limper AH. Macrophage internalization of fungal B-glucans is not necessary for initiation of related inflammatory responses. Infect Immun. 2005;73:6340–6349. doi: 10.1128/IAI.73.10.6340-6349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saijo S, Fujikado N, Furuta T, Chung S-H, Kotaki H, Seki K, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 20.Taylor PR, Tsoni V, Willment JA, Dennehy KM, Rosas M, Findon H, et al. Dectin-1 is required for B-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, et al. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 2005;1:42–54. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magee DM, Cox RA. Roles of gamma interferon and interleukin-4 in genetically determined resistance to Coccidioides immitis. Infect Immun. 1995;63:3514–3519. doi: 10.1128/iai.63.9.3514-3519.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adachi Y, Ishii T, Ikeda Y, Hoshino A, Tamura H, Aketagawa J, et al. Characterization of beta-glucan recognition site on C-type lectin, dectin 1. Infect Immun. 2004;72:4159–4171. doi: 10.1128/IAI.72.7.4159-4171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato Y, Adachi Y, Ohno N. Contribution of N-linked oligosaccharides to the expression and functions of B-glucan receptor, dectin-1. Biol Pharmacol Bull. 2006;29:1580–1586. doi: 10.1248/bpb.29.1580. [DOI] [PubMed] [Google Scholar]
- 25.Yokota K, Takashima A, Bergstresser PR, Ariizumi K. Identification of a human homologue of the dendritic cell-associated C-type lectin-1, dectin-1. Gene. 2001;272:51–60. doi: 10.1016/s0378-1119(01)00528-5. [DOI] [PubMed] [Google Scholar]
- 26.Willment JA, Gordon S, Brown GD. Characterization of the human beta-glucan receptor and its alternatively spliced isoforms. J Biol Chem. 2001;276:43818–43823. doi: 10.1074/jbc.M107715200. [DOI] [PubMed] [Google Scholar]
- 27.Fierer J. The role of IL-10 in genetic susceptibility to coccidioidomycosis on mice. Ann NY Acad Sci. 2007;1111:236–244. doi: 10.1196/annals.1406.048. [DOI] [PubMed] [Google Scholar]
- 28.Huang Q, Liu D, Majewski P, Schulte LC, Korn JM, Young RA, et al. The plasticity of dendritic cell responses to pathogens and their components. Science. 2001;294:870–875. doi: 10.1126/science.294.5543.870. [DOI] [PubMed] [Google Scholar]
- 29.Fierer J, Walls L, Eckmann L, Yamamoto T, Kirkland TN. Importance of interleukin-10 in genetic susceptibility of mice to Coccidioides immitis. Infect Immun. 1998;66:4397–4402. doi: 10.1128/iai.66.9.4397-4402.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiménez MdP, Walls L, Fierer J. High levels of interleukin-10 impair resistance to pulmonary coccidioidomycosis in mice in part through control of nitric oxide synthase 2 expression. Infect Immun. 2006;74:3387–3395. doi: 10.1128/IAI.01985-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gut-Landman SL, Grob O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 32.Dennehy KM, Ferwerda G, Faro-Trindade I, Pys E, Willment JA, Taylor PR, et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire; the Th17 lineage. Curr Opin Immunol. 2006;18:349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 35.Mayfield JA, Rine J. The genetic basis of variation in susceptibility to infection with Histoplasma capsulatum in the mouse. Genes Immun. 2007;8:468–474. doi: 10.1038/sj.gene.6364411. [DOI] [PubMed] [Google Scholar]
- 36.Walch HA, Kalvoda A. Immunization of mice with induced mutants of Coccidioides immitis. I. Characterization of mutants and preliminary studies of their use as viable vaccines. Sabouraudia. 1971;9:173–184. [PubMed] [Google Scholar]
- 37.Converse JL, Besemer AR. Nutrition of the parasitic phase of coccidioides immitis in a chemically defined liquid medium. J Bacteriol. 1959;78:231–239. doi: 10.1128/jb.78.2.231-239.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiménez MdP, Restrepo A, Garcia LF, Cano LE. Separation of Paracoccidioides brasiliensis conidia through Percoli gradients. Med Mycol. 2004;42:349–353. doi: 10.1080/13693780410001657126. [DOI] [PubMed] [Google Scholar]
- 39.Viriyakosol S, Mathison JC, Tobias PS, Kirkland TN. Structure–function analysis of CD14 as a soluble receptor. J Biol Chem. 2000;275:3144–3149. doi: 10.1074/jbc.275.5.3144. [DOI] [PubMed] [Google Scholar]
- 40.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 41.Datta SK, Okamoto S, Hayashi T, Shin SS, Mihajlov I, Fermin A, et al. Vaccination with irradiated Listeria induces protective T cell immunity. Immunity. 2006;25:1–10. doi: 10.1016/j.immuni.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 42.Dionne SO, Podany AB, Ruiz YW, Ampel NM, Galgiani JN, Lake DF. Spherules derived from Coccidioides posadasii promote human dendrite cell maturation and activation. Infect Immun. 2006;74:2415–2422. doi: 10.1128/IAI.74.4.2415-2422.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]