Abstract
Background. Tumor patients and patients after traumas are endangered by a reduced immune defense, and a silver coating on their megaprostheses may reduce their risks of infection. The aim of this study was to determine the silver ion concentration directly measured from the periprosthetic tissue and the influence on the clinical outcome. Material and Methods. Silver ions were evaluated in 5 mL wound fluids two days postoperatively and in blood patients 7 and 14 days after surgery using inductively coupled plasma emission spectrometry in 18 patients who underwent total joint replacement with a silver-coated megaendoprosthesis. Results. The concentration of silver ions averaged 0.08 parts per million. Patients who showed an increased silver concentration in the blood postoperatively presented a lower silver concentration in the wound fluids and a delayed decrease in C-reactive protein levels. There were significantly fewer reinfections and shorter hospitalization in comparison with a group that did not receive a silver-coated megaprosthesis. Conclusion. An increased concentration of silver in the immediate surroundings of silver-coated prostheses was demonstrated for the first time in cohorts of patients with trauma or tumors. An elevated concentration of silver ions in the direct periprosthetic tissue may have reduced the infection rate.
1. Introduction
Applying megaprostheses to reconstruct osseous defects after trauma, tumor, or infection has been well established for decades. These implants can replace critical size osseous defects in long bones, such as in total joint revisions or after local tumor resections [1–3]. One therapeutic challenge is the high infection rate in the latter group, which is approximately 35% in these patients compared to 1-2% in healthy individuals. Therefore, some authors advocate using silver-coated prostheses in this special cohort [4]. Silver ions have a bactericide effect because they can attach to the DNA and thus inhibit protein synthesis [4, 5]. Moreover, it is evident that silver ions induce resistance to bacteria [6, 7].
Currently, silver-coated prostheses are applied mainly due to the following two indications: (1) for infection prophylaxis in tumor endoprosthetics and (2) as the last option for patients after extensive trauma-related infection.
In vitro studies have demonstrated the efficiency of silver ions compared to other metals in killing Staphylococcus epidermidis [8]. In rabbits, Gosheger et al. showed reduced infection rates after the implantation of silver-coated prostheses [9]. In a recent clinical trial, Hardes et al. presented a reduction of the infection rate to 5.9% in tumor patients compared to 17.6% in a control group [10]. However, there is still a lack in clinical data, and the current literature almost exclusively includes patient cohorts after tumor surgery. Adequate large studies for total joint revision surgeries with silver-coated endoprostheses do not yet exist.
At this time, the typical toxic side effects of silver, such as dermal argyria (i.e., blue or bluish-grey colored skin), ocular argyrosis, gastroenteritis or fever, have not been associated with silver-coated endoprostheses. Neither Gosheger et al. nor Hardes et al. could determine evident toxicological side effects of silver in an animal experiment and in a prospective clinical study, respectively [3, 9]. This corresponds to Jung et al., who found only a mild toxicity of silver ions to human cells [11]. Recent data in the literature have demonstrated a systemic accumulation of silver ions in blood and urine as well as in local tissues adjacent to the silver-coated implant [3]. To the best of our knowledge, current studies regarding the silver concentrations in the immediate surroundings of prostheses do not exist so far.
Thus, we initiated a prospective clinical study to determine the silver ion concentration after the implantation of silver-coated prostheses that appeared in the wound fluids extracted from the immediate surroundings of the prosthesis. Questions addressed included the following.
Can the silver ion concentration be directly measured from the periprosthetic tissue using wound fluids?
Is this silver ion concentration toxic for the patient?
Does the use of silver-coated prostheses have an influence on the clinical course or outcome of the patient?
2. Material and Methods
Patients were examined with the following inclusion criteria.
Age ≥ 18 years.
An indication for the implantation of a silver-coated endoprosthesis, either due to revision surgery after trauma or due to infection prophylaxis in oncologic patients with a malignant process of the bone.
The informed consent of the patients to conduct the study.
Exclusion criteria were as follows.
The refusal of the patient to conduct the study.
The existence of other silver-coated implants (e.g., silver-coated stents) in the patient.
A known allergy or hypersensitivity reaction against silver in the previous medical history of the patient.
After the full approval by the local ethical committee, patients were recruited prospectively between 2008 and 2012. All patients underwent surgery in the Traumatology Department of the University Hospital Essen. Only modular silver-coated prostheses from the company Implantcast were implanted (Implantcast Co., Buxtehude, Germany) and fixed with polymethyl methacrylate (PMMA) bone cement. The silver coatings of the titanium-vanadium megaprostheses were realized by a galvanic deposition of elementary silver (percentage purity of 99.7%) on the prosthetic surface. The layer thickness ranged from 10 to 15 mm. Additionally, a 0.2 mm-thick gold layer between the titanium-vanadium surface of the prosthesis and the silver-coating was necessary to enable the sustained release of silver ions in the periprosthetic tissue and prevent progressive corrosion. No silver coating was applied at the articulating surfaces or the prosthetic stems [3].
2.1. Patients
Eighteen patients fulfilled the inclusion criteria and were available for evaluation; 11 of them were female. The average age at the time of surgery was 60.1 years (SD 19.4 years). The indication for the implantation of a silver-coated endoprosthesis was due to an infection for 10 patients, and 8 patients received a silver-coated primary endoprosthesis or a prosthesis replacement in the case of aseptic loosening of the primary nonsilver-coated prosthesis due to an infection prophylaxis from a tumor (Table 1). As shown in Table 1, two of the cases after trauma were secondary open fractures. In addition, Table 1 presents the corresponding total silver mass of the applied silver prostheses in grams.
Table 1.
Demographic descriptions of the patient cohort, the indication for the silver prosthesis, an illustration of the joint concerned, and the total silver mass of the respective prosthesis.
| Patient no. | Age at the time of surgery | Gender | Type of fracture/tumor | Indication silver | Type of prosthesis | Silver mass (g) |
|---|---|---|---|---|---|---|
| 1 | 37 | w | Secondary open distal femur fracture | Infection Plate osteosynthesis | Proximal Tibia, distale femur | 0.76 |
| 2 | 67 | m | Fractured acetabulum | Infection THA | Proximal femur | 0.62 |
| 3 | 83 | w | Medial fracture of the femur neck | Infection dual head prosthesis | Proximal femur | 0.46 |
| 4 | 73 | m | Periprosthetic fracture of the femur | Infection THA | Proximal femur | 1.69 |
| 5 | 50 | m | Medial fracture of the femur neck | Infection dual head prosthesis | Proximal femur | 0.46 |
| 6 | 81 | w | Medial fracture of the femur neck | Infection dual head prosthesis | Proximal femur | 0.67 |
| 7 | 83 | w | Subtrochanteric fracture of the femur | Infection Plate osteosynthesis | Proximal femur | 0.52 |
| 8 | 89 | w | Medial fracture of the femur neck | Infection dual head prosthesis | Proximal femur | 0.46 |
| 9 | 63 | w | Pertrochanteric fracture of the femur | Infection intramedullary nail | Proximal femur | 0.62 |
| 10 | 45 | w | Secondary open supracondylar humerus fracture | Infection elbow prosthesis | Distal humerus, prox. ulna | 0.4 |
| 11 | 46 | w | Osteosarcoma | Loosened TKA | Proximal tibia, distale femur | 1.33 |
| 12 | 55 | w | Metastasis renal cell carcinoma head of the humerus | Prophylaxis | Proximal humerus | 0.42 |
| 13 | 64 | w | Metastasis cervix carcinoma distal humerus | Prophylaxis | Distal humerus, prox. ulna | 0.95 |
| 14 | 71 | m | Metastasis adenoca prox. femur | Prophylaxis | Proximal femur | 1.03 |
| 15 | 24 | m | Ewing's sarcoma prox. tibia | Prophylaxis | Proximal tibia, distal femur | 1.24 |
| 16 | 66 | w | Chondrosarcoma humerus | Prophylaxis | Proximal humerus | 0.97 |
| 17 | 24 | m | Osteosarcoma femoral shaft | Prophylaxis | Total femur | 0.68 |
| 18 | 60 | w | Metastasis mamma ca prox. femur | Prophylaxis | Proximal femur | 0.75 |
Patients 1–10: infection group; patients 11–18: infection prophylaxis group; g: gram; THA: total hip arthroplasty; TKA: total knee arthroplasty.
In the infection group, an infection was noted, on average, 18 days postoperatively. Table 2 demonstrates the pathogens detected using microbiological analysis. In two cases, no pathogens from the intraoperative smears and tissue samples could be proven by either clinical or laboratory findings. Due to the small sample size of the infection group, it was not possible to demonstrate correlation between the duration of the surgical procedure, age, tumour disease, and/or trauma severity or blood loss and the infection rate. In the current literature, this relation has been clearly described by Ahrens et al. [4]. In the infection group, the infected osteosynthesis or total endoprosthesis was removed, and a temporary antibiotic-impregnated cement spacer was implanted for 6 weeks before the implantation of the silver-coated megaprosthesis. Furthermore, patients received a 6-week pathogen-specific antibiotic treatment (intravenously or orally) prior to the implantation of the silver-coated megaprosthesis. In each patient, a trial removal of the infected tissue was performed previously, and the megaprosthesis was implanted only if there were no pathogens in the tissue samples. On the day of the implantation of the silver-coated megaprosthesis, all patients—those in the infection group and those in the infection prophylaxis group—received three doses of perioperative intravenous antibiotic prophylaxis with a cephalosporin over 24 hours. No other antibiotics were administered.
Table 2.
Infection group: clinical course prior to the implantation of a silver-coated megaprosthesis.
| Patient no. | Days until clinically definite infection | Pathogen of the infection | Revision operations | Days of hospitalization prior to SMP implantation | Days after SMP implantation |
|---|---|---|---|---|---|
| 1 | 36 | Staph. epi. | 7 | 66 | 31 |
| 2 | 18 | Enterobacter cloacae, Staph. epi. | 5 | 48 | 31 |
| 3 | 15 | Staph. epi. | 12 | 73 | 44 |
| 4 | 40 | Staph. epi, Enterococcus faec., Corynebacterium | 7 | 227 | 92 |
| 5 | 18 | Staph. epi, Enterococcus faec. | 5 | 74 | 24 |
| 6 | 13 | Staph. epi. | 4 | 47 | 27 |
| 7 | 8 | Enterobacter cloacae | 5 | 60 | 34 |
| 8 | 14 | Not demonstrable | 2 | 7 | 54 |
| 9 | 12 | Enterococcus faec. | 7 | 105 | 23 |
| 10 | 9 | Not demonstrable | 2 | 14 | 5 |
Staph: Staphylococcus; epi: epidermidis; feac: faecalis; SMP: silver-coated megaprosthesis.
2.2. Sample Collection
Two days after surgery, blood samples were collected using the Redon drains that had been placed immediately surrounding the implant. The Redon bottles were shaken; directly afterwards, exactly 5 mL was extracted for analysis and preserved in serum Monovettes (S-Monovettes, Co., Sarstedt, Nümbrecht, Germany). In the case of more than one Redon bottle being used, the fluid of each Redon bottle was analyzed individually, and a total mean value was established for each evaluated patient. The measurement of the silver ion concentration—at the time when samples were collected from the Redon bottle—was the main focus, irrespective of the amount of wound secretion. The total amount of silver ions released from the prosthesis was not part of the evaluation. There were only minor differences between the groups with regard to the amount of wound secretion on the second postoperative day. In the same way, exactly 5 mL of systemic venous blood from each patient was sampled on the 7th and 14th day postoperatively. During this time, no patient in the study underwent additional surgery (Figure 1).
Figure 1.
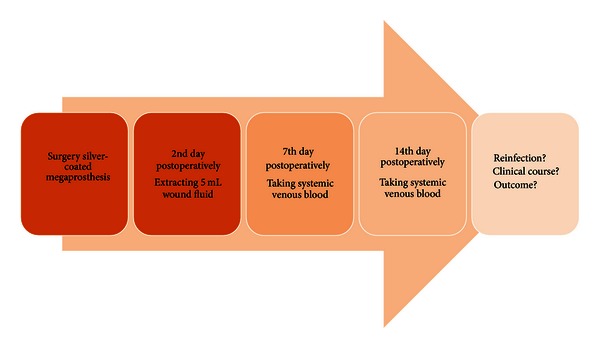
Graphic representation of test procedure.
2.3. Sample Preparation and Analytics
2.3.1. Sample Preparation
The whole blood or serum samples were heated at 300°C and denatured in 65% nitric acid (Merck Co., Darmstadt, Germany) and 35% hydrogen peroxide (Merck Co., Darmstadt, Germany). The clear fluid resulting from this was subsequently preserved for analysis in Falcon tubes at −80°C (Greiner bio-one, Heidelberg, Germany).
2.3.2. Analytics
Based on the established technique described by Rahil-Khazen et al. to verify metal ions from human tissue samples, the analysis was performed using mass spectrometrically and inductively coupled atomic absorption spectrometry (ICP-AAS) [12]. The samples were exclusively analyzed for silver ions. To receive statistically reliable results, each probe was measured in triplicate. The detection limit for silver ions of this analytic procedure was 0.01 parts per million (ppm).
2.4. Classification
The collective group was analyzed, and two group-specific analyses were carried out as follows: (1) silver-coated endoprosthesis due to an infection versus infection prophylaxis; (2) subgroup analysis depending on the mean value of the silver ions in wound fluids (MV 0.08 ppm) (high silver group: >0.08 ppm, low silver group: <0.08 ppm). Over the same period of time, 6 patients were implanted with a nonsilver-coated endoprosthesis. These patients formed the control group for detecting naturally occurring silver in the blood to determine the standard value.
Furthermore, a retrospective comparative analysis regarding the infection prophylaxis from local case material (group: no silver) in patients without silver-coated endoprosthesis implantation (n = 31 patients) was carried out from 2004 to 2011 (Table 3). The classification is shown in Figure 2.
Table 3.
Demographic descriptions of the retrospective group, an illustration of the joint concerned, and the infection rate.
| Patient no. | Age at the time of surgery | Gender | Type of fracture/tumor | Type of prosthesis | Infection yes/no |
|---|---|---|---|---|---|
| 1 | 71 | m | Periprosthetic fracture of the femur | Proximal femur | yes |
| 2 | 56 | m | Subtrochanteric fracture of the femur | Proximal femur | no |
| 3 | 83 | w | Pertrochanteric fracture of the femur | Proximal femur | yes |
| 4 | 84 | w | Medial fracture of the femur neck | Proximal femur | yes |
| 5 | 52 | m | Medial fracture of the femur neck | Proximal femur | yes |
| 6 | 85 | w | Pertrochanteric fracture of the femur | Proximal femur | no |
| 7 | 96 | w | Medial fracture of the femur neck | Proximal femur | no |
| 8 | 90 | w | Medial fracture of the femur neck | Proximal femur | yes |
| 9 | 88 | w | Pertrochanteric fracture of the femur | Proximal femur | no |
| 10 | 83 | w | Pertrochanteric fracture of the femur | Proximal femur | no |
| 11 | 85 | m | Medial fracture of the femur neck | Proximal femur | no |
| 12 | 78 | w | Pertrochanteric fracture of the femur | Proximal femur | yes |
| 13 | 85 | m | Periprosthetic fracture of the femur | Proximal femur | no |
| 14 | 82 | w | Periprosthetic fracture of the femur | Proximal femur | no |
| 15 | 88 | w | Medial fracture of the femur neck | Proximal femur | yes |
| 16 | 56 | w | Metastasis mamma carcinoma prox. femur | Proximal femur | no |
| 17 | 54 | m | Metastasis oropharyngeal Ca femoral shaft | Proximal femur | no |
| 18 | 60 | w | Metastasis mamma ca prox. femur | Proximal femur | no |
| 19 | 78 | m | Metastasis cancer of unknown primary | Proximal femur | no |
| 20 | 55 | w | Metastasis mamma ca prox. femur | Proximal femur | no |
| 21 | 70 | w | Metastasis mamma ca prox. femur | Proximal femur | no |
| 22 | 31 | m | Metastasis chondrosarcoma femoral shaft | Proximal femur | no |
| 23 | 57 | w | Metastasis hepatocellular carcinoma femur | Proximal femur | no |
| 24 | 68 | m | Metastasis Hypernephroma femur proximal |
Proximal femur | no |
| 25 | 75 | m | Plasmacytoma femur proximal |
Proximal femur | no |
| 26 | 76 | w | Metastasis corpus uteri Prox. femur |
Proximal femur | no |
| 27 | 75 | m | Plasmacytoma femur proximal |
Proximal femur | no |
| 28 | 73 | m | Metastasis kidney ca prox. femur |
Proximal femur | no |
| 29 | 75 | m | Metastasis chondrosarcoma femoral shaft | Proximal femur | no |
| 30 | 75 | w | Metastasis kidney ca prox. femur |
Proximal femur | no |
| 31 | 76 | w | Metastasis rectum ca prox. femur |
Proximal femur | no |
Patients 1–15: trauma group; patients 16–31: tumor group.
Figure 2.
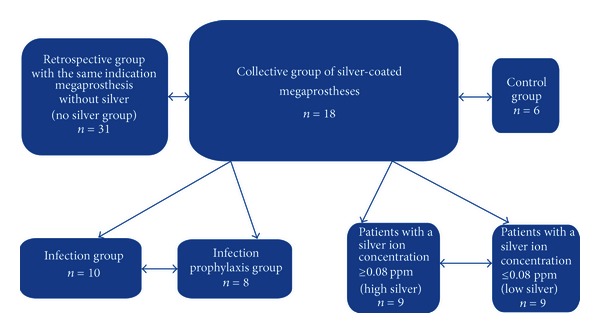
Group distribution and classification of the subgroups.
2.5. Clinical Course and Outcome
The patients were examined based on the following criteria.
Laboratory determination of C-reactive protein (CRP) (high correlation with the course of a bacterial infection) [13].
Reinfections and concomitant revision surgery (until 12 months postoperatively).
Duration of stay in the hospital.
Postoperative function (until 12 months postoperatively).
Survival rate of the implant (until 12 months postoperatively)
2.6. Statistics
The statistical analysis was carried out with the Statistic Package for Social Science (SPSS, version 19, IBM, Chicago, IL, USA). The data were analyzed for significance by the Mann-Whitney U test and the Wilcoxon signed-rank test. Differences between the groups were evaluated using a t-test for continuous variables, and correlation tests were carried out according to Pearson (c = correlation). In total, only a restricted statistical statement can be made due to the small group sizes. A P value of <0.05 was considered significant.
3. Results
3.1. Mass Spectrometry (ICP-AAS) of the Wound Fluid
In all 18 patients, increased silver ions could be detected in the samples from the wound fluids of the Redon bottles. The mean value of this silver ion concentration was 0.08 ppm (SD 0.05). There was no significant difference between the silver ion concentration in the infection group (MV: 0.07 ppm, SD 0.1) and the infection prophylaxis group (MV: 0.08 ppm; SD 0.14; P ≥ 0.05) (Figure 3). Additionally, no correlation between the silver ion concentration and the total silver mass of the implanted endoprosthesis could be determined (c = 0.2; P ≥ 0.05).
Figure 3.
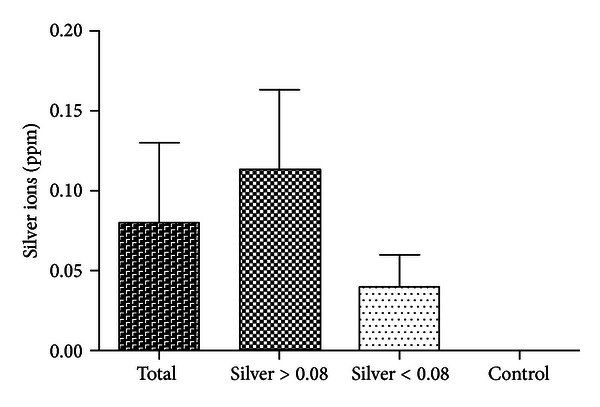
Measurement of silver ions in wound fluids (mean value). ppm: parts per million.
3.2. Mass Spectrometry (ICP-AAS) of the Venous Systemic Blood
3.2.1. 7th Day Postoperatively
In 7 patients, relevant amounts of silver ions were detected from venous blood. The mean value of the concentration was 0.03 ppm. Four patients with systemic proof of silver ions were members of the infection group (silver ion concentration MV: 0.02 ppm, SD: 0.02). In three patients from the infection prophylaxis group, the mean value of the concentration was 0.05 ppm (SD: 0.06). No significant difference between the groups was demonstrated. Moreover, there was no correlation between the silver ions in the wound fluids and the silver concentration in the systemic blood seven days postoperatively (c = 0.22; P ≥ 0.05). There was almost no statistical correlation between the total silver mass of the endoprosthesis and the silver concentration in the blood (Figure 4).
Figure 4.
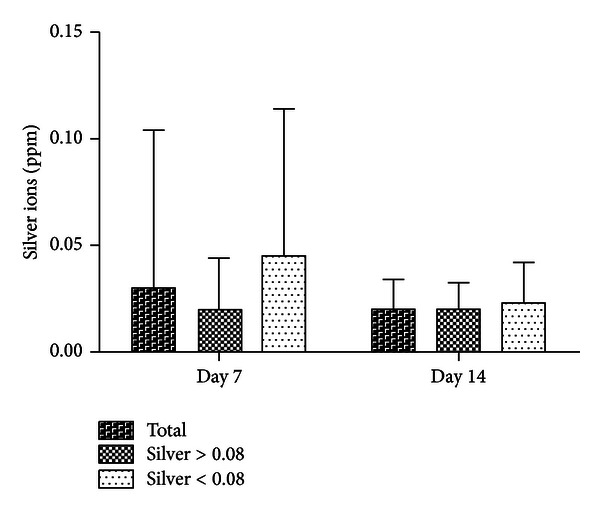
Measurement of silver ions in the venous systemic blood 7 and 14 days postoperatively. Subdivision into two groups depending on the measured mean value of the silver concentration in the wound fluids (mean value: 0.08 ppm). CRP: C-reactive protein; 7th and 14th day postoperatively; ppm: parts per million.
3.2.2. 14 Days Postoperatively
Relevant silver concentrations were noted in 11 patients (MV: 0.02 ppm, SD: 0.01). Seven of these patients belonged to the infection group; thus, this group was again the best represented (silver concentration MV: 0.02 ppm, SD: 0.01). As in the preceding sections, no significant differences among the groups could be determined. There was a correlation between the measured silver ions in the venous blood of the collective group and the silver mass of the prostheses (c = 0.6; P = 0.03). Additionally, there was a significant difference in the silver ion concentration of the venous blood compared to the control group (control group: MV 0; P = 0.03). In the subgroup analysis, the silver ions in the wound fluids increased on the 7th day postoperatively in the low-silver group compared to the high-silver group and decreased to the same level on the 14th day (Figure 4). In the control group, silver ion concentrations were discovered neither on the 7th nor 14th days postoperatively.
3.3. CRP
After seven days, the CRP mean value in the collective group was 9.6 mg/dL, and after 14 days postoperatively, it was 5.8 mg/dL (normal value < 0.05 mg/dL). Regarding the subgroups, the CRP decreased faster 7 days postoperatively in the high-silver group (n = 9; 5 of them were from the infection group) compared to the low-silver group (n = 9; 4 of them were from the infection group). Corresponding results were detected 14 days after surgery. After 14 days, the low-silver group reached a similar CRP value as that of the high-silver group on postoperative day 7 (Figure 5).
Figure 5.
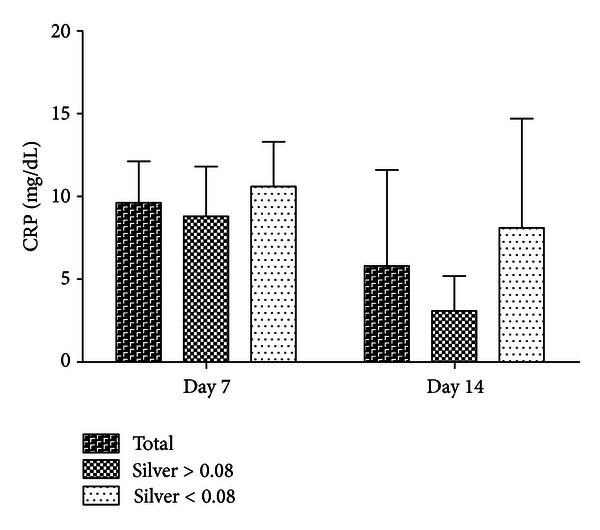
Course of the C-reactive protein in the subgroup comparison depending on the measured mean value of the silver concentration in the wound fluids (mean value: 0.08 ppm) on the 7th or rather the 14th day postoperatively. CRP: C-reactive protein.
When analyzing the infection group compared to the infection prophylaxis group, there was no significant difference in the kinetics of the CRP. A correlation between the presence of silver ions in the wound fluids or in the systemic blood and the course of the CRP could not be proven (c = 0.28; P = 0.34).
3.4. Clinical Course and Outcome
3.4.1. Reinfection Rate
Of the 18 examined patients, one patient from the infection prophylaxis group showed a reinfection (5.6%) with Enterococcus faecalis three months postoperatively. In this case, a femoral amputation was carried out. This patient had a silver ion concentration in the wound fluid that averaged 0.03 ppm and belonged to the group with less than 0.08 ppm silver ions in the wound fluid (low-silver group). In the infection group, no reinfections were detected within 12 months postoperatively.
In comparison to that result, 7 patients in the retrospective group with megaprosthesis without silver showed a significant reinfection rate (22%, P = 0.01) (Figure 6).
Figure 6.
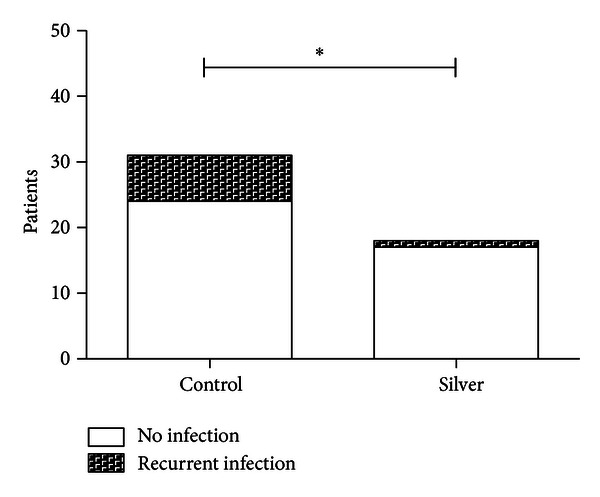
Rate of reinfections in comparison to the no silver group. *P ≤ 0.05.
3.4.2. Duration of Stay in the Hospital
The duration of the stay in hospital averaged 36.5 days in the group with silver-coated megaprostheses after implantation. In the subgroup analysis, patients in the retrospective group with megaprosthesis without silver had a significantly longer stay in the hospital (MV: 72.1 days, P ≤ 0.001) (Figure 7).
Figure 7.
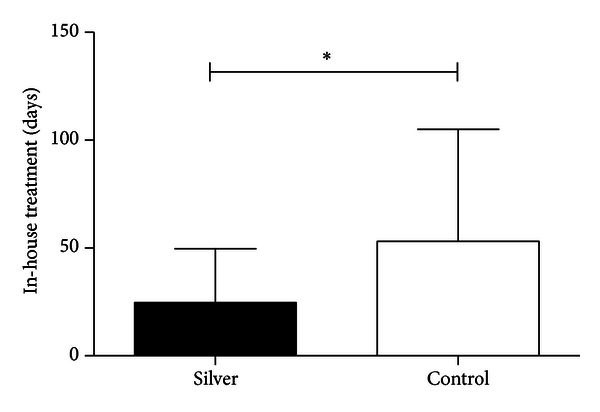
Clinical course of the group with implantation of a silver-coated megaprosthesis and the no silver group (mean value). Period of hospitalization in days. *P ≤ 0.05.
3.4.3. Functional Outcome
Seven patients were able to move the operated upon extremity without using orthopedic aids (5 patients from the infection prophylaxis group) within 12 months after surgery. Nine patients (6 patients from the infection group) were able to walk more than 200 m using auxiliary supports (e.g., forearm crutches, rolling walker). As mentioned above, a reinfection occurred in one patient such that the thigh had to be amputated (infection prophylaxis group); in one patient, the inserted megaprosthesis became dislocated (infection group). For the dislocated megaprosthesis, a closed reposition was carried out, and the following outcome was uneventful. In all patients, the X-ray control in the two planes at day 5, after 6 weeks, after 3 months, and after 12 months showed a regular implant position without any signs of loosening or infection (except for the amputated patient). The wound healing was uneventful and without prolonged secretion, and the scar 12 months postoperatively showed no inflammatory signs.
3.4.4. Survival
All 18 patients survived 12 months postoperatively. Indications of silver intoxication could not be proven clinically. Also, chemical laboratory analyses that were conducted during routine checks did not indicate any silver intoxication. Neither liver enzymes such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST) nor renal serum parameters such as creatinine concentrations changed during the entire trial period, as a sign of silver-dependent organ damage, significant when compared to the control group.
4. Discussion
Due to an increasing infection rate with a rising resistance of bacteria against the usually applied antibiotics, the development of endoprosthetic metallic coatings that can perhaps lower infection rates is essential. Such prostheses would be particularly valuable to infection-prone patients with a malign underlying disease or to patients with a disturbed immune defense from pre-existing conditions, age or as a consequence of severe trauma. Thus, Gosheger et al., Ahrens et al., and Hardes and Von Eiff demonstrated that the silver coating of a prosthesis can decrease the reinfection rate in an animal experiment or an oncological patient cohort due to the release of silver ions [4, 9, 10]. Moreover, Hardes et al. illustrated the kinetic course of the silver ion concentration in the peripheral blood [3]. In the present study, the concentration of released silver ions in the direct surroundings of the prosthesis (wound fluid) was measured for the first time. This sample represents a common area for bacterial prosthesis infection in which systematically applied antibiotics work increasingly poorly. It is well known that bacteria, especially near the prosthesis, have become increasingly resistant to antibiotic eradication due to virulence factors, such as the formation of a biofilm [14, 15]. When comparing the measured silver concentrations with those values published in the recent literature, it is evident that the values measured in this study are apparently lower. For example, Straub et al. demonstrated bactericide effects of silver ions on gram-negative periodontal pathogens starting at values of 0.5 ppm, and Zhao and Stevens Jr. achieved bactericide effects of 2.0 ppm in an in vitro study [16, 17]. As mentioned before, it is unclear—based on the current literature—whether an average silver ion concentration of 0.08 ppm that has been measured in this study with megaprostheses provides sufficient levels of bactericide action. Nevertheless, it was possible to demonstrate clinical effects. The initial objective of this study was to clarify in a first step whether silver ions can be found in the direct surroundings of the prosthesis and if there are any insights with regard to the clinical course depending on measured concentrations. In the future, further studies shall demonstrate whether the concentrations measured in wound fluid that were collected in the immediate proximity of the prosthesis may have in vivo bactericide effects.
It must be taken into account that silver ions may build complexes with serum albumin [18]. Schierholz et al. showed in their study that this serum albumin silver complexing may reduce the bactericidal effects of silver [19]. Gosheger et al. suggest in their study that bactericidal effects may be reduced due to dilution, whenever body fluids get in contact with silver ions [9]. This is a basic relationship that is true for all applications of medicines. In our study, the amount of active silver ions appeared large enough, since a significant reduction of infection rates has been observed when compared to the retrospective group with megaprosthesis without silver.
Some evidence suggests that silver coatings may not have exclusively positive effects on patient outcome. Silver coatings have been implicated in osteolyses and postoperative prosthesis loosening, and the positive effects must be measured against the negative in each individual case [20, 21]. Rosengren and Dixon described in a review that silver-impregnated dressings have demonstrated no advantage in the healing of chronic wounds in a dermatological patient cohort [22]. Again, the decision to implant a megaprosthesis must be made on a case-by-case basis.
When considering the reinfection rate, there is a significant difference between endoprostheses with and without silver coatings. Although there is no statistically significant correlation between the increased concentration of silver ions in the wound fluids or in the peripheral blood and the reinfection rate, due to the small number of cases, the indication of a direct connection seems to be reasonable. As mentioned above, this conclusion is confirmed by the current literature [10]. Interestingly, in the traumatological patient cohort of patients who already had existing or previous infections, the reinfection rate decreased. This connection can currently not be exhaustively discussed due to the lack of the current literature regarding a traumatological patient cohort.
Due to the small group size and the concomitant low level of significance, it remains unclear whether the concentration of released silver ions in the immediate surroundings of the prosthesis has an influence on the clinical course. However, our results are promising. Considering the course of the CRP, patients with a relatively large amount of released silver ions show a faster decrease in the inflammatory marker CRP. The only case of reinfection in the described patient cohort shows that an insufficient quantity of loosened silver ions may be ineffective.
However, conclusions regarding a minimum concentration can currently not be made. To show a minimum concentration, a larger patient cohort or another animal experiment is needed. Moreover, the data show that patients which initially had a higher concentration in the systemic venous blood (7th day postoperatively) had a lower concentration of silver ions in the wound fluids. Thus, the too fast removal of the loosened silver ions and the resulting decrease from the effective concentration near the prosthesis seem to delay the decrease in inflammation. This connection also cannot be exhaustively discussed due to the current literature. In a recent in vitro study, however, Wu et al. described that the surrounding level of fluid and immersion time influence the release of silver ions. Unfortunately, this study does not aim to assess the effect of the released silver ions on bacterial infections [23].
In the present study, no toxic side effects from silver were found in the patients. This corresponds to previous studies by Gosheger et al. and Hardes et al., as well as other authors [3, 9, 24]. The minimum doses mentioned in the literature of approximately 4–6 g, for example, to cause argyria, are not approached in the present study [25–27]. However, as an accumulation of silver ions is in principle possible, Hardes et al. concluded that this must be considered at all times [3]. Thus, a case report by Sudman et al. describes a thousandfold increase of the silver serum level compared to baseline. The patient described in this study suffered from this complication 5 years after the installation of a hip endoprosthesis. This hip endoprosthesis was implanted with PMMA bone cement that had been supplied with 1% silver. This patient showed a peripheral neuropathy, but it remained unclear whether the supplied silver ions alone caused the complication [28]. Further case reports have discovered other potential complications, including a greasy degeneration of liver, heart, and kidneys [29, 30].
The significant reduction of the hospitalization period and the decrease in revision surgeries, especially in the patient group with previous infections, illustrate the potential significance of silver-coated megaprostheses. Here, we have analyzed the benefit of these prostheses for the first time in a traumatological patient cohort. It is apparent that silver-coated prostheses can be used not only for prophylaxis but also for the decrease of the reinfection risk. In the group of traumatological patients, no reinfections occurred; this result is particularly relevant because the average age in the traumatological group was 69.5 years. Such patients in particular should not undergo frequent surgery, as their operative risk increases with age. The same applies to patients with multiple injuries, whose nosocomial infection risks only increase with longer stays in the intensive care unit [31].
In the traumatological cohort in particular, using the silver-coated megaprosthesis often represented the final option before amputation to treat the infection or fight sepsis. In the present study, acute infections were often in transition towards chronic inflammations in the infection group, or the patients were in sepsis due to an osseous and soft tissue-driven source of infection. Therefore, it remains remarkable that all of the described patients retained the extremity concerned and were discharged from hospital as mobile, only using auxiliaries like forearm crutches. The danger of amputation as the last option to control or treat infection is extensively described in the literature [32–36].
Finally, in the light of dwindling resources, the economic factor has to be considered. The silver-coated megaprosthesis is admittedly 5–7% more expensive than the nonsilver-coated prosthesis [9]. However, the significant decrease in the period of hospitalization and the decrease in revision surgeries must be taken into account as relevant cost factors [37, 38].
Limitations
In this study, the group size was too small to prove highly significant results. Additional prospective studies with larger cohorts are required to achieve statistically reliable results.
A prospective randomized study regarding the use of silver-coated against nonsilver-coated endoprostheses could not be carried out due to ethical concerns. Especially in the traumatological group, the use of a silver-coated endoprosthesis was often the last possible option before amputation. From a scientific point of view, the realization of such a study would be required to increase the evidence level.
The period of postoperative evaluation was 12 months. In the context of the total joint registry data, this is an early followup. In particular, this study does not allow for a statement of the implant survivorship.
A histological examination in still inserted prostheses was not yet possible. The analysis of, for example, foreign body reactions at the cellular level was thus not possible. It is important to carry out further analyses in the future, for example, by removing prostheses or post mortem.
5. Conclusions
In the present study, increased silver concentrations were detected in the immediate surroundings of silver-coated prostheses. Our data suggest that silver release does improve clinical outcome. For the first time, the positive effect of silver-coated megaprostheses was demonstrated for trauma patients and tumor patients.
Conflict of Interests
The authors declare that they have no conflict of interest regarding this submission.
Acknowledgments
Special thanks go to the Institute of Geology of the University Duisburg-Essen under the direction of Professor Schreiber and his laboratory manager, Mark Schumann, for its outstanding support. The authors also thank Heike Uhlenkott for her indescribable support in preparing the tissue samples.
References
- 1.Hardes J, Gebert C, Schwappach A, et al. Characteristics and outcome of infections associated with tumor endoprostheses. Archives of Orthopaedic and Trauma Surgery. 2006;126:289–296. doi: 10.1007/s00402-005-0009-1. [DOI] [PubMed] [Google Scholar]
- 2.Mittermayer F, Krepler P, Dominkus M, et al. Long-term followup of uncemented tumor endoprostheses for the lower extremity. Clinical Orthopaedics and Related Research. 2001;388:167–177. doi: 10.1097/00003086-200107000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Hardes J, Ahrens H, Gebert C, et al. Lack of toxicological side-effects in silver-coated megaprostheses in humans. Biomaterials. 2007;28(18):2869–2875. doi: 10.1016/j.biomaterials.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 4.Ahrens H, Gosheger G, Streitbürger A, Gebert C, Hardes J. Antimikrobielle Silberbeschichtung von Tumorprothesen. Der Onkologe. 2006;12:145–151. [Google Scholar]
- 5.Park HJ, Kim JY, Kim J, et al. Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Research. 2009;43(4):1027–1032. doi: 10.1016/j.watres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Kim JS, Kuk E, Yu KN, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3(1):95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Jones SA, Bowler PG, Walker M, Parsons D. Controlling wound bioburden with a novel silver-containing Hydrofiber dressing. Wound Repair and Regeneration. 2004;12(3):288–294. doi: 10.1111/j.1067-1927.2004.012304.x. [DOI] [PubMed] [Google Scholar]
- 8.Gordon O, Slenters TV, Brunetto PS, et al. Silver coordination polymers for prevention of implant infection: thiol interaction, impact on respiratory chain enzymes, and hydroxyl radical induction. Antimicrobial Agents and Chemotherapy. 2010;54(10):4208–4218. doi: 10.1128/AAC.01830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gosheger G, Hardes J, Ahrens H, et al. Silver-coated megaendoprostheses in a rabbit model—an analysis of the infection rate and toxicological side effects. Biomaterials. 2004;25(24):5547–5556. doi: 10.1016/j.biomaterials.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Hardes J, von Eiff C, Streitbuerger A, et al. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. Journal of Surgical Oncology. 2010;101(5):389–395. doi: 10.1002/jso.21498. [DOI] [PubMed] [Google Scholar]
- 11.Jung W, Koo HC, Kim KW, Shin S, Kim SH, Park YH. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Applied and Environmental Microbiology. 2008;74(7):2171–2178. doi: 10.1128/AEM.02001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahil-Khazen R, Bolann BJ, Myking A, Ulvik RJ. Multi-element analysis of trace element levels in human autopsy tissues by using inductively coupled atomic emission spectrometry technique (ICP-AES) Journal of Trace Elements in Medicine and Biology. 2002;16(1):15–25. doi: 10.1016/S0946-672X(02)80004-9. [DOI] [PubMed] [Google Scholar]
- 13.Castelli GP, Pognani C, Meisner M, Stuani A, Bellomi D, Sgarbi L. Procalcitonin and C-reactive protein during systemic inflammatory response syndrome, sepsis and organ dysfunction. Critical Care. 2004;8(4):R234–R242. doi: 10.1186/cc2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Eiff C, Peters G, Heilmann C. Pathogenesis of infections due to coagulase-negative staphylococci. The Lancet Infectious Diseases. 2002;2(11):677–685. doi: 10.1016/s1473-3099(02)00438-3. [DOI] [PubMed] [Google Scholar]
- 15.Sheehan E, McKenna J, Mulhall KJ, Marks P, McCormack D. Adhesion of Staphylococcus to orthopaedic metals, an in vivo study. Journal of Orthopaedic Research. 2004;22(1):39–43. doi: 10.1016/S0736-0266(03)00152-9. [DOI] [PubMed] [Google Scholar]
- 16.Straub AM, Suvan J, Lang NP, et al. Phase 1 evaluation of a local delivery device releasing silver ions in periodontal pockets: safety, pharmacokinetics and bioavailability. Journal of Periodontal Research. 2001;36(3):187–193. doi: 10.1034/j.1600-0765.2001.360308.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhao G, Stevens SE., Jr. Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. BioMetals. 1998;11(1):27–32. doi: 10.1023/a:1009253223055. [DOI] [PubMed] [Google Scholar]
- 18.Shahabadi N, Maghsudi M, Ahmadipour Z. Study on the interaction of silver(I) complex with bovine serum albumin by spectroscopic techniques. Spectrochimica Acta A. 2012;92:184–188. doi: 10.1016/j.saa.2012.02.071. [DOI] [PubMed] [Google Scholar]
- 19.Schierholz JM, Lucas LJ, Rump A, Pulverer G. Efficacy of silver-coated medical devices. Journal of Hospital Infection. 1998;40(4):257–262. doi: 10.1016/s0195-6701(98)90301-2. [DOI] [PubMed] [Google Scholar]
- 20.Fielding GA, Roy M, Bandyopadhyay A, Bose S. Antibacterial and biological characteristics of silver containing and strontium doped plasma sprayed hydroxyapatite coatings. Acta Biomaterialia. 2012;8:3144–3315. doi: 10.1016/j.actbio.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albers CE, Hofstetter W, Siebenrock KA, Landmann R, Klenke FM. In vitro cytotoxicity of silver nanoparticles on osteoblasts and osteoclasts at antibacterial concentrations. Nanotoxicology. 2013;7(1):30–36. doi: 10.3109/17435390.2011.626538. [DOI] [PubMed] [Google Scholar]
- 22.Rosengren H, Dixon A. Antibacterial prophylaxis in dermatologic surgery: an evidence-based review. American Journal of Clinical Dermatology. 2010;11(1):35–44. doi: 10.2165/11311090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Li J, Wang L, Huang D, Zuo Y, Li Y. The release properties of silver ions from Ag-nHA/TiO2/PA66 antimicrobial composite scaffolds. Biomedical Materials. 2010;5(4) doi: 10.1088/1748-6041/5/4/044105.044105 [DOI] [PubMed] [Google Scholar]
- 24.Rosenman KD, Moss A, Kon S. Argyria: clinical implications of exposure to silver nitrate and silver oxide. Journal of Occupational Medicine. 1979;21(6):430–435. [PubMed] [Google Scholar]
- 25.Brutel de la Riviere A, Dossche KME, Birnbaum DE, Hacker R. First clinical experience with a mechanical valve with silver coating. Journal of Heart Valve Disease. 2000;9(1):123–130. [PubMed] [Google Scholar]
- 26.Tweden KS, Cameron JD, Razzouk AJ, Holmberg WR, Kelly SJ. Biocompatibility of silver-modified polyester for antimicrobial protection of prosthetic valves. Journal of Heart Valve Disease. 1997;6(5):553–561. [PubMed] [Google Scholar]
- 27.Perrelli G, Piolatto G. Tentative reference values for gold, silver and platinum: literature data analysis. Science of the Total Environment. 1992;120(1-2):93–96. doi: 10.1016/0048-9697(92)90219-i. [DOI] [PubMed] [Google Scholar]
- 28.Sudmann E, Vik H, Rait M, et al. Systemic and local silver accumulation after total hip replacement using silver-impregnated bone cement. Medical Progress through Technology. 1994;20(3-4):179–184. [PubMed] [Google Scholar]
- 29.White JM, Powell AM, Brady K, Russell-Jones R. Severe generalized argyria secondary to ingestion ofcolloidal silver protein. Clinical and Experimental Dermatology. 2003;28:254–256. doi: 10.1046/j.1365-2230.2003.01214.x. [DOI] [PubMed] [Google Scholar]
- 30.Wan AT, Conyers RAJ, Coombs CJ, Masterton JP. Determination of silver in blood, urine, and tissues of volunteers and burn patients. Clinical Chemistry. 1991;37(10):1683–1687. [PubMed] [Google Scholar]
- 31.Adams SD, Cotton BA, McGuire MF, et al. Unique pattern of complications in elderly trauma patients at a Level I trauma center. The Journal of Trauma and Acute Care Surgery. 2012;72:112–118. doi: 10.1097/TA.0b013e318241f073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ham SJ, Koops HS, Veth RPH, van Horn JR, Molenaar WM, Hoekstra HJ. Limb salvage surgery for primary bone sarcoma of the lower extremities: long-term consequences of endoprosthetic reconstructions. Annals of Surgical Oncology. 1998;5(5):423–436. doi: 10.1007/BF02303861. [DOI] [PubMed] [Google Scholar]
- 33.Ritschl P, Capanna R, Helwig U, Campanacci M, Kotz R. KMFTR modular prosthesis system for the lower extremity: kotz modular femur tibia reconstruction system. Zeitschrift fur Orthopadie und Ihre Grenzgebiete. 1992;130(4):290–293. doi: 10.1055/s-2008-1039620. [DOI] [PubMed] [Google Scholar]
- 34.Wirganowicz PZ, Eckardt JJ, Dorey FJ, Eilber FR, Kabo JM. Etiology and results of tumor endoprosthesis revision surgery in 64 patients. Clinical Orthopaedics and Related Research. 1999;358:64–74. [PubMed] [Google Scholar]
- 35.Hillmann A, Gosheger G, Hoffmann C, Ozaki T, Winkelmann W. Rotationplasty—surgical treatment modality after failed limb salvage procedure. Archives of Orthopaedic and Trauma Surgery. 2000;120(10):555–558. doi: 10.1007/s004020000175. [DOI] [PubMed] [Google Scholar]
- 36.Shih LY, Sim FH, Pritchard DJ, Rock MG, Chao EYS. Segmental total knee arthroplasty after distal femoral resection for tumor. Clinical Orthopaedics and Related Research. 1993;292:269–281. [PubMed] [Google Scholar]
- 37.von Eiff C, Proctor RA, Peters G. Coagulase-negative staphylococci: pathogens have major role in nosocomial infections. Postgraduate Medicine. 2001;110(4):63–76. [PubMed] [Google Scholar]
- 38.Herrmann M, Peters G. Catheter-Associated Infections Caused By Coagulase-Negative Staphylococci: Clinical and Biological Aspects. New York, NY, USA: Marcel Dekke; 1997. [Google Scholar]


