Since sepsis has been identified as an arginine deficiency state, there is an ongoing controversial debate about arginine supplementation in the treatment of patients with sepsis. Little is known, however, about the different catabolic and anabolic downstream and upstream products or byproducts in arginine metabolism in the course of sepsis.
Using a clinical chemistry and mass spectrometric approach we analyzed metabolites related to arginine metabolism from plasma samples of polymicrobial infected mice at 6 hours and 24 hours post sepsis induction in comparison with healthy animals. Following ethical approval by the responsible animal welfare committee (Thueringer Landesamt fuer Lebensmittelsicherheit und Verbraucherschutz; TVA 02-10/10), animals (male C57BL/6N mice) were randomized into three groups: healthy controls without any intervention, mice 6 hours post sepsis induction, and mice 24 hours post sepsis induction. Peritonitis in mice was induced as described in more detail for a rat model [1] by injection of stool suspension into the right lower quadrant of the abdomen with a 21-gauge cannula. Blood samples were drawn using direct needle puncture of the right ventricle. EDTA-anticoagulated blood was immediately centrifuged, and plasma was collected and snap-frozen in liquid nitrogen and stored at -80°C until use. Mass spectrometric analysis was performed using the API4000™ LC/MS/MS system (AB SCIEX, Foster City, CA, USA) equipped with an electrospray ionization source. All animals received a standard pellet rodent diet (ssniff, Soest, Germany) and water ad libitum. The pellets contain 33% proteins, 58% carbohydrates and 9% fats. In our translational approach we did not focus on an additional nutritional support. To avoid the influence of sepsis-induced anorexia, however, we limited the observational period to 24 hours.
Our data clearly show that the arginine plasma concentration is substantially decreased in sepsis, which is not caused by lower availability of amino acids glutamine or citrulline (Table 1). The data support the concept that the arginine catabolic pathway outweighs the anabolic pathway, producing for example proline. Increased arginase activity synthesizes ornithine and urea, the latter significantly enhanced in the late phase during sepsis. The increased urea plasma concentration might reflect not only enhanced arginase activity but also a developing state of acute renal injury. Furthermore, ornithine might be catalyzed rapidly by ornithine de carboxy lase to synthesize putrescine, which is the com mitted reaction in the synthesis of polyamines. Further explanation for the observed decrease in concentration of ornithine, however, cannot be given from our present data and remains unclear.
Table 1.
Metabolites from plasma samples of healthy and polymicrobial infected mice
| Metabolite | Healthy controls | 6 hours post sepsis | 24 hours post sepsis |
|---|---|---|---|
| Arginine (μM) | 101.97 ± 8.12 | 78.25 ± 5.10 | 43.65 ± 10.06†‡ |
| Proline (μM) | 65.00 ± 4.98 | 34.79 ± 3.89† | 29.99 ± 5.18† |
| Ornithine (μM) | 55.84 ± 3.41 | 30.04 ± 3.63† | 28.31 ± 4.64† |
| Putrescine (μM) | 0.40 ± 0.03 | 0.19 ± 0.06 | 4.51 ± 1.81*‡ |
| Urea (mg/dl) | 28.08 ± 1.57 | 36.42 ± 2.09 | 55.61 ± 7.05*‡ |
| Glutamine (μM) | 590.40 ± 118.91 | 635.30 ± 107.15 | 548.50 ± 105.21 |
| Citrulline (μM) | 47.67 ± 3.56 | 45.79 ± 7.18 | 50.16 ± 4.96 |
| Acetylornithine (μM) | 2.48 ± 0.15 | 2.65 ± 0.33 | 2.08 ± 0.12 |
| Glutamic acid (μM) | 18.68 ± 3.33 | 14.04 ± 4.30 | 34.38 ± 11.64 |
| Spermidine (μM) | 0.49 ± 0.02 | 0.43 ± 0.06 | 0.08 ± 0.05†‡ |
Data presented as mean ± standard error of the mean. Investigated metabolites from plasma samples of healthy controls (n = 10) and of polymicrobial infected mice 6 hours (n = 10) and 24 hours (n = 10) post sepsis induction. Statistical analysis by one-way analysis of variance (ANOVA) and post-hoc tests using the Scheffé procedure. *Statistically significant difference compared with healthy controls (P ≤ 0.05; ANOVA). †Statistically significant difference compared with healthy controls (P ≤ 0.001; ANOVA). ‡Statistically significant difference between 6 hours and 24 hours post sepsis induction (P ≤ 0.05; ANOVA). Significant data are indicated in bold.
Interestingly, whilst the putrescine plasma concentration was significantly increased, the concentration of spermidine decreased after 24 hours. The increasing putrescine pool might indicate a restricted activity of spermidine synthase resulting in an insufficient converted concentration of spermidine. This finding might be interesting because polyamines exert pleiotropic biological activities, including modulation of cell signaling, cell growth as well as cell apoptosis [2]. Furthermore, it is important to note that administration of polyamines can function as either protective or harmful in lethal experimental sepsis [3].
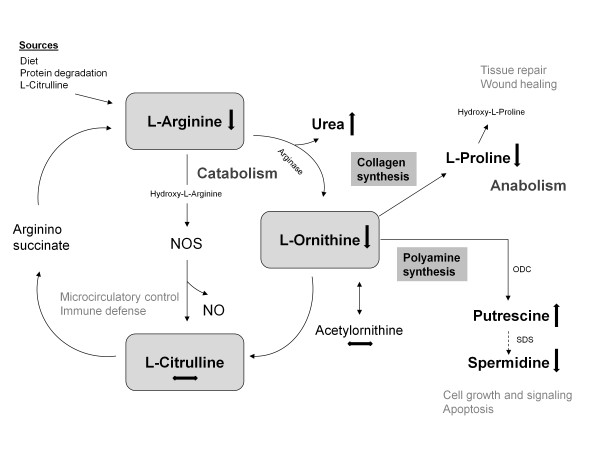
Proline plays various roles in cell metabolism and physiology; for example, in cell growth, wound healing, scavenging oxidants as well as in polyamine and protein syntheses [4]. In our model, the plasma concentration of proline was significantly decreased during sepsis compared with healthy controls. One could speculate that, beside the reduced arginine availability, increasing hydroxy lation of proline to hydroxyproline - an essential step to provide collagen for tissue repair, remodeling or wound healing - contributes to the substantially reducedproline plasma concentration during sepsis even in the early phase of the disease [5]. Consequently, targeting arginine deficiency might be essential in patients withsepsis. Owing to the complexity of arginine metabolism that underlies multiple biochemical and physiologic processes (Figure 1), however, the potential benefits and risks of arginine supplementation have to be carefully evaluated in further studies by a multiparameter approach measuring additional pathway-related products such as polyamines and ornithine, but also the activity of enzymes.
Figure 1.
Scheme of the arginine metabolism and pathway-related products. Simplified overview presenting the L-arginine pathway, including major metabolites and their related pathways and functions. NO, nitric oxide; NOS, nitric oxide synthase; ODC, ornithine decarboxylase; SDS, spermidine synthase.
Abbreviations
EDTA: ethylenediamine tetraacetic acid.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
GPO and MS designed the study and were involved in data analysis and interpretation. MS wrote the first draft of the manuscript. SN and RAC were involved in data acquisition, analysis and interpretation. All authors read and approved the final draft of the manuscript.
Contributor Information
Gordon P Otto, Email: gordon.otto@med.uni-jena.de.
Sophie Neugebauer, Email: sophie.neugebauer@med.uni-jena.de.
Ralf A Claus, Email: ralf.claus@med.uni-jena.de.
Maik Sossdorf, Email: maik.sossdorf@med.uni-jena.de.
Acknowledgements
The authors would like to gratefully thank Diana Schmerler for mass spectrometric measurements and excellent technical assistance.
References
- Gonnert FA, Recknagel P, Seidel M, Jbeily N, Dahlke K, Bockmeyer CL, Winning J, Losche W, Claus RA, Bauer M. Characteristics of clinical sepsis reflected in a reliable and reproducible rodent sepsis model. J Surg Res. 2011;16:e123–134. doi: 10.1016/j.jss.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Igarashi K, Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;16:39–51. doi: 10.1016/j.biocel.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Zhu S, Ashok M, Li J, Li W, Yang H, Wang P, Tracey KJ, Sama AE, Wang H. Spermine protects mice against lethal sepsis partly by attenuating surrogate inflammatory markers. Mol Med. 2009;16:275–282. doi: 10.2119/molmed.2009.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Knabe DA, Li P, Li X, McKnight JR, Satterfield MC, Spencer TE. Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids. 2011;16:1053–1063. doi: 10.1007/s00726-010-0715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddnas F, Koskela M, Koivukangas V, Risteli J, Oikarinen A, Laurila J, Saarnio J, Ala-Kokko T. Markers of collagen synthesis and degradation are increased in serum in severe sepsis: a longitudinal study of 44 patients. Crit Care. 2009;16:R53. doi: 10.1186/cc7780. [DOI] [PMC free article] [PubMed] [Google Scholar]