Abstract
The wound microenvironment is comprised of constituents, such as the extracellular matrix (ECM), that regulate with temporal and spatial precision, the migratory, proliferative, and contractility of wound cells. Prompt closure of the wound is an early and critical phase of healing and β1 integrins are important in this process. We previously reported a marked increase in integrin α9β1 expression in epidermal keratinocytes in cutaneous and corneal wounds. However, the functional role of keratinocyte α9β1 during re-epithelialization is unknown and analysis has been precluded by the lethal phenotype of integrin α9β1 knockout mice. We now report that in conditional integrin α9 knockout (K14–α9 null) mice normal proliferation occurs in epidermal keratinocytes and corneal basal cells. Normal epidermal keratinocyte morphology is also retained. However, corneal basal cell morphology and epithelial thickness are altered, suggesting that loss integrin α9β1 results in abnormal corneal differentiation. In cutaneous wounds, the number of proliferating epidermal keratinocytes is significantly reduced in K14–α9 null mice compared to α9fl/−; no Cre (control) mice. The decreased keratinocyte proliferation observed in K14–α9 null mice negatively impacts healing, resulting in a thinner migrating epithelium demonstrating that α9β1 is crucial for efficient and proper re-epithelialization during cutaneous wound healing.
Keywords: Integrin, α9β1, wound healing, re-epithelialization, keratinocytes
INTRODUCTION
Re-epithelialization is essential to successful wound healing and requires contributions from keratinocyte proliferation, migration and differentiation. Integrins and their interaction with the ligand(s) in the provisional matrix and ECM are one of the key regulators of keratinocyte function (Martin, 1997; Singer and Clark, 1999). Defects in one or multiple keratinocyte function(s) can have pleiotropic effects, ranging from excessive scarring to the formation of chronic ulcers.
In normal epidermis the integrin hetrodimer repertoire is mainly restricted to the basal keratinocyte and prominently includes α2β1, α3β1, α5β1, α9β1, αvβ6, and α6β4, (Hertle et al, 1991; Larjava et al, 1993; Palmer et al, 1993; Stepp et al, 2002; Stepp and Zhu, 1997). Integrin α3β1, and α6β4, bind to the basement membrane component, laminin-5 (Choma et al, 2004; DiPersio et al, 1997; Frank and Carter, 2004; Nguyen et al, 2000). Integrin α2β1, recognizes collagen (I and IV) and some forms of laminin (Grenache et al, 2007; Parks, 2007; Zweers et al, 2007). Integrin α5β1, and αvβ6, are fibronectin (FN) receptors; αvβ6, also bind to tenascin-C (AlDahlawi et al, 2006; Larjava et al, 1993; Yang et al, 1993). Integrin α9β1 is normally expressed in airway epithelial cells, the basal layer of squamous epithelia, smooth muscle, skeletal muscle, neutrophils and hepatocytes (Palmer et al, 1993; Taooka et al, 1999). ECM ligands for integrin α9β1 include tenascin-C, osteopontin, VCAM-1, ADAMs-12 and 15 and the EIIIA segment of FN (Eto et al, 2000; Liao et al, 2002; Shinde et al, 2008; Taooka et al, 1999; Yokosaki et al, 1996; Yokosaki et al, 1994; Yokosaki et al, 1999). In stratified squamous epithelial cells on the healthy ocular surface, α9 integrin expression is restricted to a subset of basal cells at the limbus (Stepp and Zhu, 1997) where it has also been shown to be expressed on the early transiently amplifying (eTA) cells adjacent to the corneal epithelial stem cell niche (Pajoohesh-Ganji and Stepp, 2005).
Integrin expression and functional profiles on keratinocyte changes following injury. Integrin heterodimers, upregulated following wounding, include α3β1, α2β1, α5β1, α9β1, αvβ1, αvβ6, αvβ5, and α6β4, suggesting that integrins have specific as well as redundant functions during wound repair (Hakkinen et al, 2000; Hertle et al, 1991; Larjava et al, 1993; Raymond et al, 2005; Singh et al, 2004; Zweers et al, 2007). However, the precise function(s) for individual integrin heterodimers during wound healing in vivo remain unclear.
Conditional ablation of integrin β1 in skin causes defective hair follicle invagination into the dermis and decreased proliferation and differentiation of the keratinocytes (Brakebusch et al, 2002; Fassler and Meyer, 1995; Grose et al, 2002; Raghavan et al, 2000). Following injury, β1 null mice exhibit severely impaired re-epithelialization (Grose et al, 2002). However, the roles of each α chain partner required along with β1 to mediate these functions are not clearly understood. Integrin α3β1 knockout mice display a neonatal lethal phenotype including severe epidermal blistering and defects in basement membrane organization and integrin α3β-regulates keratinocyte migration on a laminin 322-rich matrix and deposition of laminin 322 (Choma et al, 2004; DiPersio et al, 1997; Frank and Carter, 2004). However, the precise function of integrin α3β1 during wound healing remains unclear.
Integrin -α5β1 null mice exhibit an-embryonic lethal phenotype and the functional role of α5β1 during cutaneous wound healing remains unknown (Yang et al, 1993). Surprisingly, skin specific knockout of either α2β1 or α6β4 had no effect on the re-epithelialization process during cutaneous wound healing (Grenache et al, 2007; Parks, 2007; Raymond et al, 2005; Zweers et al, 2007). Similarly, no wound healing defects were observed in αvβ5 knockout mice (Huang et al, 2000a). However, integrin αvβ6 null mice exhibit age-dependent defects in wound healing (AlDahlawi et al, 2006). Taken together, these data suggest that integrins have redundant functions to ensure efficient and prompt re-epithelialization and eliminating -any single integrin heterodimer does not completely impair the process.
We have previously reported that integrin α9β1 expression is elevated from days 1 through 7 post injury and decreases thereafter between days 10 and 14 in epidermal keratinocytes (Singh et al, 2004). The localization of integrin α9β1 to the margins of sealing corneal epithelia sheets just prior to wound closure suggests additional -roles for α9β1 in mediating cell:cell adhesion in closing wounds (Hakkinen et al, 2000; Stepp et al, 2002; Stepp and Zhu, 1997). Although the identity of the ligand(s) for keratinocyte α9β1 is unknown, tenascin-C and EIIIA+FN are prominently expressed beneath the migrating epidermis (Mackie et al, 1988; Singh et al, 2004; Stepp and Zhu, 1997). Upregulation of integrin α9β1 expression during wound healing suggests that it is a critical contributor to the re-epithelialization process. In vitro studies have shown that integrin α9β1 promotes proliferation and migration of colon carcinoma cells on tenascin-C (Chen et al, 2004; Yokosaki et al, 1996; Young et al, 2001). However, analyses of wound keratinocyte α9β1 functions have been hampered by the loss of α9β1 from primary keratinocytes in culture (unpublished) and by the neonatal lethal phenotype of integrin α9β1 knockout mice (Huang et al, 2000b).
To overcome these limitations, we developed a genetic approach using a Cre-loxP recombination system to remove integrin α9β1 from skin and cornea. These integrin α9 knockout mice were generated by crossing mice homozygous for a floxed α9 allele (α9fl/fl) with mice heterozygous for α9 and K14 Cre transgene (α9+/−; K14 Cre+/−). K14–α9 null mice were viable and showed no obvious defects in skin morphology. However, normal cell morphology was altered in both the central and limbal regions of the eye. Following cutaneous injury, inter-follicular epidermal keratinocyte proliferation was decreased in K14–α9 null mice compared to control mice but epidermal migration remained unaffected. The decreased keratinocyte proliferation in K14–α9 null wound negatively influenced the re-epithelialization process thereby affecting the overall quality of re-epithelialization.
RESULTS
Generation of K14#x2013;α9 null mice
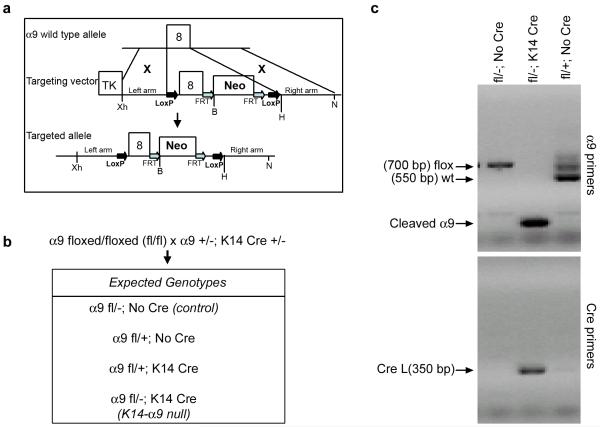
We chose to use a conditional genetic approach to inactivate the α9 gene (Huang et al, 2000b). An integrin α9 floxed targeting vector was designed to delete exon 8 within the α9 gene (Figure 1a). Correctly targeted embryonic stem cells were injected into blastocysts giving rise to two independent lines of chimeric mice. These were back-crossed into C57Bl/6 mice and the neomycin selection marker was removed by crossing with Flpe transgenic mice to generate integrin α9fl/fl mice. The α9fl/fl mice were viable and appeared normal suggesting that insertion of lox P sites did not alter the function of the α9 subunit. To generate conditional knockout of the α9 gene in skin and corneal epithelial cells, we crossed α9fl/fl mice with α9+/−; K14 Cre+/− mice and obtained the expected genotypes (Figure 1b). We confirmed by genomic PCR on tail DNA of adult mice the presence of the floxed and/or wild type alleles, as well as removal of the floxed α9 exon following K14 Cre-mediated recombination (Figure 1c). In some instances, a faint band representing the floxed allele was detected in the tail DNA of K14α9 null mice, but the cleaved α9 was more predominant.
Figure 1. Targeting strategy and molecular analysis of K14-α9 null mice.
(a) Mouse integrin α9 locus, targeting vector and targeted allele. The upper line shows a map of the α9 locus with the exon 8 defined by black boxes. The middle line shows targeting vector. The BamHI (B), XhoI (Xh), HindIII (H), and Not I (N) restriction sites used for generation and analysis of the construct as well as Neo (Neomycin) and the TK (thymidine kinase). (b) Genotypes obtained from crossing α9fl/fl mice with α9 fl/−; K14 Cre+/− mice. (c) PCR analysis of tail genomic DNA, the conditional allele of the α9 gene was detected by α9 primers (top panel) and the presence of Cre by Cre primers (bottom panel). PCR products were resolved by agarose gel electrophoresis and visualized by ethidium bromide staining. Bands corresponding to the wild type (wt) and floxed alleles (floxed) of the α9 gene as well as to the K14-Cre transgene (Cre) were indicated. Sizes of molecular weight markers were indicated in base pairs (bp).
Loss of integrin α9 from epidermis and corneal epithelial cells
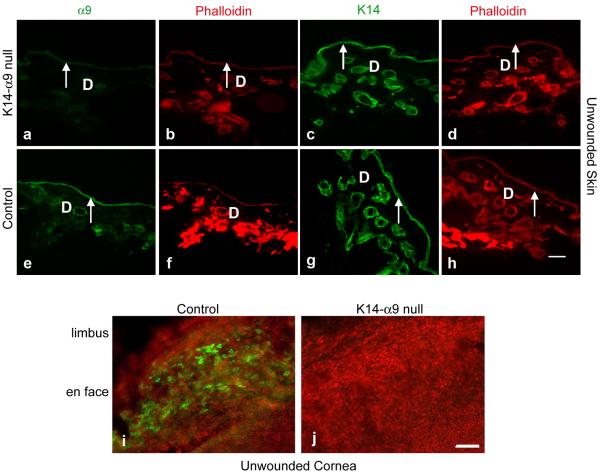
Integrin α9β1 is expressed in the basal layer of keratinocytes in unwounded skin and by a subset of basal cells at the periphery of the cornea in the limbus (Palmer et al, 1993; Singh et al, 2004; Stepp et al, 2002; Stepp and Zhu, 1997). To validate effective knockout of integrin α9 by K14 Cre-mediated recombination in skin and cornea, unwounded skin sections or whole mount corneas isolated either from K14-α9 null or control mice were used. We observed complete loss of α9 staining in basal keratinocytes of unwounded skin of K14-α9 null mice (Figure 2a–h). Similarly, using a whole cornea flat mount immunofluorescent confocal microscopy approach, we confirmed the loss of α9 from corneal epithelial cells in K14-α9 null mice (Figure 2i and j). These observations established complete knockout of α9 in epidermal keratinocytes and in corneal epithelial cells.
Figure 2. Effective knockout of α9 by K14-Cre mediated recombination in skin and cornea.
Unwounded skin (a–h) or cornea (i and j) were harvested either from K14-α9 null or control mice. Harvested skin was oriented with the epidermis on the top and panniculus carnosus below. Unwounded consecutive skin sections of K14-α9 null mice (a–d) or control mice (e–h) were treated either with rabbit anti-α9 (a, b, e and f) or K14 (c, d, g and h), as a marker for keratinocytes. No positive staining was detected for α9 in the epidermis (arrow) of K14-α9 null mice (a) compared to the positive staining observed for α9 in the control mice epidermis (e). Panels b, d, f and h depict phalloidin staining of their corresponding panels a, c, e and g respectively. Using whole mount confocal imaging, in the cornea, we confirm loss of integrin α9 staining at the limbus of cornea in K14-α9 null mice (i) compared to control mice (j). Dermis denoted as `D'. N=3, scale bar: 50 μm in (h) and 40μm in (i and j).
Characterization of uninjured skin and cornea in K14-α9 null mice
Skin
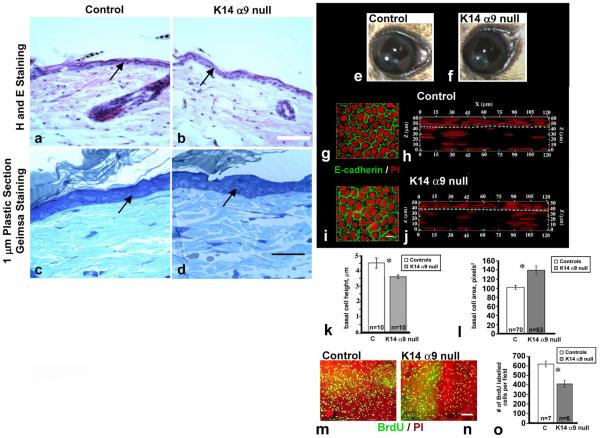
To investigate if conditional knockout of α9 affects normal skin keratinocyte morphology or organization, unwounded skin biopsies isolated from either K14-α9 null or control mice were embedded either in plastic or paraffin. Five μm or one μm sections were prepared and stained with either Haematoxylin and Eosin stain (Figure 3a and b) or Geimsa stain (Figure 3c and d), respectively, and no apparent difference in the organization of keratinocytes or morphology was observed in K14-α9 null skin compared to the control skin. To test whether basal epidermal keratinocytes cell proliferation in vivo was affected in K14-α9 null skin, we performed Ki67 labeling studies. In normal skin, 11–15% of the inter-follicular keratinocyte were labeled with Ki67 in both K14-α9 null and control skin, indicating that resting keratinocyte proliferation is not affected in K14-α9 null mice (data not shown).
Figure 3. Histological differences in cornea but not skin of unwounded K14-α9 null mice compared to controls.
Images from control mice (a, c, e, g, h and m) and K14-α9 null mice (b, d, f, i, j and n) were shown. Five μm paraffin (a and b) or one μm plastic (c and d) embedded sections of unwounded skin were stained with Hematoxylin (H) and Eosin (E) (a and b) or Geimsa stain (c and d). Arrows indicate epidermal keratinocytes. No differences in organization or morphologies of keratinocytes were observed in K14-α9 null skin compared to control skin. No differences were observed in gross images of control and K14-α9 null mouse eyes (e and f). Cross sectional representations were generated from 3-D images of flat mounted corneas by confocal microscopy show that the K14-α9 null corneal epithelium is thinner than the control epithelium (g–j). The aqua dashed lines show the border between the epithelial cells (above dotted line) and the stromal fibroblasts (below dotted line. E-cadherin and PI stained en face flat mounts (g and i) suggest that the K14-α9 null basal cells were flatter and that there were fewer cells per unit area on the corneal surface. The heights of 10 basal cells were measured for both genotypes and show that the K14-α9 null basal cells were flatter than control basal cells (k). Areas were determined from 70 and 63 control and K14-α9 null corneal epithelial basal cells respectively using Image Pro Plus software (g and i). Results confirm that the K14-α9 null basal cells were flatter and more spread so that K14-α9 null mice have ~27% fewer epithelial basal cells on their cornea. BrdU labeling of the mouse corneas were shown as flat mounts (m and n). Control (n=7) and K14-α9 null (n=7) corneas were quantified and presented normalized by area and show fewer (~30%) proliferating cells in the K14-α9 null corneas compared to controls (o), because there are also fewer overall cells on the corneal surface, taken together, these results indicate that the proliferation rates in corneal and skin are similar. N=3, scale bar: 50 μm in (b), 20 μm in (d), 3 μm in h and j, and 50 μm in n (*p < 0.05).
Cornea
On gross examination, no differences were seen in eye size or corneal clarity (Figure 3e, f). Flat mounts of control and K14-α9 null corneas were used to visualize the localization of E-cadherin and nuclei (propidium iodide, PI) and 3-D image reconstructions were generated by confocal microscopy and image analysis. Shown are representative en face (g, i) and cross sectional views of 120 μm × 120 μm sections (h, j) through the entire thickness of control and K14-α9 null mouse corneas, respectively. The cross sections show that control corneas were ~62 μm thick (entire z dimension, panel h) from corneal endothelium to the epithelial apical squames and the corneal epithelial compartment thickness was 14–15 μm and 3–5 cell layers (z above dotted line, panel h). The K14-α9 null corneas were thinner at ~50 μm (entire z dimension, panel j) and their epithelial thickness was 11–13 μm (z dimension, above dotted line, panel j) but there appeared to be similar numbers of cell layers. Further analysis showed that although the height of the control basal cells was 4.5 μm, the K14-α9 null corneal basal cells were significantly shorter at 3.3 μm (Figure 3k). Area measurements of the basal cells showed that the corneal epithelial basal cells in the K14-α9 null mice were significantly larger (~27%) than the control basal cells (Figure 3g, h and l). BrdU labeling results were normalized to the observed area and this indicated that cell proliferation in the K14-α9 null corneas was 33% less than controls (Figure 3o), Since the basal cells in the K14-α9 null corneas are flatter and occupy more space on the corneal surface than do control basal cells, the differences in basal cell size (and number) negate the proliferation difference and indicate that cell proliferation rates are similar in the uninjured cornea and skin of control and K14-α9 null mice.
Decreased keratinocytes proliferation in K14-α9 null mice during cutaneous wound healing
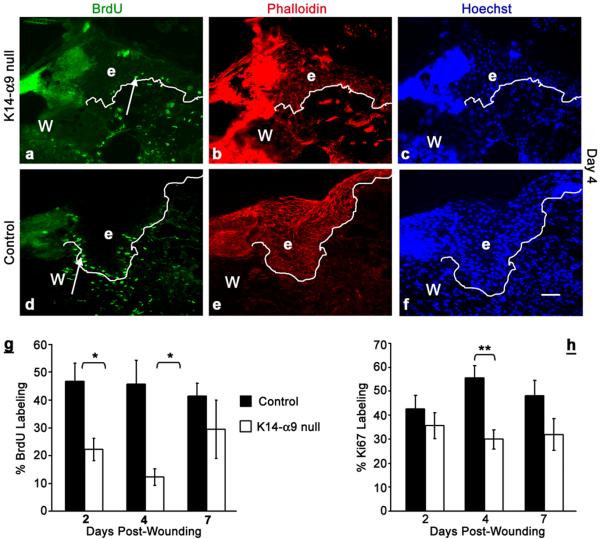
Several studies have shown that following cutaneous injury, keratinocytes proliferate to supply cells to the migrating wound epidermis and yet the mechanisms regulating this are unclear (Martin, 1997; Santoro and Gaudino, 2005). To determine if deletion of α9 from skin affects wound edge keratinocyte proliferation, we used Ki67 labeling and BrdU incorporation, detected by immunostaining. Mice were wounded and injected with BrdU, 4hours prior to harvest, at intervals of 2, 4 and 7 days and incorporation of BrdU in inter-follicular epidermal keratinocytes was determined. We observed a 54% reduction in BrdU incorporation by day 2 and up to 75% reduction by day 4 in inter-follicular keratinocytes of K14-α9 null wound sections compared to control wounds (Figure 4a–f and quantified in g). At day 7, no significant differences in BrdU incorporation were observed. Sections stained for Ki67 antigen at day 2, 4 and 7, post wounding showed no statistically significant difference in Ki67 labeling of wound edge inter-follicular keratinocytes at day 2 and 7. However, 40% decrease in Ki67 labeling was observed by day 4 in K14-α9 null compared to control wounds (Figure 4h). Our data suggest that loss of α9 impairs proliferation of wound edge inter-follicular keratinocytes, prior to re-epithelialization. In addition, inter-follicular keratinocytes residing behind the wound edges at day 2, post wounding, showed a significant decrease in the proliferation in K14-α9 null skin compared to control skin (data not shown). By contrast, no statistically significant differences were observed in the proliferation of keratinocytes within hair follicles in either K14-α9 null or control keratinocytes (data not shown). The apparent discrepancy in Ki67 and BrdU labeling is likely due to the persistent expression of Ki67 in all phases of cell cycle except G0, whereas BrdU labels only S phase cells. Our data suggest a defect in G1-S phase entry leading to delayed proliferation in K14-α9 null wound keratinocytes compared to control wounds.
Figure 4. Decreased proliferation in wound edge keratinocytes of K14-α9 null mice compared to control mice.
Excisional cutaneous wounds were harvested at 4 d post-wounding (a–f). Harvested wounds were oriented with the epidermis on top and panniculus carnosus, below. Wound sections of K14-α9 null mice (a, b and c) or control mice (d, e and f) were treated with anti-BrdU (a and d), phalloidin (b and e) and Hoechst (c and f). Note decreased numbers of BrdU positive (arrow) keratinocytes (marked as `e') in the epithelial tongue over the wound bed (W) in K14-α9 null wounds (panel a) compared to control mice wounds (panel d, and quantitative analysis in g). The white dotted line represents border between epidermis and dermis. Note decreased Ki67 labeling at day 4 in K14-α9 null wounds (h). N=3, scale bar: 50 μm in (f), *p < 0.005, **p < 0.05.
K14-α9 null cutaneous wounds re-epithelialize poorly
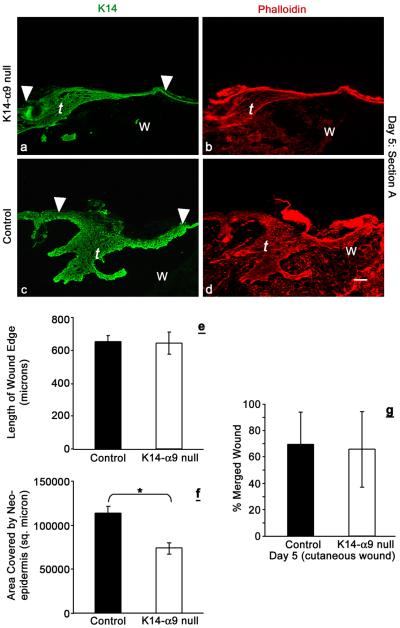
The process of re-epithelialization requires contributions from both keratinocyte migration and proliferation to ensure an efficient and proper closed wound. We hypothesized that decreased inter-follicular keratinocyte proliferation in K14-α9 null wounds would negatively affect the re-epithelialization process. To analyze this, we performed quantitative morphometric analysis of the migrating tongue at day 5 using two parameters, length and area. The migrating tongue length represents the distance traveled by the keratinocytes from the wound edge to facilitate the process of re-epithelialization. By contrast, the area of the wound edge represents the total number of keratinocytes involved in the process of re-epithelialization and is a function of keratinocyte proliferation and cell size. It has been reported earlier that post injury cell size of keratinocytes is altered relative to the uninjured epidermis (Coulombe, 2003). While we also observed this post-injury change, we did not observe any apparent differences in the size of epidermal keratinocytes within K14-α9 null wounds compared to control wounds. Quantitative morphometric analysis enabled us to distinguish between the contributions of proliferation from migration. The length was measured from the wound edge to the tip of the migrating tongue; the area was measured as the region circumscribed by the newly synthesized epidermis over the wound bed from the wound edge. We observed no difference in the length of the migrating tongues of K14-α9 null wounds compared to control wounds (Figure 5 a–d and e). By contrast, we observed a reduction in the area of the newly synthesized epidermis covering the wound bed in K14-α9 null wounds compared to the control wounds (Figure 5a–d and f). These data suggest that migration is not impaired in K14-α9 null cutaneous wounds, while decreased proliferation affects the area of the migrating tongue.
Figure 5. Poor re-epithelialization in K14-α9 null wounds compared to control mice.
Excisional cutaneous wounds were harvested at intervals of 5 d post-wounding. Harvested wounds were oriented with the epidermis on top and panniculus carnosus, below. Lengths and areas of the migrating tongues (t) were visualized by K14 (a and c) or phalloidin (b and d) staining. Graph represents average length of the wound edge (e) and area covered by the neo-epidermis (f). No statistically significant difference was observed in the length of the migrating tongue, whereas reduced area was covered by the tongue of K14-α9 null wound (*p < 0.001). The graph (g) shows the no difference in the distance traveled by the keratinocytes to cover the wound in microns. N=6, scale bar: 50μm.
By day 5 post wounding, merging of the wound edge arising from opposite ends is normally completed. To further evaluate whether migration remains unimpaired in K14-α9 null wounds compared to control wounds, we analyzed 5 day excisional wounds from skin, harvested either from K14-α9 null and control mice. Determining the contribution of keratinocytes to resurface the wound by histological analysis is often problematic due to the fact that wound sites are asymmetrical and it can be difficult to bisect the wound precisely. To ensure that we were comparing the same plane of each wound section, we used a new method to measure the distance traveled by the keratinocytes to close the wound (see Materials and Methods). On quantitative analysis, no statistically significant differences in the distance traveled by the keratinocytes to close the wounds were observed in K14-α9 null compared to control mice (Figure 5g). This finding complements our above-mentioned observation, in which the length of migrating tongue remains unaffected. Thus, the rate of merging of wound edges, per se, is not affected, while the area is affected. The decreased area of new synthesized epidermis of K14-α9 null wounds appears to represent a compromised form of re-epithelialization in which efficient resurfacing is favored over quality and suggests that this process is dictated in part, by integrin α9β1.
Keratinocyte differentiation is disturbed, following cutaneous injury in K14-α9 null mice
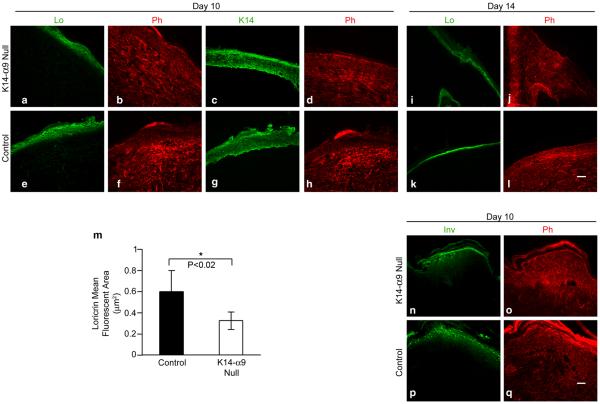
We next hypothesized that a thinner epithelium would delay differentiation and we stained 7, 10 and 14 day cutaneous wound sections either with rabbit anti-loricrin or rabbit anti-involucrin, as terminal differentiation markers. At day 7 and 10 we observed decreased staining for loricrin (Figure 6a–h and data not shown) and involucrin (Figure 6n–q and data not shown) in stratum granulosum of K14-α9 null wounds compared to control wounds. Quantitative analysis showed that the loricrin-positive stained area normalized to the total area of epidermis was significantly reduced in K14-α9 null and control wounds (Figure 6m). By day 14, post wounding, no significant differences in loricrin staining were observed in K14-α9 and control mice (Figure 6i–l). These observations suggest that decreased differentiation may not be a direct outcome of loss of α9 in skin, but could be attributed to decreased proliferation in K14-α9 null mice. This decreased proliferation negatively impacts the differentiation process, however the observed delay in differentiation is corrected with more time elapsed post re-epithelialization.
Figure 6. Terminal differentiation is delayed in K14-α9 null mice compared to control mice.
Excisional cutaneous wounds were harvested at 10 and 14 (a–h, i–l and n–q) d post-wounding. Harvested wounds were oriented with the epidermis on top and panniculus carnosus below. Wound sections of K14-α9 null mice (a–d, i, j, n and o) or control mice (e–h, k, l, p and q) were treated with rabbit anti-loricrin (Lo) antibodies (a, e, i and k), K14 on a consecutive section (c and g for panel a and e), involucrin (Inv) antibodies (n and p) or phalloidin (b, d, f, h, j, l, o and q). Note reduced staining for Lo or Inv in the stratum granulosum layer of epidermis of K14-α9 null and control wound. Graph (m) shows reduced mean fluorescent area covered with Lo staining normalized to K14 staining in K14-α9 null compared to control wounds (*p< 0.02). N=3, scale bar: 50 μm.
DISCUSSION
Prompt re-epithelialization of wounds is critical to restoration of an effective barrier to prevent wound infection and dehydration. Epidermal keratinocytes execute efficient re-epithelialization by integrating complex cellular processes including adhesion, migration, proliferation and differentiation. Integrins, cell surface receptors for extracellular matrix proteins, are known to participate in all of these cellular functions, in vitro (Hynes, 2002; Hynes, 2004; Sheppard, 1996; Sheppard, 2000). Yet, the impact of specific β1 integrin heterodimers on the re-epithelialization process during wound healing remains unclear. Using a LoxP– Cre recombinase system to introduce an epidermal-specific conditional deletion of the α9 gene, we evaluated the functional role of epidermal integrin α9β1 during epidermal wound healing. We report that deletion of the α9β1 gene reduces normal keratinocyte proliferation during re-epithelialization in healing cutaneous wounds.
Loss of α9β1 integrin disrupts squamous epithelial tissue homeostasis in the unwounded cornea but not in skin
The lack of any apparent difference in epidermal proliferation in unwounded skin in K14-α9 integrin null mice suggested that α9β1 was not required for normal epidermal development. Not surprisingly, the phenotypes of the uninjured skin observed with our K14-α9β1 null mice were significantly less pronounced than those observed in skin specific knockout of the entire β1 integrin family (Brakebusch et al, 2002; Grose et al, 2002; Raghavan et al, 2000). The epithelial cells of control and K14-α9 null cornea and skin had similar proliferation indices. Whereas the loss of integrin α9β1 caused no apparent difference in the morphologically or differentiation of epidermal keratinocytes in quiescent skin, unwounded corneal epithelium was thinner and the basal cells flatter than those of control corneas. The overall corneal thickness was also impacted by the loss of α9β1. There was no evidence of inflammation or neo-vascularization suggesting that the corneal epithelium was adherent and stable. Studies by Chung and colleagues (1992) showed that the corneal epithelium differentiates after birth in rodents prior to eyelid opening at 2 weeks. The corneal epithelium, like the epidermis, is initially two cell layers thick with flattened basal cells covered by a periderm layer (Stepp et al, 1995). Between days 10 and 14 after birth, the basal cells proliferate and corneal epithelial thickness increases from 2 to 3–5 layers of cells. At the time of eyelid opening, the corneal epithelial basal cells transition from flattened to cuboidal to columnar (Chung et al, 1992). It is unclear why these changes in cell morphology occur but they have been postulated to be induced by the differentiation of the corneal epithelial basement membrane (Rodrigues et al, 1987). Although α3β1 integrin and laminin 332 are both expressed before birth by the corneal epithelial cells (Stepp et al, 1995; Stepp, 1999), integrin α6β4 is first expressed after birth beginning between days 10 and 14. Interestingly, integrin α9β1 is expressed by all the basal cells on the mouse cornea beginning at 10 days after birth; between day 10 and day 21, as the ocular surface matures, integrin α9β1 becomes restricted to a subpopulation of basal cells at the limbus (Stepp et al, 1995) where it is retained in cells that have been shown to be early transient amplifying cells (Pajoohesh-Ganji et al, 2005). The basal cells of the K14-α9 null mouse appear to have not undergone the cell shape and differentiation changes typical of a fully mature ocular surface suggesting that integrin α9 is required for those important events.
The stem cells that maintain the ocular surface express α9 integrin as they leave their niche (Pajoohesh-Ganji et al, 2006). The expression of α9β1 in the eTA cells has been hypothesized to be required for departure of stem cell progeny from the niche to repopulate the ocular surface during normal tissue homeostasis and turnover (Pajoohesh-Ganji and Stepp, 2005). Thus the reduced numbers of cells covering the cornea in the α9β1 null mouse could also result from immigration of fewer eTA cells to the cornea from the limbus. We found no evidence for corneal epithelial stem cell deficiency on the unwounded corneas from the K14-α9 null mice (unpublished data). It remains to be determined whether inflicting wounds in the adult corneas, in vivo, resulted in α9β1-dependent stem cell deficiency.
Lack of integrin α9 in epidermal keratinocytes reduces proliferation following injury
Despite the lack of an apparent difference in epidermal proliferation in normal, unwounded skin of K14-α9 null mice, following injury, we observed a markedly reduced level of proliferation in keratinocytes residing just behind the pioneering migrating cells of the neo-epidermis in K14-α9 null mice. Conditional knock-out of β1 integrins in epidermal keratinocytes resulted in reduced proliferation in isolated keratinocytes (Fassler and Meyer, 1995; Grose et al, 2002; Raghavan et al, 2000). We did not observe any compensatory upregulation of other β1 integrins, including α3β1 in the keratinocytes in K14-α9 null mice (data not shown).
Our data from studies on -K14-α9 null mice are distinct from data obtained with K5-β1 null mice. No difference in the percentage of BrdU positive interfollicular epidermal keratinocytes at 1 or 5 days after wounding was observed in K5-β1 null mice (Grose et al, 2002). This discrepancy in wound keratinocytes proliferation could be due to the use of different keratin promoters to mediate excision of either integrin α9 (K14 promoter) or β1 (K5 promoter) from keratinocytes. Others have reaffirmed the role of β1-family integrins in keratinocyte proliferation. By grafting α3β1 integrin null skin onto the back of nude mice, Conti and colleagues reported that integrin α3β1 is the β1-family integrin that- regulates follicular proliferation (Conti et al, 2003). Because hair follicles are a source of keratinocytes during wound healing, our observations suggest that the decreased proliferation observed in the wound keratinocytes in the K14-α9 null skin would likely have been more pronounced were it not for the contribution of follicular keratinocytes for which proliferation was unaffected by α9β1 deletion. Taken together with our data, current research suggests that in addition to α9β1 integrin there are other integrins within the β1 family that regulate inter-follicular epidermal and corneal epithelial cell proliferation. Growth factors, such as keratinocytes growth factor (KGF), along with integrins, are critical regulators of wound epidermal proliferation (Werner et al, 1994). Our results support a mechanism in which integrin α9β1 exerts overall regulatory control to permit growth factor-mediated proliferation to proceed optimally.
K14-α9 null cutaneous wounds re-epithelialize poorly
Although we observed a significant decrease in keratinocyte proliferation in healing wounds, the rates of re-epithelialization remained identical in K14-α9 null wounds compared to control wounds. Interestingly, the length of the migrating keratinocyte “tongue” was comparable in either genotype while the total area in a wound section of the “neo epidermis” was significantly reduced in K14-α9 null wounds compared to control wounds. This difference in area, manifested as diminished thickness in the conditional null, is most likely attributable to the reduced numbers of migrating keratinocytes, as a downstream consequence of the diminished proliferation observed in the K14-α9 null mice. One outcome of this decreased epidermal thickness is a reduction in the “quality” of re-epithelialization. We speculate that the importance of closing the wound promptly takes precedence over other functions such as differentiation; the mutant keratinocytes compensate for decreased proliferation by reducing epidermal thickness to facilitate prompt wound coverage. One consequence of the diminished proliferation and epidermal thickness that we observed is that terminal differentiation is delayed in K14-α9 null wounds compared to control mice. Rather than being a direct outcome of the loss of integrin α9, we speculate that these results from delayed or poor wound healing. Wounding activates keratinocytes and forces them to leave their normal differentiation program and to undergo proliferation and migration to heal the wound (Coulombe, 2003). In our system, using K14-α9 null mice, we speculate that keratinocytes remain in this activated state for longer intervals resulting in a delayed differentiation program. However, differentiation is not completely impaired and eventually keratinocytes emerge from the “activated” state and differentiate normally. Several β1 integrins have been shown to be crucial for keratinocyte migration, both in vitro and in vivo, and integrin α9β1 has been reported to promote migration through a novel mechanism, in vitro (Chen et al, 2004; Choma et al, 2004; Frank and Carter, 2004; Grose et al, 2002; Raghavan et al, 2000; Young et al, 2001). Because we observed no significant differences in the rate of migration of wound edge keratinocytes in K14-α9 null wounds, we speculate that other integrins subserve this regulatory function. We find that α9β1 levels fall precipitously and permanently when epidermal or corneal keratinocytes are explanted into culture. To date, we have been unable to prevent this loss of α9β1 by a variety of culture techniques. Neither have we been able to restore expression by reconstituting cultured keratinoctyes into skin, in vitro (unpublished data). Further testing of the mechanisms through which α9β1 regulates keratinocyte proliferation and migration awaits stable transduction of α9β1 into primary keratinocytes.
Our observations indicate that while integrin α9β1 is required for normal differentiation of the corneal epithelium, it is not required for normal development of the epidermis. However, following injury to skin, integrin α9β1 is required for optimal keratinocyte proliferation and in the absence of integrin α9β1 a compromised form of re-epithelialization occurs causing a temporary delay in differentiation. Our data provide important clues about the functions of α9β1 integrin in both the differentiating corneal epithelium and in healing cutaneous wounds.
MATERIALS AND METHODS
Integrin α9 conditional knockout mice
Integrin α9 genomic DNA was amplified by three PCR reactions from mouse 129/SVJae genomic DNA. Recombinant homology sequences left arm, right arm and exon 8 are from three primer pairs (For Sequences -see supplementary Table I). Three PCR products were subcloned into the pJB1 (Chen et al, 2006) vector to generate the construct shown in Figure 1A. The targeting construct was linearized with NotI and electroporated into RF8 embryonic stem (ES) cells (provided by Robert V. Farese, Gladstone Institute of Cardiovascular Disease). Genomic DNA isolated from 400 G418-resistant ES cell colonies was analyzed by Southern blotting. Two correctly targeted ES cell clones were identified and injected into blastocysts to generate chimeras and giving rise to two independent lines. Chimeras were mated to C57BL/6 females to obtain germline transmission. Neo selection markers were removed by recombinant between two FRT sites in Flpe transgenic mice to generate integrin α9fl/fl mice. Offspring obtained from mating integrin α9fl/fl mice with α9+/−; K14 Cre+/− mice were screened for the presence of the floxed, wt allele and/or Cre by PCR analysis of tail DNA, with α9 and Cre primers (For sequences see supplementary Table I).
Wounds
Punch biopsies (4 mm diameter) were made in the flanks of female adult mice (~5–6 weeks old), as described previously (Brown et al, 1993; Brown et al, 1992). Wounds were harvested at various intervals, embedded in OCT (Electron Microscopy Sciences, Washington, PA) and frozen immediately. In some instances, tissues were fixed immediately after biopsies either in 4% paraformaldehyde and transferred to 70% ethanol or fixed in K2 buffer (Brown et al, 1993; Brown et al, 1992) and transferred to sodium cacodylate buffer. All procedures for animal care and handling had the approval of the relevant Institutional Animal Care and Use Committee.
Immunohistochemistry
Cryostat sections from mouse (10 μm) were placed on poly-lysine coated slides and rinsed in PBS to remove OCT. Sections treated with either rabbit anti-Ki67 antigen (Vector labs) or sheep anti-BrdU antibodies (Bio design) were post fixed in 3.7% formaldehyde. Unfixed sections were used for rabbit anti-loricrin (Covance, CA), rabbit anti-involucrin (Covance, CA) rabbit anti-α9 (Stepp and Zhu, 1997), and rabbit anti-K14 antibodies (Covance, CA). Tissue sections were blocked with 5% nonfat milk, 10% heat-inactivated goat serum and 0.02 % Tween 20 in PBS for 1 hour followed successively by diluted primary antibodies, wash buffer (0.02 % Tween 20 in PBS), appropriate fluorescent secondary IgG in a solution that included phalloidin Alexa 594 (Molecular Probes, Eugene, OR) and Hoechst. Tissues pre-fixed in 4% para formaldehyde and sectioned 5 μm thick, were used for H and E staining (services were provided by Mass Histology, Worchester, MA). Tissue for 1 μm sections were fixed in K2 buffer embedded in plastic, sectioned and stained with Geimsa staining. Photographs were taken under either bright field or epi-fluorescence illumination on a Nikon Eclipse 80i using a SPOT camera (Diagnostic Instruments, Sterling Heights, MI), SPOT and Metaview software were used for quantitative analysis.
Immunofluorescent labeling and confocal microscopy
Flat mounts of whole corneas were processed and used as described previously (Pajoohesh-Ganji et al, 2004). The rat anti-mouse E-cadherin monoclonal ECCD-2 (Zymed; now marketed as Invitrogen 13–1900) was used. Images were acquired using a BioRad confocal microscope and 3-D images were prepared and analyzed using the 3-D constructor module 5.1 of Image Pro Plus version 6.2 from Media Cybernetics. One μm thick sections were acquired throughout the cornea and corneas typically are 40–60 μm thick. To measure the height of the basal cell nuclei, 10 individual nuclei were visualized in sequential layers starting at the basal aspect at the basement membrane zone; p values were less than 0.05 using the two-tailed student's t-test.
BrdU labeling
Mice were injected with BrdU intraperitoneally at a concentration of 50 mgs/ kg body wt of mice, 4 hours prior to harvest. For detection of BrdU, cryostat sections (10 μm) were placed on polylysine coated slides and rinsed in PBS to remove OCT. Tissues were fixed in 3.7% formaldehyde for 10 min, washed in wash buffer for 10 min and permeabilized with 0.5 % Triton-X for 5 min, followed by wash buffer for 10 min. Tissue sections were blocked with 5% nonfat milk in 0.02 % Tween 20 in PBS, and subjected to immunostaining (as described above).
Quantitative analysis of proliferation
For corneas, eyes were fixed in methanol, corneas dissected away from the rest of the eye, and BrdU labeling was performed using Roche's in situ Cell Proliferation kit with modifications as described in (Pajoohesh-Ganji et al, 2006). Six control and seven conditional K14-α9 null mouse corneas were assessed with 4 fields quantified per cornea. Therefore, no fewer than 3 mice were used for each analysis. Statistical significance was determined using the student t-test and p values are less than 0.05.
Skin proliferation was analyzed using two different markers, Ki67 or BrdU incorporation. In each field either Ki67 or BrdU positive keratinocytes were counted in the basal layer and a layer above it within a fixed area marked by a box, followed by counting the total number of nuclei in the same area either manually or with Metaview software. For each section, multiple fields were counted and the same area was used for every field and section. The percentage ratio of Ki67 or BrdU positive/total number of nuclei was calculated and plotted on the graph. Statistical analysis was determined using Student t-test.
Wound closure analysis
Harvested wounds oriented with the stratum corneum on top and panniculus carnosus at the bottom were sectioned vertically (10 μm thick). Each section was numbered and the first section showing the wound edges un-merged was marked as (A). As the wound was sectioned deeper, the first section observed with two wound edges merged was marked as (B). Both section A and B were stained with rabbit anti-K14 antibody to confirm unmerged or merged wound edges, respectively. To calculate the % wound closure, the section number with wound edge merged (B) minus section number with the wound edges un-merged (A), was determined yielding the total number of sections with no merged wound edges (NR). This number was multiplied by 10 (as each section is 10 μm thick). The resulting number (R) is the total distance with no wound edge merged in μm. Because the initial wound was 4000 μm in diameter, subtracting R from 4000 μm gives the total distance migrated by the keratinocytes to close the wound (M). The % average M values were plotted on the graph.
Area and length measurements
Section `A' (as described above) was used for epithelial tongue area and length measurements. The method used as described previously (Tscharntke et al, 2007) using Spot imaging analysis software. Statistical analysis was determined using student t-test.
Quantitative analysis of Differentiation
Images of 10 day wound sections from either K14-α9 null or control wounds stained for loricrin and consecutive section for K14 were used to calculate the mean fluorescent area. Total area covered by loricrin staining was normalized to total area of epidermis (K14 stained sections were used to determine the total area of the epidermis). Area was calculated using Image Pro Analysis software. Student's t-test was used for statistical analysis.
Supplementary Material
ACKNOWLEGDEMENTS
This work was supported by NIH GM-56442 (L. V.D.W.), NIH EYO 08512 and 13559 (M.A.S), NIH HL-64353, HL53949, HL-66600 (D.S.), and AHA #0425034Y (C.C.). We thank Dr. Janice Nagy and Ms. Ellie Manseau (Beth Israel Deaconess Medical Center, Boston) for preparation of one micron sections. We are grateful to Debbie Moran for her care in the preparation of this manuscript.
Abbreviations Used
- ECM
extracellular matrix
- VCAM-1
vascular cell adhesion molecule-1
- ADAM
disintegrin and metalloprotease
- FN
fibronectin
- K14
Keratin14
- ES
embryonic stem
- eTA
early transit amplifying
Footnotes
CONFLICT OF INTEREST The authors declare no conflict of interest.
References
- AlDahlawi S, Eslami A, Hakkinen L, Larjava HS. The αvβ6 integrin plays a role in compromised epidermal wound healing. Wound Repair Regen. 2006;14:289–297. doi: 10.1111/j.1743-6109.2006.00123.x. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Fillatreau S, Potocnik AJ, et al. β1 integrin is not essential for hematopoiesis but is necessary for the T cell-dependent IgM antibody response. Immunity. 2002;16:465–477. doi: 10.1016/s1074-7613(02)00281-9. [DOI] [PubMed] [Google Scholar]
- Brown LF, Dubin D, Lavigne L, Logan B, Dvorak HF, Van De Water L. Macrophages and fibroblasts express embryonic fibronectins during cutaneous wound healing. Am J Pathol. 1993;142:793–801. [PMC free article] [PubMed] [Google Scholar]
- Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, et al. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176:1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Huang X, Sheppard D. ADAM33 is not essential for growth and development and does not modulate allergic asthma in mice. Mol Cell Biol. 2006;26:6950–6956. doi: 10.1128/MCB.00646-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Young BA, Coleman CS, Pegg AE, Sheppard D. Spermidine/spermine N1-acetyltransferase specifically binds to the integrin α9 subunit cytoplasmic domain and enhances cell migration. J Cell Biol. 2004;167:161–170. doi: 10.1083/jcb.200312166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choma DP, Pumiglia K, DiPersio CM. Integrin α3β1 directs the stabilization of a polarized lamellipodium in epithelial cells through activation of Rac1. J Cell Sci. 2004;117:3947–3959. doi: 10.1242/jcs.01251. [DOI] [PubMed] [Google Scholar]
- Chung EH, Bukusoglu G, Zieske JD. Localization of corneal epithelial stem cells in the developing rat. Invest Ophthalmol Vis Sci. 1992;33:2199–206. [PubMed] [Google Scholar]
- Conti FJ, Rudling RJ, Robson A, Hodivala-Dilke KM. α3β1-integrin regulates hair follicle but not interfollicular morphogenesis in adult epidermis. J Cell Sci. 2003;116:2737–2747. doi: 10.1242/jcs.00475. [DOI] [PubMed] [Google Scholar]
- Coulombe PA. Wound epithelialization: accelerating the pace of discovery. J Invest Dermatol. 2003;121:219–230. doi: 10.1046/j.1523-1747.2003.12387.x. [DOI] [PubMed] [Google Scholar]
- DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. α3β1 Integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto K, Puzon-McLaughlin W, Sheppard D, Sehara-Fujisawa A, Zhang XP, Takada Y. RGD-independent binding of integrin alpha9beta1 to the ADAM-12 and -15 disintegrin domains mediates cell-cell interaction. J Biol Chem. 2000;275:34922–34930. doi: 10.1074/jbc.M001953200. [DOI] [PubMed] [Google Scholar]
- Fassler R, Meyer M. Consequences of lack of β1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Frank DE, Carter WG. Laminin 5 deposition regulates keratinocyte polarization and persistent migration. J Cell Sci. 2004;117:1351–1363. doi: 10.1242/jcs.01003. [DOI] [PubMed] [Google Scholar]
- Grenache DG, Zhang Z, Wells LE, Santoro SA, Davidson JM, Zutter MM. Wound healing in the α2β1 integrin-deficient mouse: altered keratinocyte biology and dysregulated matrix metalloproteinase expression. J Invest Dermatol. 2007;127:455–466. doi: 10.1038/sj.jid.5700611. [DOI] [PubMed] [Google Scholar]
- Grose R, Hutter C, Bloch W, et al. A crucial role of β1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development. 2002;129:2303–2315. doi: 10.1242/dev.129.9.2303. [DOI] [PubMed] [Google Scholar]
- Hakkinen L, Hildebrand HC, Berndt A, Kosmehl H, Larjava H. Immunolocalization of tenascin-C, α9 integrin subunit, and αvβ6 integrin during wound healing in human oral mucosa. J Histochem Cytochem. 2000;48:985–998. doi: 10.1177/002215540004800712. [DOI] [PubMed] [Google Scholar]
- Hertle MD, Adams JC, Watt FM. Integrin expression during human epidermal development in vivo and in vitro. Development. 1991;112:193–206. doi: 10.1242/dev.112.1.193. [DOI] [PubMed] [Google Scholar]
- Huang X, Griffiths M, Wu J, Farese RV, Jr, Sheppard D. Normal development, wound healing, and adenovirus susceptibility in β5-deficient mice. Mol Cell Biol. 2000a;20:755–759. doi: 10.1128/mcb.20.3.755-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Jr, et al. Fatal bilateral chylothorax in mice lacking the integrin α9β1. Mol Cell Biol. 2000b;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Hynes RO. The emergence of integrins: a personal and historical perspective. Matrix Biol. 2004;23:333–340. doi: 10.1016/j.matbio.2004.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava H, Salo T, Haapasalmi K, Kramer RH, Heino J. Expression of integrins and basement membrane components by wound keratinocytes. J Clin Invest. 1993;92:1425–1435. doi: 10.1172/JCI116719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao YF, Gotwals PJ, Koteliansky VE, Sheppard D, Van De Water L. The EIIIA segment of fibronectin is a ligand for integrins α9β1 and α4β1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J Biol Chem. 2002;277:14467–14474. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- Mackie EJ, Halfter W, Liverani D. Induction of tenascin in healing wounds. J Cell Biol. 1988;107:2757–2767. doi: 10.1083/jcb.107.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Nguyen BP, Ryan MC, Gil SG, Carter WG. Deposition of laminin 5 in epidermal wounds regulates integrin signaling and adhesion. Curr Opin Cell Biol. 2000;12:554–562. doi: 10.1016/s0955-0674(00)00131-9. [DOI] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Ghosh SP, Stepp MA. Regional distribution of α9β1 integrin within the limbus of the mouse ocular surface. Dev Dyn. 2004;230:518–528. doi: 10.1002/dvdy.20050. [DOI] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Pal-Ghosh S, Simmens SJ, Stepp MA. Integrins in slow-cycling corneal epithelial cells at the limbus in the mouse. Stem Cells. 2006;24:1075–1086. doi: 10.1634/stemcells.2005-0382. [DOI] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Stepp MA. In search of markers for the stem cells of the corneal epithelium. Biol Cell. 2005;97:265–276. doi: 10.1042/BC20040114. [DOI] [PubMed] [Google Scholar]
- Palmer EL, Ruegg C, Ferrando R, Pytela R, Sheppard D. Sequence and tissue distribution of the integrin α9 subunit, a novel partner of β1 that is widely distributed in epithelia and muscle. J Cell Biol. 1993;123:1289–1297. doi: 10.1083/jcb.123.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks WC. What is the α2β1 integrin doing in the epidermis? J Invest Dermatol. 2007;127:264–266. doi: 10.1038/sj.jid.5700573. [DOI] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of β1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond K, Kreft M, Janssen H, Calafat J, Sonnenberg A. Keratinocytes display normal proliferation, survival and differentiation in conditional β4-integrin knockout mice. J Cell Sci. 2005;118:1045–1060. doi: 10.1242/jcs.01689. [DOI] [PubMed] [Google Scholar]
- Rodrigues M, Ben-Zvi A, Krachmer J, Schermer A, Sun TT. Suprabasal expression of a 64-kilodalton keratin (no. 3) in developing human corneal epithelium. Differentiation. 1987;34:60–7. doi: 10.1111/j.1432-0436.1987.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res. 2005;304:274–286. doi: 10.1016/j.yexcr.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Sheppard D. In vivo functions of integrins: lessons from null mutations in mice. Matrix Biol. 2000;19:203–209. doi: 10.1016/s0945-053x(00)00065-2. [DOI] [PubMed] [Google Scholar]
- Sheppard D. Epithelial integrins. Bioessays. 1996;18:655–660. doi: 10.1002/bies.950180809. [DOI] [PubMed] [Google Scholar]
- Shinde AV, Bystroff C, Wang C, Vogelezang MG, Vincent PA, Hynes RO, et al. Identification of the peptide sequences within the EIIIA (ED-A) segment of fibronectin that mediate integrin α9β1-dependent cellular activities. J Biol Chem. 2008;283:2858–2870. doi: 10.1074/jbc.M708306200. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Eng J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Singh P, Reimer CL, Peters JH, Stepp MA, Hynes RO, et al. The spatial and temporal expression patterns of integrin α9β1 and one of its ligands, the EIIIA segment of fibronectin, in cutaneous wound healing. J Invest Dermatol. 2004;123:1176–1181. doi: 10.1111/j.0022-202X.2004.23485.x. [DOI] [PubMed] [Google Scholar]
- Stepp MA. α9 and β8 integrin expression correlates with the merger of the developing mouse eyelids. Dev Dyn. 1999;214:216–28. doi: 10.1002/(SICI)1097-0177(199903)214:3<216::AID-AJA5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Gibson HE, Gala PH, et al. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J Cell Sci. 2002;115:4517–4531. doi: 10.1242/jcs.00128. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Zhu L, Sheppard D, Cranfill RL. Localized distribution of alpha 9 integrin in the cornea and changes in expression during corneal epithelial cell differentiation. J Histochem Cytochem. 1995;43:353–62. doi: 10.1177/43.4.7534781. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Zhu L. Upregulation of α9 integrin and tenascin during epithelial regeneration after debridement in the cornea. J Histochem Cytochem. 1997;45:189–201. doi: 10.1177/002215549704500205. [DOI] [PubMed] [Google Scholar]
- Taooka Y, Chen J, Yednock T, Sheppard D. The integrin α9β1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J Cell Biol. 1999;145:413–420. doi: 10.1083/jcb.145.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscharntke M, Pofahl R, Chrostek-Grashoff A, et al. Impaired epidermal wound healing in vivo upon inhibition or deletion of Rac1. J Cell Sci. 2007;120:1480–1490. doi: 10.1242/jcs.03426. [DOI] [PubMed] [Google Scholar]
- Werner S, Smola H, Liao X, Longaker MT, Krieg T, Hofschneider PH, et al. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science. 1994;266:819–822. doi: 10.1126/science.7973639. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in α5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Yokosaki Y, Matsuura N, Sasaki T, et al. The integrin α9β1 binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J Biol Chem. 1999;274:36328–36334. doi: 10.1074/jbc.274.51.36328. [DOI] [PubMed] [Google Scholar]
- Yokosaki Y, Monis H, Chen J, Sheppard D. Differential effects of the integrins α9β1, αvβ3, and αvβ6 on cell proliferative responses to tenascin. Roles of the β subunit extracellular and cytoplasmic domains. J Biol Chem. 1996;271:24144–24150. doi: 10.1074/jbc.271.39.24144. [DOI] [PubMed] [Google Scholar]
- Yokosaki Y, Palmer EL, Prieto AL, Crossin KL, Bourdon MA, Pytela R, et al. The integrin α9β1 mediates cell attachment to a non-RGD site in the third fibronectin type III repeat of tenascin. J Biol Chem. 1994;269:26691–26696. [PubMed] [Google Scholar]
- Young BA, Taooka Y, Liu S, Askins KJ, Yokosaki Y, Thomas SM, et al. The cytoplasmic domain of the integrin α9 subunit requires the adaptor protein paxillin to inhibit cell spreading but promotes cell migration in a paxillin-independent manner. Mol Biol Cell. 2001;12:3214–3225. doi: 10.1091/mbc.12.10.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweers MC, Davidson JM, Pozzi A, et al. Integrin α2β1 is required for regulation of murine wound angiogenesis but is dispensable for reepithelialization. J Invest Dermatol. 2007;127:467–478. doi: 10.1038/sj.jid.5700546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.