Abstract
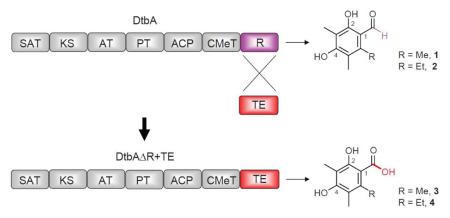
We re-annotated the A. niger NR-PKS gene, e_gw1_19.204 and its downstream R domain gene, est_GWPlus_C_190476 as a single gene which we name dtbA. Heterologous expression of dtbA in A. nidulans demonstrated that DtbA protein produces two polyketides, 2,4-dihydroxy-3,5,6-trimethylbenzaldehyde 1 and 6-ethyl-2,4-dihydroxy-3,5-dimethylbenzaldehyde 2. Generation of DtbAΔR+TE chimeric PKSs by swapping the DtbA R domain with the AusA (austinol biosynthesis) or ANID_06448 TE domain enabled the production of two metabolites with carboxylic acids replacing the corresponding aldehydes.
Filamentous fungi produce numerous polyketides that exhibit a wide range of bioactivities. Fungal polyketides are synthesized by multi-domain polyketide synthases (PKSs) which can be classified as non-reducing (NR), partially reducing (PR), and highly reducing (HR) PKSs.1-5 Canonical NR-PKSs catalyze the production of aromatic polyketides and contain starter unit ACP transacylase (SAT), β-ketoacyl synthase (KS), acyl transferase (AT), product template (PT), and acyl carrier protein (ACP) domains at a minimum, and sometimes contain a methyltransferase (CMeT) domain as well.6-8 Release of the polyketide chain from an NR-PKS can be mediated by several different types of domains found in NR-PKSs including thioesterase/Claisen-cyclase (TE/CLC), thioesterase (TE), and reductase (R) domains.9-11 An additional discrete β-lactamase can also release the polyketide from NR-PKSs lacking a TE/CLC domain.12 NR-PKS genes are common and widespread among Aspergillus species. Using a variety of genome mining methods, we and others have successfully identified the products of all 14 NR-PKSs of A. nidulans. As an extension to our study in A. nidulans we have examined the eight species in the Broad Institute Aspergillus Comparative Database (http://www.broadinstitute.org/) and identified at least 71 NR-PKSs that can be classified into seven major groups.13 We are interested in deciphering the chemical products of these NR-PKSs, especially ones with non-canonical domain architectures. Ultimately, our goal is to understand the relationship between NR-PKS DNA sequences and chemical structures in order to develop bioinformatic tools capable of predicting the chemical products of NR-PKSs from DNA sequences generated by fungal genome projects.
Through bioinformatic analyses of A. niger strain ATCC 1015, we identified a putative TE-less NR-PKS encoded by e_gw1_19.204, belonging to Group VI NR-PKSs. A PKS in this group typically has a SAT-KS-AT-PT-ACP-CMeT-TE domain architecture.13 Sequence analysis indicated that the 3′ coding region of e_gwl_19.204 is incomplete. Interestingly, the gene next to e_gw1_19.204, est_GWPlus_C_190476, encodes a protein with a putative R domain, but both 5′ and 3′ coding regions are missing. These data combined suggested that e_gw1_19.204 and est_GWPlus_C_190476 are both mis-annotated, and the two fragments are in fact part of a single gene. If this is true, then the fused NR-PKS should have the SAT-KS-AT-PT-ACP-CMeT-R domain architecture, which is unusual in Group VI. To verify this hypothesis, we used an in vivo heterologous expression approach to determine the PKS product of this fused gene.
First, a PCR fragment ranging from the start site of e_gwl_19.204 to a predicted stop site of est_GWPlus_C_190476 was generated and fused to a selectable marker and an inducible alcA promoter cassette (Figure S1).14 This DNA construct was heterologously expressed in A. nidulans. The parent strain used for transformation featured an nkuAΔ, stcJΔ genetic background which not only facilitates transformation success rate but also eliminates the major secondary metabolite sterigmatocystin.15, 16 After induction, total RNA was extracted, and the cDNA was synthesized using the reverse primer specific to the stop site of est_GWPlus_C_190476. The cDNA was then PCR amplified and underwent sequencing analysis. The cDNA sequence differed from the predicted coding sequence (Figure S2). NCBI protein blast analysis (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) of the deduced amino acids revealed that a coding region was incorrectly annotated, thereby splitting the actual R domain from the C terminus of the NR-PKS.
Next, we focused on determining the product of this re-annotated NR-PKS. Upon induction, the heterologous expression strain produced two UV-Vis detectable aromatic compounds 1 and 2 (Figure 1). To determine the structure of compounds 1 and 2, both compounds were isolated from a large-scale culture and structurally elucidated by spectroscopic methods. Compound 2 has been structurally elucidated as 6-ethyl-2,4-dihydroxy-3,5-dimethylbenzaldehyde by our group previously.13 Compound 1 has a similar UV-Vis spectrum to compound 2 (Figure S5). The 1H and 13C NMR spectra of 1 exhibited three methyl groups attached to the benzene ring (Figure S6, S7). This together with the fact that compound 1 is 14 amu less than compound 2 suggested that 1 is 2,4-dihydroxy-3,5,6-trimethylbenzaldehyde. We designated this intact NR-PKS gene as dtbA, from 2,4-dihydroxy-3,5,6-trimethylbenzaldehyde. The result implies that the SAT domain of DtbA is capable of accepting both acetyl-CoA and propionyl-CoA as starter units, which is then extended with three malonyl-CoAs, and modified with two S-adenosyl methionines (SAMs) followed by the reductive release.
Figure 1.
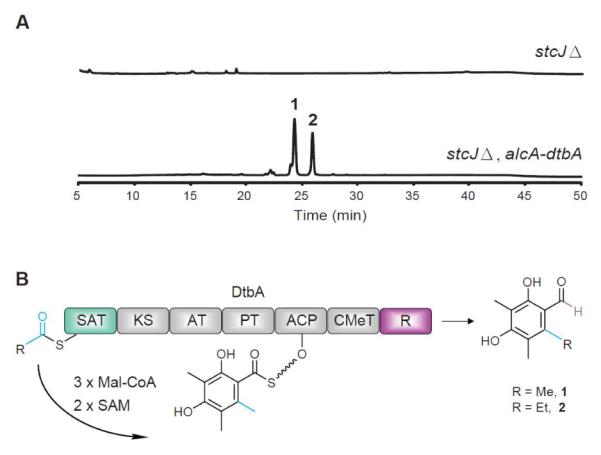
(A) HPLC-DAD-MS analysis of metabolite profiles. (B) Domain architecture, putative starter units, unreleased intermediates, and released polyketides for DtbA.
Phylogenetic analysis revealed this re-annotated NR-PKS belongs in Group VI NR-PKS all members of which possess a TE domain but not an R domain for product release (Figure S3). To date, it is still not clear whether a programmed releasing mechanism can be modulated by modifying the native building blocks. We reasoned that if we could engineer this NR-PKS with a TE release domain instead of the R domain, we would be able to further expand the structural diversity of products produced by fungal NR-PKSs. To test this hypothesis, by taking advantage of the fact that all chemical products of NR-PKSs from A. nidulans have been solved recently,13 we generated chimeric modules in which the SAT-KS-AT-PT-ACP-CMeT fragment from DtbA (DtbAΔR) was fused to a TE or TE/CLC domain from other NR-PKSs of A. nidulans (Figure S4).
We analyzed the phylogenetic relationship of the recipient NR-PKS DtbA with the donor NR-PKSs from A. nidulans (Figure 2). Phylogenetic analysis revealed that ANID_08383 (AusA) and ANID_06448 from A. nidulans are positioned at the shortest phylogenetic distance from DtbA and both of them contain a TE domain (Figure 2). Therefore, first, we replaced the R domain in DtbA with the TE from AusA or ANID_06448 of A. nidulans, respectively.
Figure 2.
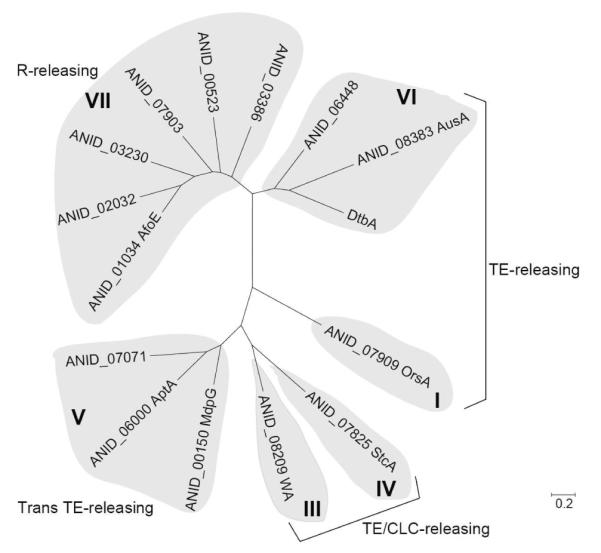
Phylogenetic analysis of DtbA and fourteen NR-PKSs in A. nidulans.
Heterologous expression of the chimeric PKS, DtbAΔR+TE(AusA) and DtbAΔR+TE(ANID_06448), led to the production of compounds 3 and 4 after 48 hr of induction (Figure 3). Compounds 3 and 4 were purified from a large-scale culture for structural elucidation. Spectroscopic data confirmed that compound 3 is 2,4-dihydroxy-3,5,6-trimethylbenzoic acid, an aromatic polyketide produced by AusA.13 Compound 4 has a similar UV-Vis spectrum to compound 3 (Figure S5). The 1H and 13C NMR spectra of 4 exhibited two methyl groups and one ethyl group attached to the benzene ring (Figure S8 and Figure S9). This together with the fact that compound 4 is 14 amu more than compound 3 suggested that compound 4 is 6-ethyl-2,4-dihydroxy-3,5-dimethylbenzoic acid. Therefore, by swapping the R domain with the TE domain, we were able to engineer the chimeric PKS to produce the predicted carboxylic derivatives of 1 and 2.
Figure 3.
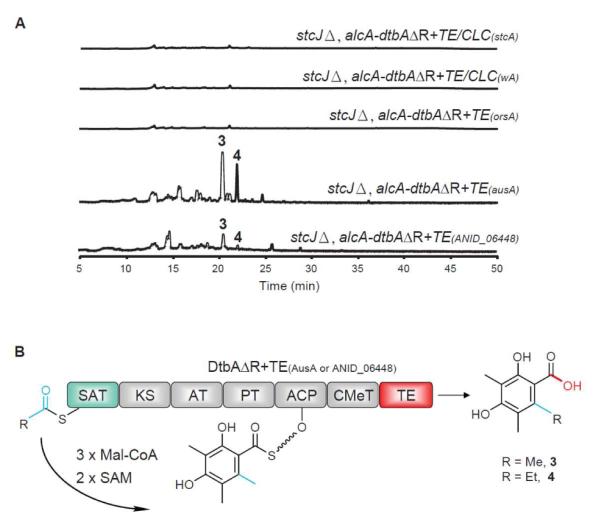
(A) HPLC-DAD-MS analysis of metabolite profiles. (B) Domain architecture, putative starter units, unreleased intermediates, and released polyketides for hybrid DtbAΔR+TE(AusA or ANID_06448).
Next we tested whether TE or TE/CLC from NR-PKSs further away phylogenetically would also allow polyketide production. We created three chimeras by fusing DtbAΔR with the TE domain from OrsA, or the TE/CLC domain from StcA or WA. However, no metabolites were detected by LC/MS analysis from any strains expressing the chimeric proteins (Figure 3). These results suggested that phylogenetic analysis could serve as a guide for the success of the domain swapping experiment. It seems that the close phylogenetic relationship between domain swapped synthases might provide better domain-domain interaction of the engineered synthases which leads to the successful product release. It is not surprising that TE/CLC did not release the polyketides since TE/CLC catalyzes Claisen cyclization and exhibits low sequence similarity with TE.5 In the cases of NR-PKSs in Group VI and VII, the location of CMeT domain after ACP domain might imply that the two domains in the CMeT-TE or CMeT-R didomains have evolved together. This might explain why TE from OrsA failed to release the product since OrsA lacks the CMeT domain,10 moreover the TE from OrsA is phylogenetically far away from that of AusA and ANID_06448 (Figure S10). We also performed phylogenetically analysis on the DtbA R domain, five TE domains for which we conducted domain swapping, and six R domains excised from the NR-PKSs of A. nidulans. This analysis indicated that the DtbA R domain is phylogenetically more closely related to the R domains than other TE domains (supplementary Figure 11). Lastly we determined the production yield of the products for both WT DtbA and the chimeric DtbAΔR+TE(AusA). We showed that under induction conditions, we were able to isolate 11.0 mg/l of 1 and 8.4 mg/l of 2 from WT DtbA and 3.1 mg/l of 3 and 2.3 mg/l of 4 from the chimeric DtbAΔR+TE(AusA). Therefore, we observed a three-fold decrease in production of the chimeric NR-PKS compared with that of native DtbA (Figure S12).
The TE from ANID_06448, TE(ANID_06448), is responsible for the releasing of 2,4-dihydroxy-3,6-dimethylbenzoic acid whereas the TE from AusA, TE(AusA), is responsible for the releasing of 2,4-dihydroxy-3,5,6-trimethylbenzoic acid (3) which harbors an additional methyl group at the C-5 position in contrast to 2,4-dihydroxy-3,6-dimethylbenzoic acid. This implies that TE(AusA) may provide a larger and more flexible pocket for substrate binding, which might explain the observation that the production yield of compounds 3 and 4 are more abundant in DtbAΔR+TE(AusA) than that in DtbAΔR+TE(ANID_06448) (Figure 3).
TEs have been classified into 23 families based on enzyme function and substrate identity.17 We performed phylogenetic analysis of TE domains that we applied for domain swapping and 47 representative TE family members that belong to 23 known TE families, TE1 to TE23. Interestingly, phylogenetic results showed that TE(AusA) and TE(ANID_06448) are phylogenetically close to TE21 whereas TE(OrsA), TE/CLC(StcA) and TE/CLC(WA) are phylogenetically close to TE16 (Figure S10). Members of TE21 possess the canonical TE catalytic triad consisting of a conserved serine-histidine-aspartate active site in which serine served as the reactive residue which relied on the histidine and aspartate residues to stabilize the transition state.17-20 To better define the role of TE(AusA) in product release, we applied a RaptorX protein structure prediction server (http://raptorx.uchicago.edu)21 to predict the tertiary structure of TE(AusA). Together with the fact that nucleophilic S123 is located within the characteristic active site motif G-X-S-X-G, the putative tertiary structure suggested that S123-H317-D285 in TE(AusA) could be the catalytic triad that is responsible for TE(AusA)-mediated product release (Figure S13). To address this hypothesis, we generated active site mutants followed by heterologous expression and metabolite profile analysis. The proposed TE catalytic triad residues were individually mutated (S123A, H317F and D285N) to assess their involvement in TE-catalyzed product releasing. These mutants showed no detectable product (Figure 4). As a control, two serines which are not involved in the triad were individually mutated (S152A and S162A) and gave only a slightly reduced level of product generation (Figure S14). Our results confirmed that the proposed catalytic triad (S123-H317-D285) is essential for product release.
Figure 4.
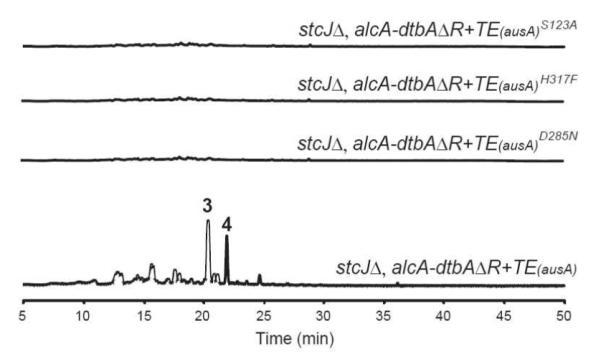
HPLC-DAD-MS analysis of TE triad mutant strains.
In summary, using a heterologous expression approach, we first identified the products of DtbA, a re-annotated NR-PKS in A. niger. We further created chimeric PKSs by replacing the R domain with the TE domain which led to the production of new polyketides with functional groups that were generated by the releasing domains. Our experiments provided evidence that the replacement of the R domain with a phylogenetically close TE domain can facilitate alternative NR-PKS product releasing, creating a carboxylic moiety instead of an aldehyde functionality. These results indicated that rational domain swaps may provide a route for engineering the functionalization of valuable chemicals.
Supplementary Material
Acknowledgment
The project described was supported by Grant Number PO1-GM084077 from the National Institute of General Medical Science to B.R.O. and C.C.C.W. Research conducted at the Pacific Northwest National Laboratory was supported by the Department of Energy, Office of the Biomass Program.
Footnotes
Supporting Information Available: The primers and Aspergillus nidulans strains used in this study, nucleotide alignment analysis, experimental details, and characterization of compounds 1 and 4 (1H NMR, 13C NMR and HRMS) are provided in the Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
References
- (1).Nicholson TP, Rudd BA, Dawson M, Lazarus CM, Simpson TJ, Cox RJ. Chem. Biol. 2001;8:157–178. doi: 10.1016/s1074-5521(00)90064-4. [DOI] [PubMed] [Google Scholar]
- (2).Kroken S, Glass NL, Taylor JW, Yoder OC, Turgeon BG. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15670–15675. doi: 10.1073/pnas.2532165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Cox RJ. Org. Biomol. Chem. 2007;5:2010–2026. doi: 10.1039/b704420h. [DOI] [PubMed] [Google Scholar]
- (4).Chiang YM, Oakley BR, Keller NP, Wang CC. Appl. Microbiol. Biotechnol. 2010;86:1719–1736. doi: 10.1007/s00253-010-2525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Chooi YH, Tang Y. J. Org. Chem. 2012;77:9933–9953. doi: 10.1021/jo301592k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Crawford JM, Dancy BC, Hill EA, Udwary DW, Townsend CA. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16728–16733. doi: 10.1073/pnas.0604112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Crawford JM, Korman TP, Labonte JW, Vagstad AL, Hill EA, Kamari-Bidkorpeh O, Tsai SC, Townsend CA. Nature. 2009;461:1139–1143. doi: 10.1038/nature08475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Fisch KM, Skellam E, Ivison D, Cox RJ, Bailey AM, Lazarus CM, Simpson TJ. Chem. Commun. (Camb) 2010;46:5331–5333. doi: 10.1039/c0cc01162b. [DOI] [PubMed] [Google Scholar]
- (9).Fujii I, Watanabe A, Sankawa U, Ebizuka Y. Chem. Biol. 2001;8:189–197. doi: 10.1016/s1074-5521(00)90068-1. [DOI] [PubMed] [Google Scholar]
- (10).Schroeckh V, Scherlach K, Nutzmann HW, Shelest E, Schmidt-Heck W, Schuemann J, Martin K, Hertweck C, Brakhage AA. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14558–14563. doi: 10.1073/pnas.0901870106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Bailey AM, Cox RJ, Harley K, Lazarus CM, Simpson TJ, Skellam E. Chem. Commun. (Camb) 2007:4053–4055. doi: 10.1039/b708614h. [DOI] [PubMed] [Google Scholar]
- (12).Awakawa T, Yokota K, Funa N, Doi F, Mori N, Watanabe H, Horinouchi S. Chem. Biol. 2009;16:613–623. doi: 10.1016/j.chembiol.2009.04.004. [DOI] [PubMed] [Google Scholar]
- (13).Ahuja M, Chiang YM, Chang SL, Praseuth MB, Entwistle R, Sanchez JF, Lo HC, Yeh HH, Oakley BR, Wang CC. J. Am. Chem. Soc. 2012;134:8212–8221. doi: 10.1021/ja3016395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Waring RB, May GS, Morris NR. Gene. 1989;79:119–130. doi: 10.1016/0378-1119(89)90097-8. [DOI] [PubMed] [Google Scholar]
- (15).Nayak T, Szewczyk E, Oakley CE, Osmani A, Ukil L, Murray SL, Hynes MJ, Osmani SA, Oakley BR. Genetics. 2006;172:1557–1566. doi: 10.1534/genetics.105.052563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Chiang YM, Szewczyk E, Davidson AD, Keller N, Oakley BR, Wang CC. J. Am. Chem. Soc. 2009;131:2965–2970. doi: 10.1021/ja8088185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Cantu DC, Chen Y, Reilly PJ. Protein Sci. 2010;19:1281–1295. doi: 10.1002/pro.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Carter P, Wells JA. Nature. 1988;332:564–568. doi: 10.1038/332564a0. [DOI] [PubMed] [Google Scholar]
- (19).Kim KK, Song HK, Shin DH, Hwang KY, Choe S, Yoo OJ, Suh SW. Structure. 1997;5:1571–1584. doi: 10.1016/s0969-2126(97)00306-7. [DOI] [PubMed] [Google Scholar]
- (20).Korman TP, Crawford JM, Labonte JW, Newman AG, Wong J, Townsend CA, Tsai SC. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6246–6251. doi: 10.1073/pnas.0913531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Kallberg M, Wang H, Wang S, Peng J, Wang Z, Lu H, Xu J. Nat. Protoc. 2012;7:1511–1522. doi: 10.1038/nprot.2012.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


