Summary
Background
Mechanical forces regulate cell behavior and function during development, differentiation, and tissue morphogenesis. In the vascular system, forces produced by blood flow are critical determinants not only of morphogenesis and function, but also pathological states such as atherosclerosis. Endothelial cells (ECs) have numerous mechanotransducers, including platelet endothelial cell adhesion molecule-1 (PECAM-1) at cell-cell junctions and integrins at cell-matrix adhesions. However, the processes by which forces are transduced to biochemical signals and subsequently translated into downstream effects are poorly understood.
Results
Here, we examine mechanochemical signaling in response to direct force application on PECAM-1. We demonstrate that localized tensional forces on PECAM-1 result in, surprisingly, global signaling responses. Specifically, force-dependent activation of phosphatidylinositol 3-kinase (PI3K) downstream of PECAM-1 promotes cell-wide activation of integrins and the small GTPase RhoA. These signaling events facilitate changes in cytoskeletal architecture, including growth of focal adhesions and adaptive cytoskeletal stiffening.
Conclusions
Taken together, our work provides the first evidence of a global signaling event in response to a localized mechanical stress. In addition, these data provide a possible mechanism for the differential stiffness of vessels exposed to distinct hemodynamic force patterns in vivo.
Introduction
Mechanical forces are involved in nearly all aspects of biology [1]. Within the vascular system, hemodynamic forces produced by blood flow play a critical role in EC biology and maintenance of the vascular hemostasis. Cells respond to mechanical stresses on mechanosensitive proteins, such as integrins, by employing an adaptive cellular stiffening response in an effort to resist increased tensile strain [2-4]. Adaptive cellular stiffening requires the coordination of mechanically activated signaling cascades, including the small GTPase RhoA and its effectors, which mediate local changes in focal adhesion growth and actomyosin contractility [3, 5, 6].
Within the vascular system, ECs lining the lumen of blood vessels are positioned to experience constant force as a result of the shear stress of blood flow. Hemodynamic forces influence EC biology and play an integral role in determining the health and integrity of the vessel. To this regard, ECs are decorated with numerous mechanosensors that function to convert mechanical forces into defined biochemical signaling cascades. We have previously identified PECAM-1 as a key endothelial mechanosensor that influences vessel physiology and pathology [7-9], yet insights into cellular responses directly linked to PECAM-1-dependent force transduction are lacking.
Here, we use a magnetic tweezers system and permanent ceramic magnet to investigate cellular responses to mechanical tension on PECAM-1. We reveal a mechanotransduction pathway that involves integration of signaling between two mechanosensors at distinct cellular sites. Specifically, force transduction via PECAM-1 promotes integrin-dependent RhoA activation, leading to focal adhesion growth and adaptive cellular stiffening. Furthermore, we provide evidence that local mechanical stimulation of PECAM-1 can initiate a global cellular response, providing new insights into the spatial regulation of mechanochemical signaling cascades.
Results
Tensional forces on PECAM-1 result in adaptive cellular stiffening and mechanosignaling
In order to investigate mechanoresponses downstream of PECAM-1, we applied tensional forces, using magnetic tweezers [10], to paramagnetic beads bound to endogenous PECAM-1 on ECs adherent on fibronectin (FN) (Fig. S1A). Brief force application (~100pN) revealed a typical viscoelastic-creep response similar to those seen with bead-integrin linkages [3] (Figure S1B). Application of successive pulsatile forces on PECAM-1 resulted in a significant decrease in pulse-to-pulse bead displacement in latter pulses, indicative of force-dependent adaptive stiffening (Fig. 1A). Furthermore, average bead displacement decreased approximately 40% by the end of the 2-minute time course, indicating a 40% increase in cell stiffness with a calculated time constant of 39.93 seconds (Fig. S1C). It is important to note that bead displacement decreased without significant displacement of cellular organelles, such as the nucleus (Fig. S1D), and analysis of bead recovery following each pulse of force revealed a 90-95% recovery from each pulse of force (Fig. S1E), suggesting that baseline drift in bead recovery is negligible in the adaptive response. Importantly, adaptive stiffening was specific to anti-PECAM-1-coated beads, as force application to poly-lysine-coated beads did not initiate a mechanical response (Supplementary Fig. S1F). Previous work demonstrated activation of PI3K downstream of PECAM-1[7]. In order to examine PI3K activation in response to localized tensional forces on PECAM-1, magnetic beads bound to ECs expressing a GFP-PH fusion protein (which serves as a sensor for PI3-lipids) were subjected to force using a permanent ceramic magnet. Brief force application was sufficient to induce PI3K activation around anti-PECAM-1-coated beads (Fig. 1B). Recruitment around the bead was specific to PECAM-1 and not due to perturbation of the membrane, as ECs transfected with GFP alone (Fig. S1G) or ECs incubated with poly-lysine-coated beads (Fig. S1H) did not display recruitment in response to force. In order to examine if PI3K activation is required for adaptive cytoskeletal stiffening, pharmacological inhibitors were used to block PI3K activation. Pretreatment of ECs with PI3K inhibitors, LY294002 (Fig. 1C) and wortmannin (data not shown), or inhibition of actin polymerization with Cytochalasin D (Fig. 1C), abolished adaptive stiffening, suggesting a requirement for both biochemical signaling and cytoskeletal remodeling. It is important to note that, in addition to an impaired response to mechanical force on PECAM-1, cells pretreated with Cytochalasin D also exhibited a decrease in basal cell stiffness, indicated by a significant increase in absolute bead displacement during the first pulse of force (Fig. S1I).
Figure 1. Tensional forces on PECAM-1 result in adaptive cellular stiffening and PI3-kinase activation.
(A) Schematic of experimental design. Two-second pulses of force (~100 pN) separated by 10-second intervals over a 2-minute time course. Representative example of bead displacement in response to the pulsatile force regimen. Stiffening is indicated by decreased displacement during latter pulses. (B) ECs expressing GFP-PH were incubated with anti-PECAM-1-coated magnetic beads and subjected to force with a permanent ceramic magnet for the indicated times. Cells were fixed and scored for GFP-PH recruitment around the bead (box). Location of the bead is highlighted by the yellow circle (n > 50 cells/condition from 3 independent experiments; scale bar = 10μm). (C) Average relative anti-PECAM-1 bead displacements induced by the pulsatile force regimen. In some experiments, cells were pretreated with LY294002 (30μM for 20 min) or Cytochalasin D (10μM for 30 min) prior to incubation with magnetic beads. Average displacements were calculated relative to the first pulse of force. (n >15 beads/condition from 3 independent experiments). Error bars represent s.e.m., *p<0.05. (See also Fig. S1)
PECAM-1-mediated adaptive stiffening is an integrin-dependent process
Growing evidence suggests that the integrin-extracellular matrix (ECM) adhesions function as sites of mechanotransduction, where upon application of external forces on integrins, an intracellular response is activated that leads to local focal adhesion assembly and associated cytoskeletal strengthening [2, 4, 11]. We therefore examined if integrin ligation with the underlying ECM plays a role in force transmission via PECAM-1. We used a blocking (16G3) or nonblocking (11E5) antibody to inhibit new integrin-FN connections without disrupting existing adhesions [12]. Inhibition of force-induced integrin engagement with the FN blocking antibody attenuated PECAM-1-mediated adaptive stiffening, whereas the nonblocking antibody had no effect (Fig. 2A). These data suggest that new integrin-FN connections are required for adaptive stiffening, and indicate that the mechanical response requires input from more than one mechanosensor. Next, we tested the possibility that new integrin-FN connections are required for force-induced PI3K activation. To this regard, inhibition of new integrin-FN connections had no effect on force-dependent PI3K activation (Fig. 2B), as ECs subjected to force showed similar levels of activation in the presence of the blocking and nonblocking antibodies. These data suggest that PI3K activation is upstream of integrin ligation with the underlying ECM.
Figure 2. PECAM-1-mediated adaptive cellular stiffening, but not PI3K activation, is integrin-dependent.
(A) ECs were incubated with 20μg/ml of FN blocking (16G3) or nonblocking antibody (11E5) for 20 min prior to force application. Average displacements were calculated relative to the first pulse of force to anti-PECAM-1-coated beads (n > 15 beads/condition from 3 independent experiments) (B) ECs expressing GFP-PH were incubated with 16G3 or 11E5 (20μg/ml, 20min) antibodies prior to being subjected to force with a permanent ceramic magnet. Cells were fixed and scored for GFP-PH recruitment around the bead (n > 50 cells/condition from 3 independent experiments; scale bar = 10μm). Error bars represent s.e.m., *p<0.05.
Tensional forces on PECAM-1 activate the RhoA pathway via GEF-H1 and LARG
Local activation of the small GTPase RhoA has been implicated in adaptive cellular stiffening in response to mechanical stresses on integrins [3, 11]. To investigate the role of the RhoA pathway in adaptive stiffening downstream of PECAM-1, ECs were pretreated with C3 transferase or Y27632, Rho and ROCK inhibitors, respectively, prior to force application. Inhibition of either Rho or ROCK attenuated adaptation to force (Fig. 3A), suggesting a role for the RhoA pathway in adaptive stiffening. We therefore hypothesized that tensional forces on PECAM-1 leads to RhoA activation, which is required for cytoskeletal adaptation to force. To test this hypothesis, we performed Rho pulldown assays to detect levels of active RhoA. ECs were incubated with anti-PECAM-1-and stimulated with continuous force (~10pN) using a permanent magnet for biochemical analyses. Indeed, ECs subjected to tensional force on PECAM-1 displayed robust and sustained RhoA activation in response to force, as levels of GTP-loaded RhoA increased at 5 minutes of force application and remained elevated at 30 minutes of sustained force (Fig. 3B). Force-induced RhoA activation was specific to PECAM-1, as poly-lysine-coated beads did not increase levels of active RhoA in response to force (Fig. S2). Interestingly, PECAM-1-mediated RhoA activation was integrin-dependent, as inhibition of new integrin-FN connections quenched force-induced RhoA activity (Fig. 3C).
Figure 3. Tensional forces on PECAM-1 activate the RhoA pathway.
(A) Adherent ECs were incubated with anti-PECAM-1-coated magnetic beads and subjected to pulsatile tensional forces. For some conditions, cells were pretreated with C3 (2.0μg/ml, 2hrs) or Y27632 (5μM, 10min) prior to force application. Average displacements were calculated relative to the first pulse of force. (n>15 cells/condition from 3 independent experiments). Error bars represent s.e.m., *p<0.05. (B-C) ECs were incubated with anti-PECAM-1-coated beads and subjected to force with a permanent ceramic magnet for the indicted times (B) Active RhoA (RhoA-GTP) was isolated with GST-RBD and analyzed by western blot (n=5). (C) ECs were incubated with 20μg/ml of FN blocking (16G3) or nonblocking antibody (11E5) for 20 min prior to force application. Active RhoA (RhoA-GTP) was isolated with GST-RBD and analyzed by western blot (n=3). Blots (B, C) are representative of at least 3 independent experiments. (See also Fig. S2)
We next sought to identify the guanine nucleotide exchange factors (GEFs) that mediate force-induced RhoA activation by performing affinity pulldowns with a nucleotide-free RhoA mutant (G17A) [13]. Analysis revealed a force-dependent increase in GEF-H1 and LARG activity, while the activity of other GEFs, such as Dbl, Vav, and Net1 were unaffected (Fig. 4A). Interestingly, these GEFs also mediate RhoA activation in response to tensional forces on FN-binding integrins [11]. It was also reported that mechanical activation of GEF-H1 relies on activation of a FAK/ERK pathway. Previous studies have demonstrated force-dependent activation of ERK downstream of PECAM-1 [14-16]. In agreement with previous reports, in response to tensional forces on PECAM-1, we observed a force-dependent increase ERK activation, as well as FAK phosphorylation (Fig. 4B). Furthermore, inhibition of FAK or ERK activity with pharmacological inhibitors (FAK 14 or U0126, respectively), attenuated force-induced GEF-H1 activation, while LARG activity was unaffected (Fig. 4C). These data suggest a common pathway employed for GEF activation in response to tension on diverse adhesion molecules, and, therefore, may represent a conserved mechanosensitive pathway. In order to confirm a role for GEF-H1 and LARG in PECAM-1-mediated RhoA activation, siRNAs were used to knockdown these GEFs in ECs. Depletion of GEF-H1 and LARG with specific siRNAs attenuated RhoA activation and adaptive cellular stiffening in response to tensional forces on PECAM-1 (Fig. 4D,E), further supporting a role for these GEFs in PECAM-1-dependent stiffening.
Figure 4. Tensional forces on PECAM-1 elicit RhoA activation and adaptive cellular stiffening via GEF-H1 and LARG.
(A-D) Tension was applied to anti-PECAM-1-coated beads using a permanent ceramic magnet for the indicated times, and (A) cells were lysed and active GEFs were precipitated with GST-(G17A)RhoA and analyzed by western blot (n=3), (B) Cells were lysed, subjected to SDS-PAGE, and immunoblotted with indicated antibodies. Blots are indicative of 3 independent experiments. (C) Cells were lysed and active GEFs were precipitated with GST-(G17A)RhoA and analyzed by Western blot (n=3). For some conditions, cells were pretreated with FAK inhibitor 14 (5μM, 30min) or U0126 (5μM, 30min) to inhibit FAK and ERK, respectively (n=3). (D,E) siRNA-transfected ECs were incubated with anti-PECAM-1-coated beads and subjected to force for the indicated times. Active RhoA was isolated with GST-RBD and analyzed by western blot (n=3). All blots (A-D) are indicative of at least 3 independent experiments. (E) siRNA-transfected ECs on FN were incubated with anti-PECAM-1-coated beads and subjected to pulsatile forces. Average displacements were calculated relative to the first pulse of force (n >15 beads/condition from 3 independent experiments, *p<0.05).
Localized tensional forces on PECAM-1 a global mechanotransduction response
Our data suggest that force-induced RhoA activation downstream of PECAM-1 is integrin-dependent (Fig. 3C). In order to assay integrin activation in response to tensional force on PECAM-1, ECs were immunostained for ligated β1 integrin (Fig. 5A). Unexpectedly, we observed a global increase in β1 integrin ligation, rather than a local response confined to the region proximal to the bead under tension. This result was surprising, as previous studies applying tensional forces on other adhesion receptors demonstrated a local cellular response restricted to the site of mechanical stress [4]. Cells were assayed for the ratio of “global” versus “local” integrin activation, where “local” was defined as the region with a 5 micron radius from the site of bead attachment and the rest of the cell was deemed “global.” As seen in Supplementary Figure S3, while total intensity of activated integrin staining increases with force, the ratio of local to global integrin activation does not significantly change. These data suggest that all areas of the cell can activate integrins to a similar level and indicate that there is no preferential localization of integrin activation.
Figure 5. Local tensional forces on PECAM-1 elicit global β1 integrin activation.
(A) ECs were incubated with anti-PECAM-1-coated beads (4.5μm) and subjected to force with a permanent ceramic magnet for the indicated times. ECs were fixed and stained with HUTS-4, which recognizes ligated β1 integrin, and phalloidin to mark the actin cytoskeleton. (n > 30 cells/condition from 3 independent experiments; scale bar = 10μm, *p<0.05, **p<0.02). (B) ECs were incubated with anti-PECAM-1-coated beads (4.5μm) and subjected to force for the indicated times. ECs were fixed and stained for activated β1 integrins and the actin cytoskeleton. Cells were pretreated with LY294002 (30μM, 20min) to inhibit PI3K activation prior to force application (n > 25 cells/condition from 3 independent experiments; scale bar = 10μm, *p<0.05). (C) ECs overexpressing GFP or GFP-PH were incubated with anti-PECAM-1-coated beads (4.5μm) and force was applied. ECs were fixed and stained for activated β1 integrins (n > 25 cells/condition from 3 independent experiments, *p<0.05). For all panels, integrin activation was quantified using thresholded images and ImageJ software. Values were normalized to the “No Force” condition. Error bars represent s.e.m. (See also Fig. S3)
PI3K has been implicated in integrin activation in numerous cell types, including ECs in response to shear stress. To this regard, pharmacological inhibition of PI3K attenuated PECAM-1-mediated integrin ligation with the ECM (Fig. 5B), suggesting that PI3K activation is required for global integrin activation. We hypothesized that soluble lipid products produced by activated PI3K may promote cell-wide integrin activation. Previous studies have employed the overexpression of a GFP-PH construct to sequester cellular phospholipid messengers [17], as overexpression of GFP-PH restricts the mobility of cellular lipid messengers and affects downstream signaling. In order to test the hypothesis that mobility of PI3K lipid products is required for global integrin activation, we applied force to PECAM-1 on ECs overexpressing GFP-PH and assayed integrin-ECM ligation. Overexpression of the PH domain (which sequesters PI3K-mediated lipids) inhibited global integrin activation (Fig. 5C). This effect was specific, as overexpression of GFP alone did not affect force-induced β1 integrin activation. These data suggest that activation of PI3K and production of a soluble second messenger promotes global integrin activation at sites remote from the applied force.
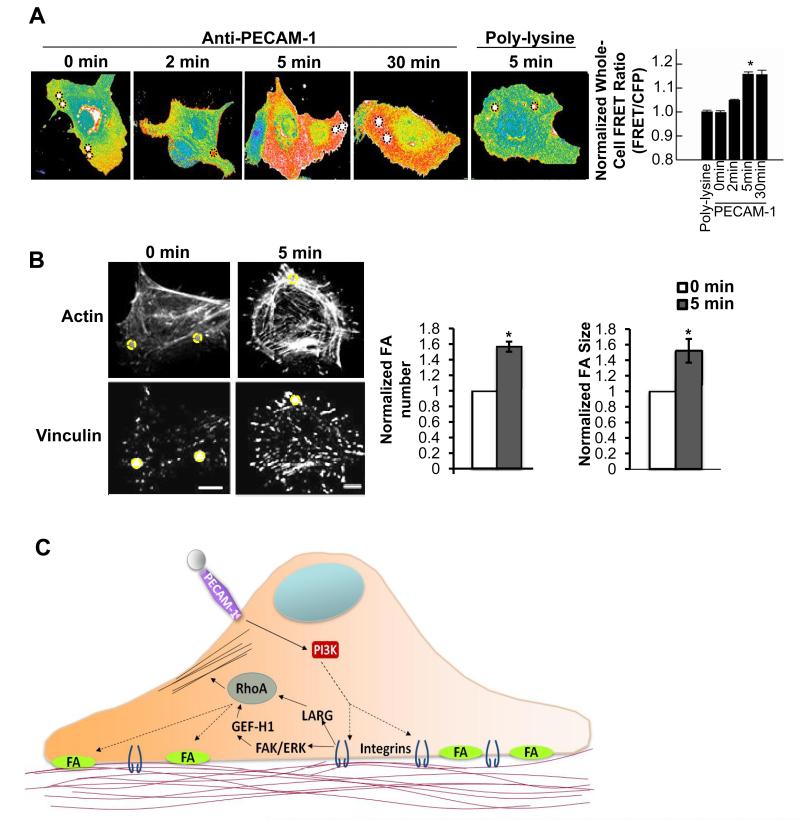
In light of our data indicating global integrin activation in response to a localized force on PECAM-1, we next tested if downstream RhoA activation was also a global response using a RhoA biosensor that detects RhoA activation via fluorescence resonance energy transfer (FRET) [18]. ECs transfected with the biosensor were subjected to force for the indicated times and fixed for subsequent FRET analysis. Importantly, fixation did not significantly affect the FRET signal intensity or localization (Fig. S4A). Consistent with our biochemical assays, we detected a statistically significant increase in RhoA activation after 5 minutes of force (Fig.6A). A trend for increased RhoA activation remained at 30 minutes, but was no longer significant, as 30% of the population had returned to basal levels by this time point. These results are not surprising, as it has been demonstrated that, under chronic force, ECs dampen activated signaling networks to maintain homeostasis. Importantly, a significant increase in FRET was exclusive to anti-PECAM-1-coated beads, as poly-lysine-coated beads did not display increased activation in response to force. In agreement with the ligated β1 integrin immunostaining, ECs also displayed a remarkable cell-wide increase in RhoA activity in response to force on PECAM-1 (Fig. 6A), as RhoA activity increased equally in local and global regions of the cell (Fig. S4B). In contrast to PECAM-1, tensional forces on FN-binding integrins did not induce global RhoA activation (data not shown).
Figure 6. Local tensional forces on PECAM-1 elicit global RhoA activation and focal adhesion growth.
(A) ECs expressing the RhoA biosensor were incubated with poly-lysine or anti-PECAM-1-coated beads (4.5μm) and subjected to force with a permanent ceramic magnet for the indicated times. Cells were fixed and analyzed for FRET. Whole cell FRET ratios were calculated for each condition. Autofluorescent beads are highlighted in black dotted circles (n > 45 cells/condition from 4 independent experiments, *p < 0.05). (B) Adherent ECs on FN were incubated with anti-PECAM-1-coated magnetic beads and subjected to force for the indicated times. ECs were fixed stained with phalloidin and an anti-vinculin antibody to mark focal adhesions. Focal adhesion number and size were quantified using NIH ImageJ software. Values were normalized to the “No Force” condition. Location of the beads are highlighted in yellow circles (n > 30cells/condition from 3 independent experiments, *p < 0.05, scale bar = 10μm). (C) Model of PECAM-1-mediated mechanotransduction. Local tensional forces on PECAM-1 results in global mechanosignaling and changes in cytoskeletal architecture. (See also Fig. S4)
Adaptive cellular stiffening is mediated, in part, by a local increase in focal adhesions at the site of mechanical stress that function to resist the applied force [5, 19, 20]. To further explore the possibility that localized force on PECAM-1 could lead to a global cellular response, we assessed focal adhesion growth by immunostaining for the focal adhesion marker vinculin. Remarkably, ECs exhibited a cell-wide increase in focal adhesion number, as well as individual focal adhesion size in response to tensional forces on PECAM-1 (Fig. 6B). These results further support the notion that a local force on PECAM-1 promotes a global signaling and cytoskeletal response.
Discussion
The present study provides insights into a mechanochemical signaling pathway downstream of PECAM-1 that relies on signals from multiple inputs, including mechanosensors at other transduction sites, such as integrins (Fig. 6C). We propose that force application on PECAM-1 results in PI3K activation, which leads to global activation of integrins and subsequent global RhoA activation via GEF-H1 and LARG. Activation of the GTPase promotes changes in cytoskeletal organization, including adaptive stiffening of the cytoskeleton and a cell-wide growth of focal adhesions. Using pharmacological inhibitors, we show that numerous signaling molecules work in concert to facilitate adaptive cellular stiffening. Interestingly, cells treated with cytoskeletal inhibitors (Cytochalasin D, C3, and Y27632) are immediately impaired and cannot respond to force (pulses 2-11, Figs.1C, 3A), whereas inhibition of new FN-integrin connections do not impair the mechanical response until latter pulses (pulses 5-11, Fig. 2A). These data suggest that pre-existing tension within the cytoskeleton is required for the immediate response to force, while new FN-integrins interactions are required for strengthening of adhesions and adaptive stiffening. At the present time it is difficult to determine if adaptive stiffening is a cell-wide phenomenon or a local event that occurs proximal to the site of force application. However, a global increase in focal adhesion size and number suggests that regions of the cell distal from the site of mechanical stress are responsive to exogenous force.
Previous studies probing integrins reported rapid mechanosignaling propagated through tensile cytoskeletal elements to remote cytoplasmic locations away from the site of mechanical stress [21-23]. However, these signals were not global and diffuse, but rather confined to distinct foci that corresponded with sites of cytoskeletal deformation. Thus, we provide the first evidence of a global signaling event in response to a localized mechanical stress.
While PECAM-1 has not been shown to directly interact with the cytoskeleton, indirect association via cytoplasmic interactions with β-catenin and γ-catenin have been proposed [21,24]. Although we cannot rule out that mechanical signaling through tensile cytoskeletal elements may contribute to PECAM-1-mediated mechanotransduction, our data suggest that chemical signaling (via activated PI3K) is required for a global cellular response, such as integrin activation. We also observed a delayed cellular stiffening response following force application on PECAM-1 compared to the immediate stiffening response reported when probing integrins [3, 11]. This delayed response further suggests involvement of a chemical signaling component, as one would anticipate an exclusively mechanical response to occur on a millisecond timescale. Future studies with PECAM-1 cytoplasmic tail truncation mutants may provide insight into the relative contributions of mechanical and chemical signaling components in adaptive stiffening.
Our study also highlights cooperation of two mechanosensors (PECAM-1 and integrins) in the EC response to force. Previous studies have highlighted a complex relationship between PECAM-1 and integrins. PECAM-1/PECAM-1 homophilic engagement can upregulate function of β1 integrins in numerous cell types. Crosslinking of PECAM-1 on specific subsets of T-lymphocytes increases β1-mediated adhesion [25]. In addition, engagement of PECAM-1 on platelets increases integrin-dependent adhesion and aggregation [26]. Our data suggest that PECAM-1-mediated mechanosensing may also promote β1-mediated adhesion in ECs, as tension on PECAM-1 initiates β1 ligation with the underlying extracellular matrix. Furthermore, β1 integrin engagement has also been shown to mediate tyrosine dephosphorylation of the cytoplasmic tail of PECAM-1, which may influence PECAM-1-mediated signaling [27]. Therefore, it is possible that a complex feedback loop may be present in our system. Importantly, a complex relationship between PECAM-1 and αvβ3 also exists. αvβ3 serves as a heterotypic ligand for PECAM-1, and interaction between these proteins may be important for endothelial functions such as leukocyte transendothelial migration and angiogenesis [28]. While we focus on the β1 integrin subtype, αvβ3 integrins may also contribute to the EC response to force. At the present time, we cannot differentiate the contribution of the different FN-binding integrin subtypes to the cellular response to tension on PECAM-1. However, previous studies have implicated a role for α5β1 clustering in the formation of adhesions that experience strong matrix forces, while αvβ3 integrin heterodimers strengthen integrin-cytoskeleton linkages in a talin-dependent manner [29]. Therefore, it is likely that multiple integrin subtypes may also be involved in PECAM-1-mediated mechanotransduction.
Atomic force microscopy (AFM) studies have revealed that ECs in regions of the vasculature that experience disturbed hemodynamics, and are thus predisposed to development of atherosclerotic plaques, exhibit increased stiffness when compared to ECs in healthy regions of the vessel [30]. Interestingly, regions of disturbed shear stress are also rich in FN deposition [31, 32], which, we now show, promotes a stiffer cellular phenotype. Therefore, our work may provide insights into early signaling events that contribute to cellular stiffening and plaque development.
Experimental Procedures
Cell culture, reagents, and antibodies
Bovine aortic endothelial cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, CellGro) with 10% fetal bovine serum, and 1% penicillin/streptomycin solution. Cells were plated on fibronectin (10μg/ml) 4 hours prior to experiments. LY294002, Cytochalasin D, wortmannin, and Y27632 were purchased from Calbiochem. Cell permeable C3 transferase was purchased from Cytoskeleton. The PECAM-1 antibody (PECAM 1.3) was a generous gift from D.K. Newman (BloodCenter of Wisconsin). Integrin blocking (16G3) and nonblocking (11E5) antibodies were kindly provided by K. Yamada (NIH). The LARG antibody was a generous gift from Kozo Kaibuchi (Nagoya University, Japan). Antibodies to RhoA (26C4) and Dbl (sc-89) were purchased from Santa Cruz Biotechnologies. The GEF-H1 was from Cell Signaling and the antibody to Vav was from BD Transduction. The antibody to Net1 was obtained from Abcam and the vinculin antibody was purchased from Sigma. The HUTS-4 antibody (which recognizes ligated β1 integrin) was purchased from Millipore.
Transfections and RNA interference
For GFP-PH and FRET experiments, cells were seeded at 50% confluence and transfected with 2.5μg of the GFP-PH construct or RhoA biosensor using Effectene reagents (Qiagen) according to the manufacturer’s protocol and experiments were performed 48 hours after transfection. For RNA interference experiments, control (Dharmacon siGLO RISC-free control siRNA), GEF-H1, or LARG siRNAs (Dharmacon) were transfected into cells using DharmaFECT4 (Dharmacon), according to the manufacturer’s instructions. Cells were plated on FN 72 hours post-transfection and experiments were performed. The following siRNA sequences were used in this study: GEF-H1: 5′-AGACAGAGGAUGAGGCUUAUU-3′; and LARG: 5′-GGGAAUAUGGAGAGAAUUAUU-3′.
Preparation of beads
Tosyl-activated paramagnetic beads (2.8 or 4.5 micron, Invitrogen) were washed with PBS and coated with an anti-PECAM-1 antibody (PECAM 1.3) or poly-lysine solution (Sigma) according to the manufacturer’s instructions. Beads were quenched in 0.2M Tris prior to use to remove any remaining tosyl group and resuspended in DMEM containing 10% fetal bovine serum and 1% penicillin/streptomycin solution. Immediately before experiments ECs were incubated with beads (2-6 beads/cell) for 30 min at 37°C. Cells were briefly washed with fresh media to remove unbound beads prior to force application.
Pulsatile force application
The UNC 3D Force Microscope (3DFM) was used to apply controlled pulsatile forces (~100pN) to anti-PECAM-1-coated magnetic beads (2.8μm diameter). Bead displacements were recorded with a high-speed video camera (Pulnix, JAI) and tracked using Video Spot Tracker (Center for Computer Integrated Systems for Microscopy and Manipulation). Cells were monitored for changes in morphology, movement of the nucleus, cell edges, and particulates. No significant changes in cell morphology or movement of organelles were noticeable.
Permanent force application
For all immunostaining and biochemical analyses, continuous force (~10pN) was applied to anti-PECAM-1-coated beads (4.5μm diameter) using a permanent ceramic magnet (K&J Magnetics) parallel to the culture dish surface at a distance of 1cm from the adherent cells. No significant changes in cell morphology or movement of the nucleus, cell edges, or organelles was noticeable.
Immunofluorescence
To examine activation of PI3K, GFP-PH-transfected cells subjected to force (permanent magnet, 4.5μm beads) were fixed for 20 min in PBS containing 2% formaldehyde and mounted in Vectashield mounting medium (Vector laboratories). For all other experiments, cells were fixed for 20 min in PBS containing 2% formaldehyde, permeabilized with 0.2% Triton X-100, and blocked with PBS containing 10% goat serum for 1hr at room temperature. Antibody incubations were performed as previously described [33] and mounted in Vectashield mounting medium. Images were acquired using a confocal microscope (Olympus FV500) with a 63x oil lens.
GST-RBD and GST-RhoA(G17A)
Adherent cells were incubated with anti-PECAM-1-coated beads (4.5 micron, Invitrogen) for 30 min and subjected to force for indicated times. Active RhoA pulldowns were performed as previously described[34]. Briefly, following force application, cells were lysed in 50mM Tris (pH 7.6), 500mM NaCl, 1% Triton X-100, 0.1% SDS, 0.5% deoxycholate, 10mM MgCl2, and protease inhibitors. Anti-PECAM-1-coated magnetic beads were removed from lysates with a magnetic separator. Lysates were centrifuged for 5min and supernatants were transferred to a new tube and incubated at 4° with 80μg of purified (GST-RBD) bound to glutathione-sepharose beads. Bead pellets were washed in 50mM Tris (pH 7.6), 150mM NaCl, 1% Triton X-100, 10mM MgCl2, and protease inhibitors, and subsequently resuspended in Laemmli sample buffer and subjected to SDS-PAGE. Precipitation of active GEFs with the nucleotide-free RhoA mutant (G17A) were performed as previously described[13]. Briefly, following force application cells were lysed in 20mM HEPES (pH 7.6), 150mM NaCl, 1% Triton X-100, 5mM MgCl2, and protease inhibitors. Lysates were incubated at 4°C for 45 minutes with 100μg of purified GST-G17A RhoA bound to glutathione-sepharose beads. Pelleted beads were then wash in lysis buffer, resuspended in Laemmli sample buffer, and subjected to SDS-PAGE.
FRET analysis
RhoA activation was measured by FRET in fixed cells by monitoring the ratio of FRET (ECFP excitation and Citrine emission) to ECFP emission (ECFP excitation and emission) as previously described[18, 35]. Cells were chosen for similar, low expression levels. Single-frame images were acquired on an inverted epifluorescence microscope (model IX81; Olympus), using a 40X UPlan FLN 1.3 N/A DIC lens (Olympus), a CCD camera (CoolSnapESII; Roper Industries), and MetaMorph software (Universal Imaging). For emission ratio imaging, the following filter sets were used (Chroma Technology Corp.): CFP: D436/20, D470/40; FRET: D436/20, HQ535/30; YFP: HQ500/20, HQ535/30. A dichroic mirror was custom-manufactured by Chroma for compatibility with all of these filters. Cells were illuminated with a 100 W Hg arc lamp through an ND 1.0 neutral density filter. At each time point, three images were recorded with the following exposure times: CFP (1.2 s) and FRET (0.6 s)
Metamorph software was used to perform image analysis. All images were first shading-corrected and background-subtracted. The FRET image, because it had the largest signal-to-noise ratio and therefore provided the best distinction between the cell and the background, was thresholded to generate a binary mask with a value of zero outside the cell and a value of one inside the cell. After multiplication by this mask, the FRET image was divided by the CFP image to yield a ratio image reflecting RhoA activation throughout the cell. A linear pseudocolour lookup table was applied, and the ratio values were normalized to the lower scale value which was chosen to exclude the bottom 5% of the total histogram distribution, thereby avoiding spurious low intensity pixels. In each experiment, all images were inspected to verify that all portions used to create the ratio image had a sufficiently high signal-to-noise ratio. We targeted at least 300 gray level values (12-bit dynamic range) above background in the lowest intensity regions within the cell (S/n > 3). This was especially important in thin parts of the cell where fluorescence was low. The ratio was corrected for bleaching using a method described elsewhere [35].
Whole-Cell FRET Analysis
To calculate whole-cell average FRET ratio for the determination of the effects of mechanical force on RhoA activation, FRET ratio (FRET/CFP) images acquired and processed as described above were loaded into Metamorph, thresholded to generate masks for each cell, and regions were drawn around each cell using the mask. From these regions, a number of parameters, including average pixel intensity, could be measured and recorded. Average FRET ratio intensity was calculated for each image for at least 10 cells per condition and averaged for each treatment condition.
Quantification of integrin activation and focal adhesions
ECs stained for ligated β1 integrin- or vinculin were analyzed with NIH ImageJ software. Confocal image planes at the basal surface of the cell were chosen for analysis and RGB images were converted to 8-bit black and white images. Activated integrins and focal adhesions were defined by setting an intensity threshold to remove any background signal. Integrin activation and focal adhesion size and number were analyzed using the ‘Analyze particles’ function.
Statistical analysis
Data are presented as means ± s.e.m. p-values were determined using a two-tailed unpaired Student’s t-test.
Supplementary Material
Highlights.
Tension on PECAM-1 results in adaptive cellular stiffening and mechanosignaling
PECAM-1-mediated mechanosensing requires activation of integrins
Tensional forces on PECAM-1 stimulate integrin-dependent RhoA activation
Localized tensional forces on PECAM-1 elicit a global mechanoresponse
Acknowledgements
We would like to thank Marie Rougié for assistance with FRET experiments, Dr. Robert Bagnell and the UNC Lineberger Cancer Center Microscopy Facility for help with microscopy studies, Luke Osborne and Ben Rardin for calculating magnetic forces used in this study, and Vinay Swaminathan for initial technical assistance with the magnetic tweezers system. C.G. is supported by a Marie Curie Outgoing International Fellowship from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 254747. E.T. is an Ellison Medical Foundation New Scholar. This work was supported by NIH grants HL088632 (to E.T.), GM094663 (to K.H), and 5P41EB002025 (to R.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
C.C. and E.T. designed the experiments. C.C. and C.G. performed the experiments and C.C., C.G., and C.W. analyzed the data. E.T.O., R.S., K.H., and K.B. helped with experimental design and procedures. C.C. and E.T. wrote the manuscript. All authors provided detailed comments.
References
- 1.Hoffman BD, Crocker JC. Cell mechanics: dissecting the physical responses of cells to force. Annu Rev Biomed Eng. 2009;11:259–288. doi: 10.1146/annurev.bioeng.10.061807.160511. [DOI] [PubMed] [Google Scholar]
- 2.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 3.Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci. 2006;119:508–518. doi: 10.1242/jcs.02760. [DOI] [PubMed] [Google Scholar]
- 4.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 5.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Rico C, Pincet F, Thiery JP, Dufour S. Integrins stimulate E-cadherin-mediated intercellular adhesion by regulating Src-kinase activation and actomyosin contractility. J Cell Sci. 2010;123:712–722. doi: 10.1242/jcs.047878. [DOI] [PubMed] [Google Scholar]
- 7.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 8.Goel R, Schrank BR, Arora S, Boylan B, Fleming B, Miura H, Newman PJ, Molthen RC, Newman DK. Site-Specific Effects of PECAM-1 on Atherosclerosis in LDL Receptor-Deficient Mice. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.108.172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harry BL, Sanders JM, Feaver RE, Lansey M, Deem TL, Zarbock A, Bruce AC, Pryor AW, Gelfand BD, Blackman BR, et al. Endothelial Cell PECAM-1 Promotes Atherosclerotic Lesions in Areas of Disturbed Flow in ApoE-Deficient Mice. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.108.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tim O’Brien E, Cribb J, Marshburn D, Taylor RM, 2nd, Superfine R. Chapter 16: Magnetic manipulation for force measurements in cell biology. Methods Cell Biol. 2008;89:433–450. doi: 10.1016/S0091-679X(08)00616-X. [DOI] [PubMed] [Google Scholar]
- 11.Guilluy C, Swaminathan V, Garcia-Mata R, Timothy O’Brien E, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011 doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Mata R, Wennerberg K, Arthur WT, Noren NK, Ellerbroek SM, Burridge K. Analysis of activated GAPs and GEFs in cell lysates. Methods Enzymol. 2006;406:425–437. doi: 10.1016/S0076-6879(06)06031-9. [DOI] [PubMed] [Google Scholar]
- 14.Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol. 2002;158:773–785. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu YJ, McBeath E, Fujiwara K. Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited PECAM-1 phosphorylation. J Cell Biol. 2008;182:753–763. doi: 10.1083/jcb.200801062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chretien ML, Zhang M, Jackson MR, Kapus A, Langille BL. Mechanotransduction by endothelial cells is locally generated, direction-dependent, and ligand-specific. J Cell Physiol. 2010;224:352–361. doi: 10.1002/jcp.22125. [DOI] [PubMed] [Google Scholar]
- 17.Raucher D, Stauffer T, Chen W, Shen K, Guo S, York JD, Sheetz MP, Meyer T. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell. 2000;100:221–228. doi: 10.1016/s0092-8674(00)81560-3. [DOI] [PubMed] [Google Scholar]
- 18.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 19.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 20.Galbraith CG, Skalak R, Chien S. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil Cytoskeleton. 1998;40:317–330. doi: 10.1002/(SICI)1097-0169(1998)40:4<317::AID-CM1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci U S A. 2008;105:6626–6631. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poh YC, Na S, Chowdhury F, Ouyang M, Wang Y, Wang N. Rapid activation of Rac GTPase in living cells by force is independent of Src. PLoS One. 2009;4:e7886. doi: 10.1371/journal.pone.0007886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- 24.Ilan N, Mahooti S, Rimm DL, Madri JA. PECAM-1 (CD31) functions as a reservoir for and a modulator of tyrosine-phosphorylated beta-catenin. J Cell Sci. 1999;112(Pt 18):3005–3014. doi: 10.1242/jcs.112.18.3005. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka Y, Albelda SM, Horgan KJ, van Seventer GA, Shimizu Y, Newman W, Hallam J, Newman PJ, Buck CA, Shaw S. CD31 expressed on distinctive T cell subsets is a preferential amplifier of beta 1 integrin-mediated adhesion. J Exp Med. 1992;176:245–253. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varon D, Jackson DE, Shenkman B, Dardik R, Tamarin I, Savion N, Newman PJ. Platelet/endothelial cell adhesion molecule-1 serves as a costimulatory agonist receptor that modulates integrin-dependent adhesion and aggregation of human platelets. Blood. 1998;91:500–507. [PubMed] [Google Scholar]
- 27.Lu TT, Yan LG, Madri JA. Integrin engagement mediates tyrosine dephosphorylation on platelet-endothelial cell adhesion molecule 1. Proc Natl Acad Sci U S A. 1996;93:11808–11813. doi: 10.1073/pnas.93.21.11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckley CD, Doyonnas R, Newton JP, Blystone SD, Brown EJ, Watt SM, Simmons DL. Identification of alpha v beta 3 as a heterotypic ligand for CD31/PECAM-1. J Cell Sci. 1996;109(Pt 2):437–445. doi: 10.1242/jcs.109.2.437. [DOI] [PubMed] [Google Scholar]
- 29.Roca-Cusachs P, Gauthier NC, Del Rio A, Sheetz MP. Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc Natl Acad Sci U S A. 2009;106:16245–16250. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyazaki H, Hayashi K. Atomic force microscopic measurement of the mechanical properties of intact endothelial cells in fresh arteries. Med Biol Eng Comput. 1999;37:530–536. doi: 10.1007/BF02513342. [DOI] [PubMed] [Google Scholar]
- 31.Nigro P, Abe JI, Berk BC. Flow Shear Stress and Atherosclerosis: A Matter of Site Specificity. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2010.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J Cell Biol. 2005;169:191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Sweet DT, Irani-Tehrani M, Maeda N, Tzima E. Shc coordinates signals from intercellular junctions and integrins to regulate flow-induced inflammation. J Cell Biol. 2008;182:185–196. doi: 10.1083/jcb.200709176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.