Abstract
Cytoplasmic transport of large molecules such as plasmid DNA (pDNA) has been shown to increase when cells are subjected to mild levels of cyclic stretch for brief periods. In the case of pDNA, this is in part due to the increased active transport of pDNA along stabilized, acetylated microtubules in the cytoplasm, whose levels are increased in response to stretch. It also has been shown that disruption of the dense actin network leads to increased pDNA and macromolecule diffusion as well. We hypothesize that stretch not only increases active transport of pDNA but also, similar to actin disrupting drugs, decreases cytoplasmic stiffness leading to a less restive pathway for macromolecules to diffuse. To test this we used particle tracking microrheology to measure cytoplasmic mechanics. We conclude that while cyclic stretch transiently decreases cytoplasmic stiffness and increases diffusivity, stretch-independent modulation of the levels of acetylated, stable microtubules has no effect on cytoplasmic stiffness. Furthermore, stretching cells that have maximally acetylated microtubules increases cytoplasmic trafficking of pDNA, without increasing levels of acetylated microtubules. These findings suggest that stretchenhanced gene transfer may occur by two independent mechanisms: increased levels of acetylated microtubules for directed active transport, and reduced cytoplasmic stiffness for increased diffusion.
Keywords: particle tracking microrheology, stiffness, cytoskeleton reorganization, nanorheology, intracellular transport, gene transfer, HDAC6, acetylated microtubules
Introduction
The cytoplasm, a complex meshwork of microtubules, microfilaments, and intermediate filaments is a major impediment to the intracellular movement of any macro-molecule. This is especially true for plasmid DNA (pDNA), which must cross the cytoplasm to reach the nucleus for transcription during transfection and gene therapy procedures (Lukacs et al., 2000). Two possible scenarios have been proposed for how pDNA travels to the nucleus: passive diffusion or active transport. The idea that DNA simply diffuses towards the nucleus has been investigated by various groups (Dowty et al., 1995; Lukacs et al., 2000; Shimizu et al., 2005). The diffusion rate for naked DNA determined by photobleaching experiments suggests that DNA diffusion through the cytoplasm is minimal at best. The limited diffusion of DNA within cells increased, however, when the actin network was destabilized with Cytochalasin D, suggesting that the actin network is the major contributor of hindrance to DNA simple diffusion (Dauty and Verkman, 2005). The alternative mechanism proposed for DNA movement is active transport (Suh et al., 2003; Vaughan and Dean, 2006). Microinjected and electroporated pDNA has been shown to bind to microtubules through adaptor proteins and actively transported to the nucleus via the molecular motor dynein (Vaughan and Dean, 2006). Taken together, all these results indicate that pDNA diffusion is minimal and that active transport of pDNA on microtubules is responsible for the rather small expression observed in static cells.
The cytoskeleton is not static, but rather it is constantly undergoing assembly and disassembly to aid in cell motility, maintain cell shape, and facilitate cell division. External mechanical forces on cells such as cyclic stretch and shear stress have also been shown to reorganize the cytoskeleton (Geiger et al., 2006; Sivaramakrishnan et al., 2008). Upon exposure to mild levels of cyclic stretch for brief periods of time, actin filaments become shortened and concentrated at the periphery of the cell, while the microtubule network is largely disassembled (Geiger et al., 2006). This reorganization of the cytoskeleton is accompanied by up to a 10-fold increase in the efficiency of gene transfer and expression after just a few minutes of cyclic stretch at 0.5 Hz and 10% change in basement membrane surface area (BMSA) in a variety of cells including smooth muscle cells, fibroblasts, and human alveolar epithelial cells (Geiger et al., 2006; Taylor et al., 2003). The reorganization of the cytoskeleton by itself, however, is not sufficient to account for the increase in gene trafficking and expression, since the disruption of the microtubule and actin networks are not sufficient to increase expression levels following transfection (Vaughan and Dean, 2006).
Indeed, we have also found that cyclic stretch inhibits the activity of histone deacetylase 6 (HDAC6) a cytoplasmic enzyme that does not act on histones, but rather deacetylates tubulin (Haggarty et al., 2003; Vaughan et al., 2008). Inhibition of HDAC6 by stretch leads to increased levels of acetylated microtubules, which have been shown to represent a stable pool of microtubules that are resistant to depolymerization and bind more motor proteins than unmodified microtubules (Reed et al., 2006; Vaughan et al., 2008). This increased recruitment of molecular machinery in the cytoplasm may allow for more binding of pDNA and transport it to the nucleus for transcription. This indicates that cyclic stretch may enhance gene delivery through more than one mechanism and that the sequence of events by which cyclic stretch enhances expression levels are dependent on each other.
In this report we examine the effect of cyclic stretch on the stiffness of the dense and complex cytoplasm in alveolar epithelial cells. Mechanical forces such as stretch and cytoskeletal reorganization are particularly relevant in the lung, a dynamic organ that undergoes continuously modulating exogenous forces due to cyclical respiratory patterns. Indeed, alveolar epithelial cells undergo distension of approximately 10% change in BMSA with normal tidal breathing, a value that we have shown causes reorganization of the cytoskeleton and increased gene trafficking in cells isolated from the lung (Geiger et al., 2006; Taylor et al., 2003). Since Dauty and colleagues have shown that destabilizing the actin network by drugs increased the diffusion of pDNA in a size dependent matter, we hypothesize that cyclic stretch mimics the effect of cytoskeleton disrupting drugs by reducing the stiffness of the cytoskeleton, and thus increasing the contribution of passive diffusion of pDNA towards the nucleus (Dauty and Verkman, 2005; Geiger et al., 2006). We also investigated the role of modulating the levels acetylated microtubules in the cytoplasm on the stiffness of the cytoskeleton to determine if the two pathways utilized by cyclic stretch to enhance gene expression are interconnected.
Materials and Methods
Cell Culture
Human adenocarcinoma alveolar epithelial cells A549 cells (#CCL-185; American Type Culture Collection, Rockville, MD) were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and antibiotic/antimycotic solution (Invitrogen, Carlsbad, CA). For cytoskeletal inhibitor experiments, cells were plated on 35 mm Petri dishes, 14 mm Microwell No. 1.5 coverglass (MatTek Corporation, Ashland, MA) and incubated with drugs for 10–30 min at 378C followed by a change of medium and microinjection. Drugs were used at the following final concentrations: Latrunculin B 0.625 µM, nocadazole 20 µM.
Cyclic Stretch
A549 cells were grown to 80% confluency on Pronectin coated 6-well plates and stretched at 10% change in the BMSA at 30 cycles per minute using the Flexcercell baseplate and the Flexcell FX3000 (Flexcell International, McKeesport, PA) (Geiger et al., 2006).
Microinjection
One hundred nanometer green fluorescent carboxylated beads (Invitrogen, Carlsbad, CA) were injected using a Femtojet and Injectman microinjection system (Eppendorf, Westbury, NY) mounted on an inverted Leica DMI 6000 B microscope.
Image Acquisition
Series of images were collected at 110–250 frames per second for 5 s using an inverted Leica microscope equipped with a 100× oil immersion objective (NA 1.47) and a Hamamatsu C9100-13 CCD (Hamamatsu, Hamamatsu City, Japan) using Volocity software (Improvision, Waltham, MA). Each pixel in the CCD corresponds to 0.64 µm.
Particle Tracking
A custom Matlab algorithm was used as described (Rogers et al., 2007). Briefly, after choosing the region of interest in the images where the particles move, the particles were fitted to a fourth order polynomial weighted by a two-dimensional Gaussian distribution. The center of the particle was then the maximum point in the fit. Images with signal to noise ratio (SNR) less than 10 were not used as the error in the tracking algorithm increases rapidly (Cheezum et al., 2001; Ehrenberg and McGrath, 2005; Rogers et al., 2007). To determine the effects of noise and vibration on our system, beads tightly attached to a cover slip by electrostatic salt interactions were tracked to establish the system’s spatial resolution.
Microrheology
Mean squared displacement (MSD) was calculated using the following equation:
where
N is the number of steps in the trajectory. x(i) and y(i) are the location of the center of the tracer. From the MSD, the stiffness or G was calculated from the generalized Stokes– Einstein equation as described (Mason, 2000). Briefly:
where G*(ω) is the complex shear modulus, KB is Boltzmann’s constant, T is the absolute temperature, α is the radius of the tracer, and Γ is the gamma function, which is approximated by:
and
The storage (stiffness) and loss moduli were described by:
and
siRNA Methods
siRNAs against HDAC6 and scrambled siRNAs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). A549 cells were plated on cover slips at 30% confluency 24 h prior to transfection. Cells were transfected with 100-nmol/L of HDAC6 siRNA and allowed to grow for 48 h prior to microinjection.
Statistics
Stiffness results were compared at selected frequencies using the unpaired one sided Student’s t-testt-test. Differences between groups were analyzed by one-way ANOVA with a P-value <0.05 considered significant. All computations were done using Matlab.
Plasmid Labeling and Microinjection
The green fluorescent protein-expressing plasmid pDD306 which contains 10 tandem peptide nucleic acid (PNA) binding sites and the SV40 DNA nuclear targeting sequence was labeled with Cy3-labeled PNA and resuspended in Tris–EDTA buffer at 0.5 mg/mL as described (Gasiorowski and Dean, 2005). Cy3-labeled plasmids were microinjected in the cytoplasm of A549 cells and imaged following microinjection to determine the localization in cytoplasm or nucleus.
Western Blotting
Cell lysates were separated by SDS–PAGE, transferred to nitrocellulose and reacted with antibodies against acetylated or unmodified tubulin be Western blots as described (Geiger et al., 2009).
Results
Sensitivity of Measurements
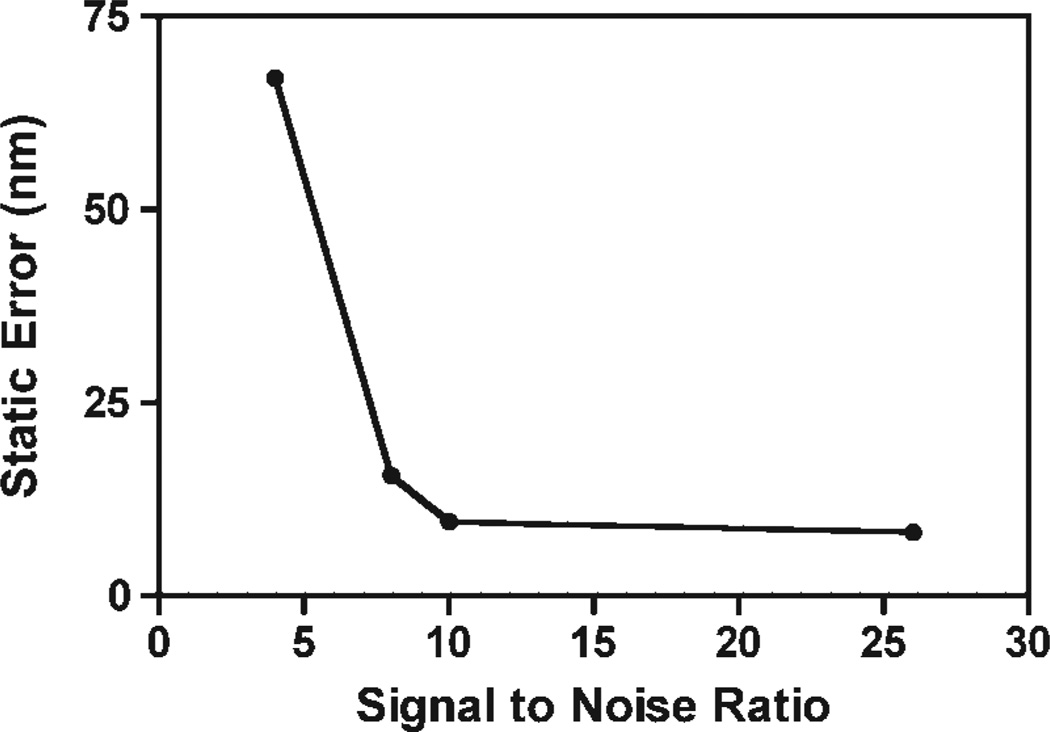
To establish the effects of vibration and other sources of noise on our measurements, tracers were affixed to a cover slip by electrostatic interactions and their trajectory was calculated to determine the static error as described previously (Rogers et al., 2007). Noise was added to these images in order to examine the effect of SNR on the accuracy of the tracking algorithm. At a SNR ratio of 26, the static error is 8.2 nm. The static error increased to 9.8 at a SNR of 10 and 15.6 nm at a SNR of 8 (Fig. 1). The accuracy of the tracking algorithm decreased rapidly with further decrease in the SNR to 67 nm at SNR of 4. These results indicate that in order to accurately determine the location of a particle, collected images must have a high SNR. Further, at a high SNR, the resolution of our system is sufficient for microrheology experiments (Ehrenberg et al., 2009; Wirtz, 2009).
Figure 1.
Noise and vibrations levels are insignificant to the tracking measurements in cells. A particle stuck to a cover slip by electrostatic interactions was imaged and tracked in order to calculate the static error at various signal to noise ratios. The static error increases with decreasing signal to noise ratio and thus the ability to track the particle accurately is sensitive to noise levels in the images.
Cyclic Stretch Decreases Cytoplasmic Stiffness
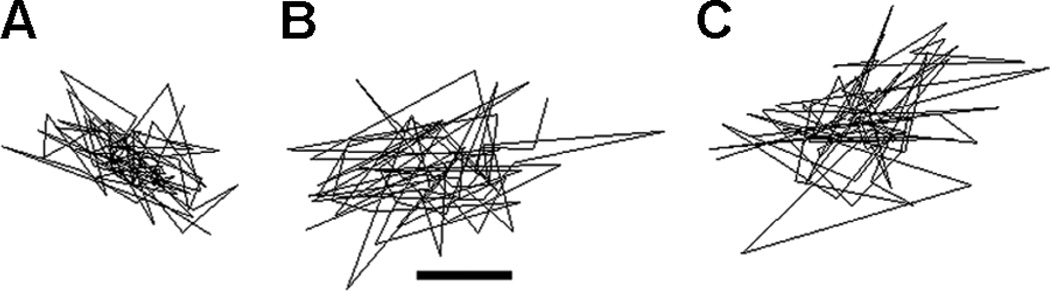
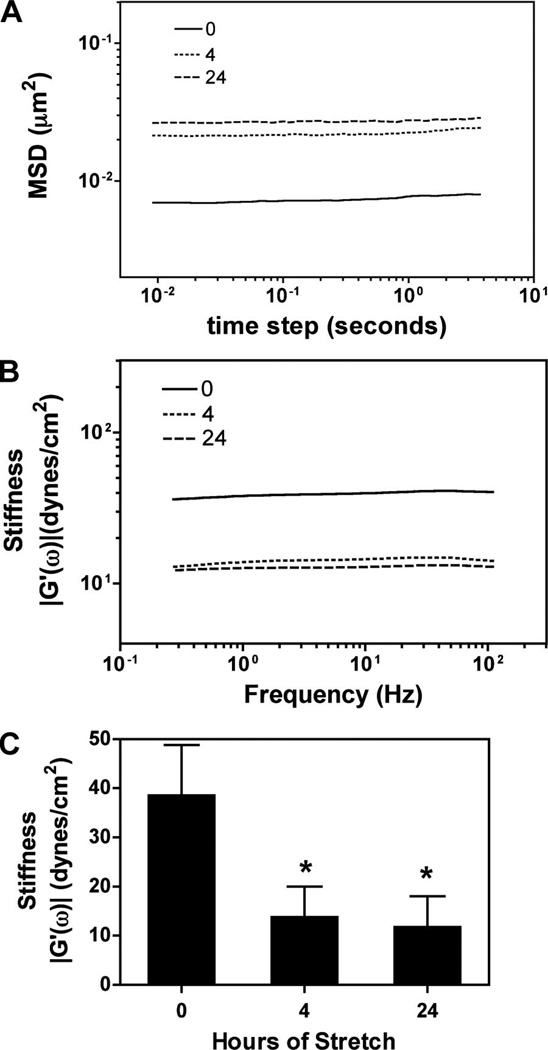
To investigate whether cyclic stretch affects cytoplasmic stiffness of A549 cells, cells were grown on pronectin coated silastic bioflex plate and subjected to 0, 4, or 24 h of eqiubiaxial stretch (10% Δ in BMSA, 0.5 Hz). Cells were then microinjected with 100 nm carboxylated beads and the Brownian random thermal fluctuation movements of the beads were traced for microheologic calculations within 20–30 min of cessation of stretch. Figure 2 shows representative trajectory of beads embedded in the cytoskeleton of unstretched and stretched cells. The trajectory of beads within the unstretched cells was much more restricted than the stretched ones suggesting that the cytoskeleton of static cells is denser and more rigid than that of stretched cells. From the trajectory of the beads an ensemble average MSD, a statistic used to describe Brownian motion, was calculated (Fig. 3A). The MSD of stretched cells for 4 h was 3.6-fold higher than that of unstretched cells, indicating that the cytoskeleton of stretched cells was much more fluid. There was no statistical difference in MSD and stiffness between cells stretched for 4 and 24 h. The elastic storage modulus or stiffness, G′, was calculated to be 14 and 12 dynes/cm2 after being subjected for cells stretched for 4 and 24 h, respectively, while the stiffness of unstretched cells was calculated to be 38 dynes/cm2 (Fig. 3B). These results demonstrate that cyclic stretch decreases cytoplasmic stiffness, which could lead to enhanced diffusion within the cytoplasm.
Figure 2.
Beads in stretched cells show greater movement and larger step sizes. Representative 75 step trajectory of a 100 nm carboxylated fluorescent beads microinjected into the cytoplasm of A549 cells after (A) 0 h, (B) 4 h, or (C) 24 h of cyclic stretch (20% area strain, 0.5 Hz). The trajectory of the beads is much more restricted in unstretched cells indicating higher stiffness. Scale bar = 50 nm.
Figure 3.
Cyclic stretch increases MSD of microinjected beads and decreased cytoplasmic stiffness. (A) Mean squared displacement (MSD) as a function of lag time of A549 cells subjected to 0 h (n = 22 beads, in seven cells), 4 h (n = 21 beads, in seven cells), or 24 h (n = 23 beads, in eight cells) of cyclic stretch as in Figure 1. MSD is more than threefold greater in stretched cells, suggesting reduced cytoplasmic resistance to diffusion (P < 0.001). (B) Stiffness or storage modulus of epithelial cells in (a) subjected to cyclic stretch have significantly less cytoplasmic stiffness (P < 0.001). (C) Storage modulus averaged over the three decades of frequency (± standard deviation).
Cytoplasmic Stiffness Returns to Pre-Stretch Levels Within 24 h
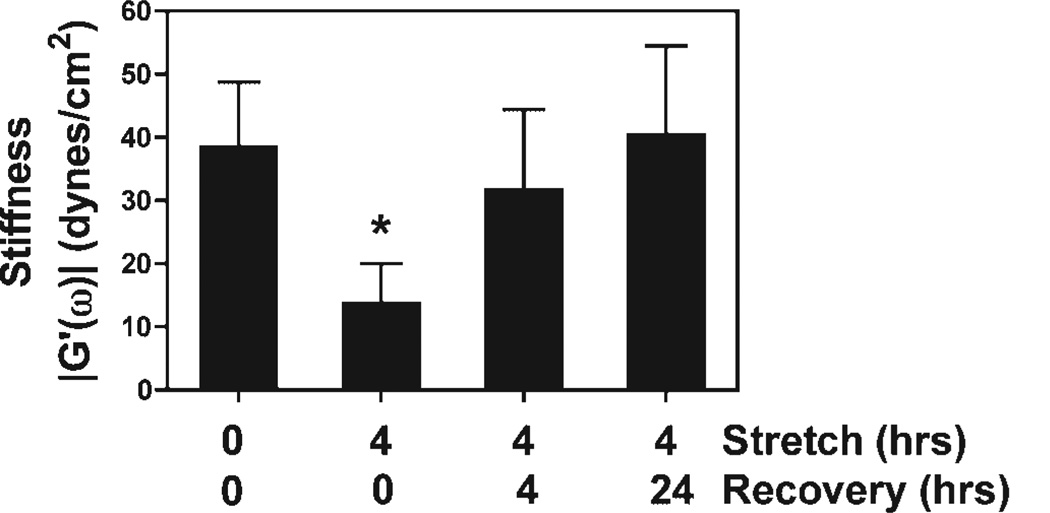
We next asked whether the decreased cytoplasmic stiffness seen immediately following the cessation of cyclic stretch remained low over time when stretched cells were grown statically for up to 24 h or recovered to levels seen prior to stretching. To accomplish this, A549 cells were stretched for 4 h and then injected within 20 min with 1–10 carboxylated fluorescent beads and returned to the incubator. After 4 or 24 h, the trajectory of the beads were traced and rheologic properties were calculated as described above. After 4 h of static growth following the 4 h of stretch, cytoplasmic stiffness increased from 14 to 32 dynes/cm2 (Fig. 4). By 24 h after the cessation of cyclic stretch, cytoplasmic stiffness returned to pre-stretch levels (41 dynes/cm2 vs. 38 dynes/cm2). These results indicate that the decrease in the cytoplasmic stiffness resulting from mild cyclic stretch is transient and reversible.
Figure 4.
Cytoplasmic stiffness recovers to pre-stretch levels within 24h of static growth after stretch. A549 cells were stretched for 4 h as in Figure 1. Stiffness was measured immediately after stretch (n = 21 beads, in seven cells) or after the cells were allowed to recover under static conditions for 4 h (n = 20 beads in eight cells) or 24 h (n = 21 beads in eight cells). Stiffness recovered to pre-stretch levels within 23 h of static growth (± standard deviation; P < 0.05).
Disrupting the Microtubules and Actin Networks Decreases Cytoplasmic Stiffness
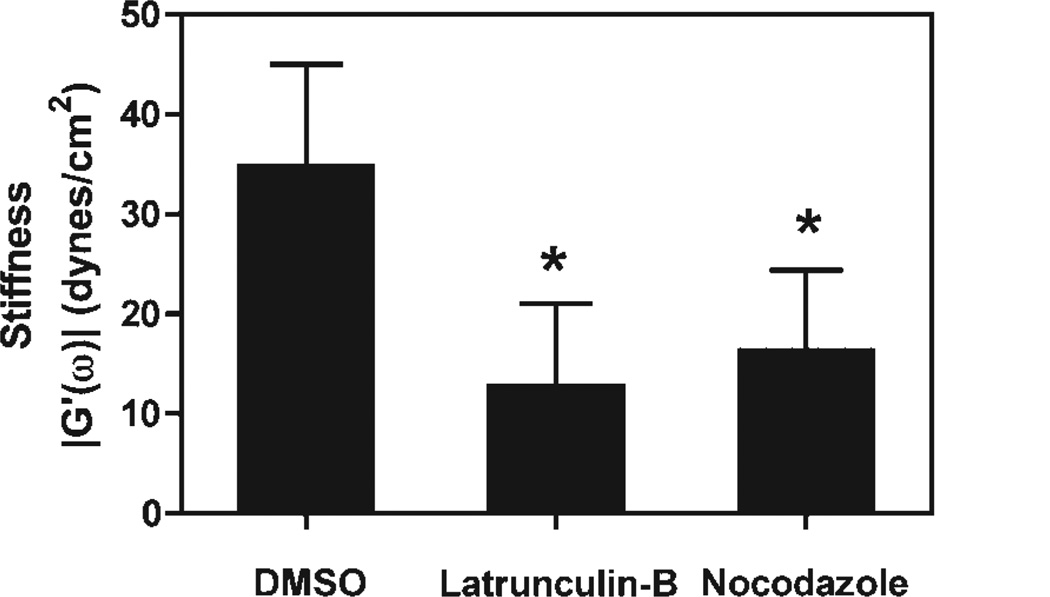
We and others have shown that cyclic stretch results in reorganization of the cytoskeleton (Geiger et al., 2006). Immunofluorescence microscopy experiments on stretched cells have shown that the fine actin filaments are rendered much shorter and concentrated within the periphery of the cell and the microtubules network largely disappears in favor of acetylated microtubules (Geiger et al., 2006). These observations are similar to the effects of pharmacologic agents that depolymerize actin filaments and microtubules which led us to our previously stated hypothesis that stretch mimics of the effects of these drugs in alveolar epithelial cells. To investigate if these drugs alter cytoplasmic stiffness similar to cyclic stretch, the actin filaments and microtubule networks were disrupted by Latrunculin B and nocodazole, respectively (Fig. 5). When the actin network was disrupted in A549 cells, the cytoplasmic stiffness decreased more than twofold compared to cells treaded with dimethyl sulfoxide (DMSO; vehicle alone). In the case of cells treated with nocodazole to disrupt the microtubules network, the cytoplasmic stiffness also decreased by twofold. These results are consistent with the observation that both the actin network and the microtubule networks are responsible for cell stiffness (Van Citters et al., 2006; Fig. 5).
Figure 5.
Disruption of actin filaments and/or microtubules decreases cytoplasmic stiffness. A549 cells were treated for 15 min with 0.625 µM Latrunculin B to disrupt the actin network, for 30 min with 20 µM nocadazole to disrupt the microtubule network, or with vehicle only (DMSO) before microinjection of beads to measure stiffness (n = 20 beads in seven cells for Latrunculin B, n = 22 beads in eight cells for nocadazole, and n = 20 beads in seven cells for DMSO). Disruption of either network decreased cytoplasmic stiffness by more than twofold compared to treatment with DMSO alone (± standard deviation; P < 0.001).
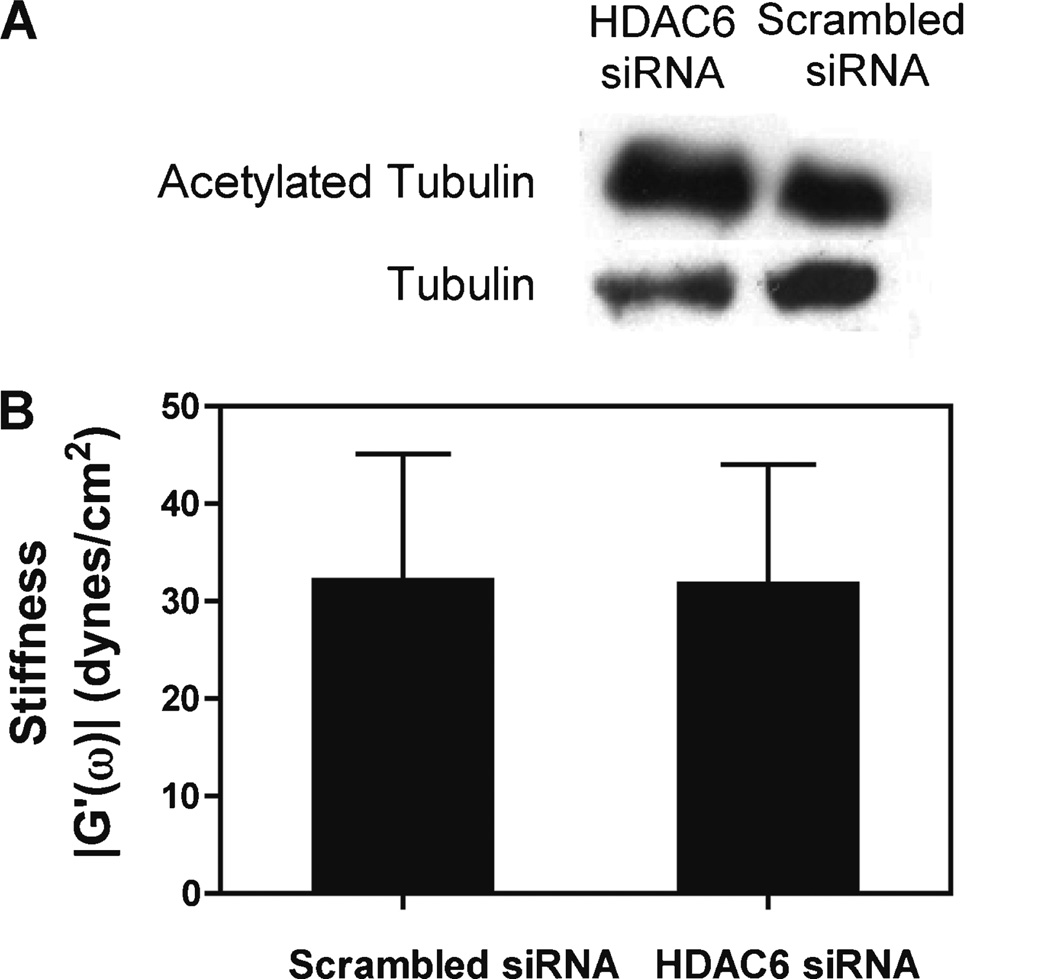
Microtubule Acetylation Does Not Affect Cytoplasmic Stiffness
We have shown that when cyclic stretch is applied to various types of cells, levels of acetylated microtubules in the cytoplasm increase while unmodified microtubules are largely disassembled (Geiger et al., 2006). Since acetylation is a hallmark of stabilized microtubules that are resistant to depolymerization by a variety of stimuli, we wanted to test whether the levels of microtubule acetylation could affect cytoplasmic stiffness, independent of cyclic stretch. To investigate this, HDAC6, the enzyme responsible for microtubule deacetylation, was knocked down via RNA interference, resulting in increased microtubule acetylation by 2.5-fold, and the cytoplasmic stiffness was assayed (Fig. 6). Results of this experiment show that cytoplasmic stiffness of cells with maximally acetylated microtubules is the same as that of wild type cells, even though the two cell lines have greatly different levels of acetylated tubulin. These results indicate that microtubule acetylation and levels of stabilized microtubules do not affect cytoplasmic stiffness.
Figure 6.
Increased levels of stabilized, acetylated microtubules does not affect cytoplasmic stiffness. (A) Western blot of siRNA treated cells indicating a 2.5-fold increase in acetylated microtubules levels for cells treated with siRNAs against HDAC6. (B) A549 cells were transfected with siRNA against HDAC6 or a scrambled, control siRNA and 48 h later, when levels of acetylated microtubules in the cytoplasm were maximal, cells were microinjected with fluorescent beads for PTM. In HDAC6 knockdown cells (± standard deviation; n = 22 beads in seven cells), cytoplasmic stiffness was identical to that of cells treated with a scrambled siRNA (± standard deviation; n = 21 beads in seven cells), suggesting that the levels of stabilized microtubules do not alter cytoplasmic stiffness.
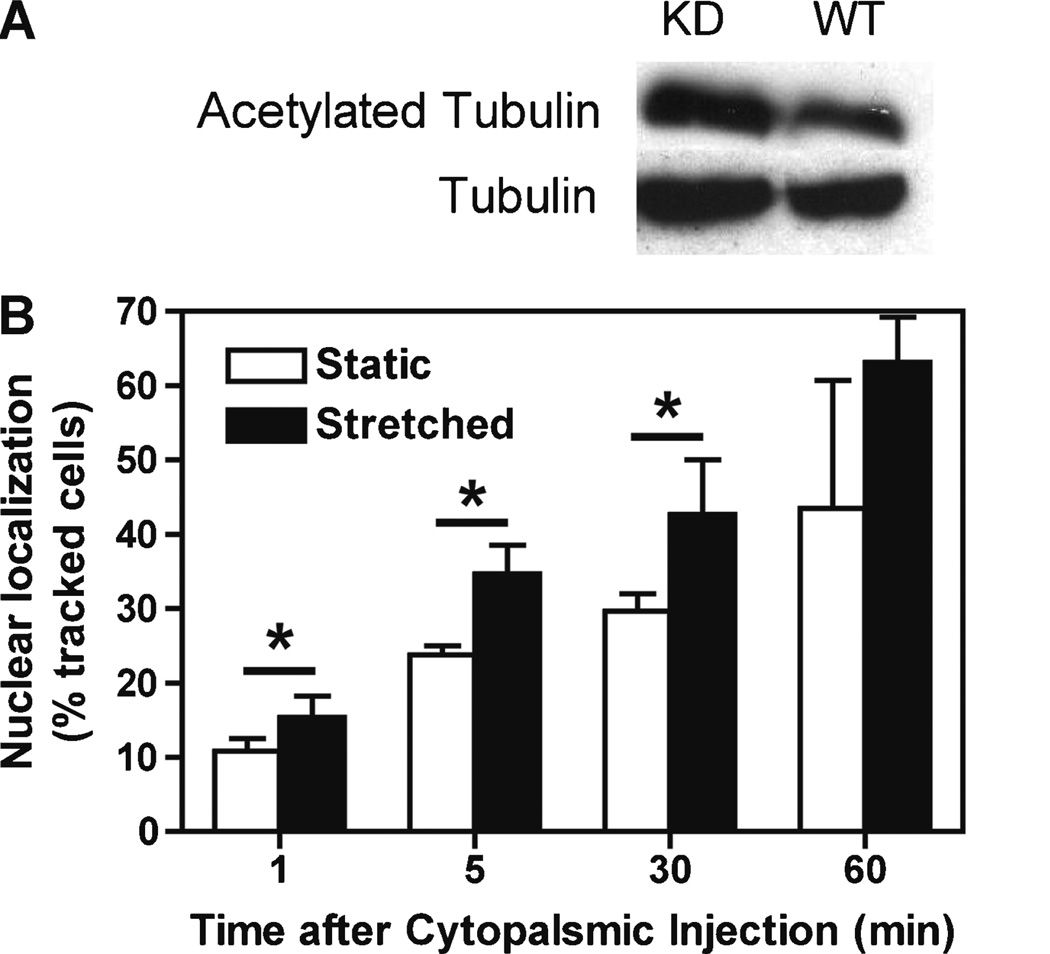
Cyclic Stretch Increases Cytoplasmic Transport of Plasmid DNA Independent of the Levels of Cytoplasmic Acetylated Microtubules
We next investigated whether cyclic stretch enhances the cytoplasmic trafficking of pDNA independent of the levels of acetylated microtubules. Cy3-labeled plasmids were microinjected in the cytoplasm of stretched or static A549 cells that are stably transfected with plasmids expressing short hairpin RNA against HDAC6 to reduce its expression, resulting in greatly increased levels of acetylated tubulin, even in the absence of cyclic stretch (Fig. 7A). Moreover, tubulin appears maximally acetylated since the levels of acetylated tubulin do not increase with stretch (Geiger et al., 2009). The subcellular location of the plasmids was then determined at various times after microinjection to determine rates of directed DNA movement. Our results indicate that pDNA shows faster cytoplasmic transport at all time points in stretched cells compared to that of statically grown cells even when levels of acetylated microtubules do not further increase (Fig. 7B). This suggests that cyclic stretch enhances pDNA cytoplasmic transport in addition to and independent from the levels of acetylated microtubules.
Figure 7.
Cyclic stretch enhances the cytoplasmic movement of plasmids independent of microtubule acetylation. Stably transfected A549 cells expressing short hairpin RNA against HDAC6 were grown statically or stretched for 4 h. (A) Cell lysates were then prepared and evaluated for levels of acetylated tubulin for (B) the cells were cytoplasmically microinejected with Cy3-labeled plasmids (over 100 static or stretched cells were injected and the experiment was repeated twice). Mean levels of nuclear and nuclear rim localization (± standard deviation) are shown. Paired t-tests show that stretched cells show statistically increased directed movement than do static cells (P < 0.01).
Discussion
While gene trafficking of both viral and non-viral plasmids has been studied in depth, the effect of external mechanical stimuli has not been considered in detail. In recent years, several investigators have reported an increase in gene expression when cyclic stretch is applied to variety of cells (Kaneko et al., 2009; Korff et al., 2007; Taylor et al., 2003). In human alveolar epithelial cells, this increase in gene expression is in part due to the reorganization of the cytoskeleton (Geiger et al., 2006). Our results in this report show that cyclic stretch decreases the stiffness of the cytoskeleton rendering it less resistive to diffusion of large molecules the size of pDNA. By microinjecting fluorescent 100 nm beads into the cytoplasm of cells grown statically or with mild levels of cyclic stretch (10% change in the BMSA at 0.5 Hz) we find that the cytoplasmic stiffness decreases by a factor of ~ 2 following 4 or 24 h of stretch. Stiffness recovers slowly over the next 24 h, but is indistinguishable from untreated cells by 24 h. Furthermore, in this report we have shown that cyclic stretch affects cytoskeletal stiffness in a manner similar to cytoskeleton disrupting drugs, which have been shown to increase pDNA diffusion when cells where treated with Cytochalasin D (Dauty and Verkman, 2005).
While non-viral gene therapy presents a safe and effective treatment for various diseases, it is very inefficient. For pDNA to reach the nucleus, it must travel across the plasma membrane, through the cytoplasm and gain entry to the nucleus. While several methods have been devised to get the pDNA across the plasma membrane, cytoskeletal transport is still not very well understood. It has been shown that once the pDNA is in the cytoplasm, it simply does not diffuse through the dense cytoskeleton in static cells and that the actin network is the major obstacle for pDNA diffusion (Dauty and Verkman, 2005; Lukacs et al., 2000). This indicates that the low expression levels must be blamed on an active process. Much like viruses, it has been shown that pDNA utilizes the cytoskeleton to travel through the cytoplasm (Zhou et al., 2004). Naked DNA binds to microtubules through adaptor proteins and is transported to the nucleus by an energy consuming process (Vaughan and Dean, 2006). All this taken together indicates that pDNA needs the cytoskeleton and is hindered by it.
In addition to reorganizing the cytoskeleton and decreasing the cytoplasmic stiffness, cyclic stretch has been also shown to inhibit the activity of HDAC6, an enzyme that deacetylates tubulin rather than histones (Vaughan et al., 2008). When the activity of HDAC6 is inhibited, the levels of acetylated microtubules increase. Acetylated microtubules have been shown to bind more motor proteins, which result in greater movement of DNA. It could be argued that the decrease in cytoplasmic stiffness observed here in this report did not arise for cyclic stretch, but due to the increase in the levels of acetylated microtubules. We have shown in this report that modulating the levels of acetylated microtubules in the cytoplasm has no effect on cytoplasmic stiffness. Further, even when tubulin is maximally acetylated by genetic means independent of cyclic stretch, the application of cyclic stretch still increases the cytoplasmic movement of the DNA. In cells where HDAC6 is knocked down, higher levels of gene expression were also observed in cells subjected to stretch but not static ones (Vaughan et al., 2008), resulting from the increased movement that we have reported here. Taken together, our results here suggest that cyclic stretch improves gene transfer by two self-regulating methods: increasing acetylated microtubules and decreasing cytoplasmic resistance to pDNA diffusion.
Several methods have been introduced to measure cell rheologic properties including atomic force microscopy, magnetic twisting cytometry, and micropipette aspiration. While each of those methods is superior for particular applications, they all share two major drawbacks. All these methods probe for cortical rheologic properties, not just the cytoplasm. For the question we are asking in this report, we needed a method that probed for the changes in the cytoskeleton rheology when cyclic stretch is applied, not the cell membrane or the nucleus. Conclusions from cortical rheology methods about the changes in cytoskeleton usually require significant assumptions about cellular structures (Wirtz, 2009). On the other hand, PTM measurements are specified to the location of the beads measuring only the cytoplasmic contribution to cell rheology. The second drawback for the methods mentioned above is that they are active measurements, which means that an external stimulus must be applied and the cell’s response to that input is measured. This is a major setback for those methods especially when studying cytoplasmic rheology due to mechanical stimuli such as cyclic stretch because it would be difficult to determine if the measurement reflects the cell’s response to the mechanical stimulus under investigation or due to the input energy from the instrument. In contrast, PTM is a passive measurement with thermal fluctuations being the driving energy for the bead’s Brownian motion. PTM also has some limitations that hinder its ability to measure cytoplasmic rheology. Firstly, the beads are microinjected into the cytoplasm, which may induce cell injury. Microinjection, however, is a well-established technique and cells have been shown to recover and function normally following microinjection. Another disadvantage to PTM is that the beads are not completely resistant to protein absorption, nevertheless it has been shown that carboxylated beads used in our experiments are inert (Ehrenberg and McGrath, 2005).
In recent years, the rheologic properties of cells under cyclic stretch and shear stress have been studied in verity of cells (Berrios et al., 2001; Dangaria and Butler, 2007; Lehoux et al., 2006; Owatverot et al., 2005; Sivaramakrishnan et al., 2008; Trepat et al., 2004). To our knowledge, however, we are the first to investigate the cytoplasmic rheology in human alveolar epithelial cells. In rat type II alveolar epithelial cells, and colleagues observed a decrease in apparent stiffness after being subjected to 10 min of cyclic deformation at 0.3 Hz by ~ 10% from 100 dynes/cm2 (Berrios et al., 2001). Trapet and colleagues have shown that when cyclic stretch is applied to A549 cells, stiffness increased using optical magnetic twisting cytometry from 7,000 to 12,000 dynes/cm2 (Trepat et al., 2004). While these results contradict our findings in this report, there are a few differences in the experimental setup and instrumentation. Most importantly, magnetic twisting cytometry experiments does not probe for cytoplasmic rheology, instead it measures cortical rheology rendering their conclusion that cytoskeleton reorganization is responsible for the increase in stiffness unfounded. Interestingly, it was found that alveolar epithelial cells recruit more lipids to the plasma membrane when deformed increasing its resistance to deformation to prevent membrane failure (Vlahakis et al., 2002). Since optical magnetic twisting cytometry measures whole cell stiffness and PTM measures cytoplasmic stiffness, the difference in measured stiffness can be blamed on nucleus stiffness, which has been shown to be three to four times stiffer than the cytoplasm as well as the cell membrane stiffness (Guilak et al., 2000).
Acknowledgments
The authors would like to thank Drs. R. Chris Geiger, James McGrath, Salman Rogers for technical and scientific advice.
References
- Berrios JC, Schroeder MA, Hubmayr RD. Mechanical properties of alveolar epithelial cells in culture. J Appl Physiol. 2001;91(1):65–73. doi: 10.1152/jappl.2001.91.1.65. [DOI] [PubMed] [Google Scholar]
- Cheezum MK, Walker WF, Guilford WH. Quantitative comparison of algorithms for tracking single fluorescent particles. Biophys J. 2001;81(4):2378–2388. doi: 10.1016/S0006-3495(01)75884-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangaria JH, Butler PJ. Macrorheology and adaptive microrheology of endothelial cells subjected to fluid shear stress. Am J Physiol Cell Physiol. 2007;293(5):C1568–C1575. doi: 10.1152/ajpcell.00193.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauty E, Verkman AS. Actin cytoskeleton as the principal determinant of size-dependent DNA mobility in cytoplasm: A new barrier for non-viral gene delivery. J Biol Chem. 2005;280(9):7823–7828. doi: 10.1074/jbc.M412374200. [DOI] [PubMed] [Google Scholar]
- Dowty ME, Williams P, Zhang G, Hagstrom JE, Wolff JA. Plasmid DNA entry into postmitotic nuclei of primary rat myotubes. Proc Natl Acad Sci USA. 1995;92(10):4572–4576. doi: 10.1073/pnas.92.10.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg M, McGrath JL. Binding between particles and proteins in extracts: Implications for microrheology and toxicity. Acta Biomater. 2005;1(3):305–315. doi: 10.1016/j.actbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Ehrenberg MS, Friedman AE, Finkelstein JN, Oberdorster G, McGrath JL. The influence of protein adsorption on nanoparticle association with cultured endothelial cells. Biomaterials. 2009;30(4):603–610. doi: 10.1016/j.biomaterials.2008.09.050. [DOI] [PubMed] [Google Scholar]
- Gasiorowski JZ, Dean DA. Postmitotic nuclear retention of episomal plasmids is altered by DNA labeling and detection methods. Mol Ther. 2005;12(3):460–467. doi: 10.1016/j.ymthe.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger RC, Taylor W, Glucksberg MR, Dean DA. Cyclic stretch-induced reorganization of the cytoskeleton and its role in enhanced gene transfer. Gene Ther. 2006;13(8):725–731. doi: 10.1038/sj.gt.3302693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger RC, Kaufman CD, Lam AP, Budinger GR, Dean DA. Tubulin acetylation and histone deacetylase 6 activity in the lung under cyclic load. Am J Respir Cell Mol Biol. 2009;40(1):76–82. doi: 10.1165/rcmb.2007-0307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Tedrow JR, Burgkart R. Viscoelastic properties of the cell nucleus. Biochem Biophys Res Commun. 2000;269(3):781–786. doi: 10.1006/bbrc.2000.2360. [DOI] [PubMed] [Google Scholar]
- Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci USA. 2003;100(8):4389–4394. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko D, Sasazaki Y, Kikuchi T, Ono T, Nemoto K, Matsumoto H, Toyama Y. Temporal effects of cyclic stretching on distribution and gene expression of integrin and cytoskeleton by ligament fibroblasts in vitro. Connect Tissue Res. 2009;50(4):263–269. doi: 10.1080/03008200902846270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korff T, Aufgebauer K, Hecker M. Cyclic stretch controls the expression of CD40 in endothelial cells by changing their transforming growth factor-beta1 response. Circulation. 2007;116(20):2288–2297. doi: 10.1161/CIRCULATIONAHA.107.730309. [DOI] [PubMed] [Google Scholar]
- Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med. 2006;259(4):381–392. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- Lukacs GL, Haggie P, Seksek O, Lechardeur D, Freedman N, Verkman AS. Size-dependent DNA mobility in cytoplasm and nucleus. J Biol Chem. 2000;275(3):1625–1629. doi: 10.1074/jbc.275.3.1625. [DOI] [PubMed] [Google Scholar]
- Mason TG. Estimating the viscoelastic moduli of complex fluids using the generalized Stokes–Einstein equation. Rheol Acta. 2000;39:371–378. [Google Scholar]
- Owatverot TB, Oswald SJ, Chen Y, Wille JJ, Yin FC. Effect of combined cyclic stretch and fluid shear stress on endothelial cell morphological responses. J Biomech Eng. 2005;127(3):374–382. doi: 10.1115/1.1894180. [DOI] [PubMed] [Google Scholar]
- Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16(21):2166–2172. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Rogers SS, Waigh TA, Zhao X, Lu JR. Precise particle tracking against a complicated background: Polynomial fitting with Gaussian weight. Phys Biol. 2007;4(3):220–227. doi: 10.1088/1478-3975/4/3/008. [DOI] [PubMed] [Google Scholar]
- Shimizu N, Kamezaki F, Shigematsu S. Tracking of microinjected DNA in live cells reveals the intracellular behavior and elimination of extrachromosomal genetic material. Nucleic Acids Res. 2005;33(19):6296–6307. doi: 10.1093/nar/gki946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan S, DeGiulio JV, Lorand L, Goldman RD, Ridge KM. Micromechanical properties of keratin intermediate filament networks. Proc Natl Acad Sci USA. 2008;105(3):889–894. doi: 10.1073/pnas.0710728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J, Wirtz D, Hanes J. Efficient active transport of gene nanocarriers to the cell nucleus. Proc Natl Acad Sci USA. 2003;100(7):3878–3882. doi: 10.1073/pnas.0636277100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W, Gokay KE, Capaccio C, Davis E, Glucksberg M, Dean DA. The effects of cyclic stretch on gene transfer in alveolar epithelial cells. Mol Ther. 2003;7(4):542–549. doi: 10.1016/s1525-0016(03)00041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepat X, Grabulosa M, Puig F, Maksym GN, Navajas D, Farre R. Viscoelasticity of human alveolar epithelial cells subjected to stretch. Am J Physiol Lung Cell Mol Physiol. 2004;287(5):L1025–L1034. doi: 10.1152/ajplung.00077.2004. [DOI] [PubMed] [Google Scholar]
- Van Citters KM, Hoffman BD, Massiera G, Crocker JC. The role of Factin and myosin in epithelial cell rheology. Biophys J. 2006;91(10):3946–3956. doi: 10.1529/biophysj.106.091264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan EE, Dean DA. Intracellular trafficking of plasmids during transfection is mediated by microtubules. Mol Ther. 2006;13(2):422–428. doi: 10.1016/j.ymthe.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan EE, Geiger RC, Miller AM, Loh-Marley PL, Suzuki T, Miyata N, Dean DA. Microtubule acetylation through HDAC6 inhibition results in increased transfection efficiency. Mol Ther. 2008;16(11):1841–1847. doi: 10.1038/mt.2008.190. [DOI] [PubMed] [Google Scholar]
- Vlahakis NE, Schroeder MA, Pagano RE, Hubmayr RD. Role of deformation-induced lipid trafficking in the prevention of plasma membrane stress failure. Am J Respir Crit Care Med. 2002;166(9):1282–1289. doi: 10.1164/rccm.200203-207OC. [DOI] [PubMed] [Google Scholar]
- Wirtz D. Particle-tracking microrheology of living cells: Principles and applications. Annu Rev Biophys. 2009;38:301–326. doi: 10.1146/annurev.biophys.050708.133724. [DOI] [PubMed] [Google Scholar]
- Zhou R, Geiger RC, Dean DA. Intracellular trafficking of nucleic acids. Expert Opin Drug Deliv. 2004;1(1):127–140. doi: 10.1517/17425247.1.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]