Abstract
Background
CD70 has been regarded as a novel potential therapeutic target for multiple cancers. In this study, we characterized the expression of the CD70 protein in ovarian carcinomas and assessed its clinical-pathological prognostic value.
Materials and methods
The expression of CD70 in advanced ovarian cancer specimens was assessed by immunohistochemistry. Our results indicated that 16 out of 92 (17.4%) advanced ovarian serous carcinoma tumors showed a high level of CD70 expression. Furthermore, CD70 overexpression was significantly associated with cisplatin-based chemotherapy responses. The high CD70 expression subgroup demonstrated a higher incidence of chemotherapy resistance than the low CD70 subgroup (68.8% versus 25.0%, P = 0.001). Furthermore, univariate analysis conducted on subsets of ovarian carcinoma indicated that high CD70 expression was also associated with decreased survival rates; retained significance was observed on multivariate analysis.
Conclusion
Given the elevated expression of CD70 and its relationship with drug resistance and poor prognosis, our findings suggest that a minor proportion of ovarian carcinomas with CD70 overexpression might be a candidate for the emerging anti-CD70 antibody drug conjugates or therapeutic anti-CD70 antibodies.
Keywords: ovarian carcinoma, CD70, immunohistochemistry, survival, chemotherapy resistance
Introduction
Ovarian carcinoma is one of the leading causes of death in gynecological malignancies worldwide.1 Although systemic cisplatin-based chemotherapy following cytoreductive surgery is administered, ovarian carcinoma patients still have a high relapse rate of approximately 60%–75%, leading to a poor prognosis. Recently, immunotherapies have been proposed as promising strategies for ovarian carcinoma patients, especially for those with chemotherapy resistance.2 A number of immunotherapy agents have activity in ovarian carcinoma, but none of these agents have been unequivocally demonstrated to significantly improve survival; novel therapeutic agents, targets, and strategies are therefore needed to improve the outcome for patients with ovarian carcinoma.
CD70 is a type 2 transmembrane surface antigen belonging to the tumor necrosis factor super family 7.3 CD70 is upregulated in the cancer cells of several malignancies, which could induce cytotoxic effects on B- and T-lymphocytes, and then lead to immune escape.4 Recently, aberrant high CD70 expression in cancer cells has been observed in ovarian carcinomas, and CD70 is regarded as an ideal immunotherapeutic target.5 Ryan et al6 reported that cancerous CD70 was positive in the cancer cells of ovarian cancer tissues, and ovarian cancer cell lines were also demonstrated to be sensitive to the auristatin-based anti-CD70 antibody-drug conjugate, SGN-75, in vitro and in vivo. Aggarwal et al7 used a proteomics approach to show that expression of CD70 was associated with the cisplatin resistance phenotype of ovarian cancer cell lines.
Novel antibody-based therapeutic agents targeting CD70 for tumor elimination have been investigated in early clinical trials.8 It has been proposed that such efficacy is based on various mechanisms such as antibody effector functions, immune enhancement, a blockade of the paracrine growth loop, and the delivery of cytotoxic payloads.9 Therefore, a comprehensive evaluation of CD70 in clinical ovarian samples with cisplatin treatment history and prognosis is warranted. In this present study, we immunohistochemically detected CD70 expression in ovarian carcinoma specimens, and their clinicopathological and prognostic significance was also evaluated.
Materials and methods
Patients and samples
A total of 92 advanced (stage 3 and stage 4) ovarian carcinoma patients who had undergone primary surgery from 2005 to 2008 were included in this study. Histopathology types and cytoreduction levels were assessed according to World Health Organization criteria. Grades and tumor–node–metastasis stages were determined according to the Gynecologic Oncology Group criteria and the International Federation of Gynecology and Obstetrics system, respectively. All the ovarian cancer patients received postoperative cisplatin-based adjuvant chemotherapies following cytoreduction, and the response to chemotherapy was classified into the “sensitive” subgroup when complete response and partial response were obtained, and they were classified into the “resistant” subgroup when disease stabilization or progression were observed. None of patients had received neoadjunctive chemotherapy. Overall survival rates were calculated from the date of histological diagnosis to the date of cancer-caused death or to the date of the last follow-up examination. The study was approved by the ethics committee of Shandong Cancer Hospital and Institute.
Immunohistochemistry
Immunohistochemistry was performed to detect the expression of CD70 protein in ovarian carcinoma specimens, as described previously.10 Briefly, 4 μm of formalin-fixed paraffin-embedded sections were deparaffinized; endogenous peroxidase activity was blocked by using 0.3% hydrogen peroxide. The slides were incubated with 20% normal goat serum for 30 minutes at room temperature, and they were then incubated overnight at 4°C using mouse monoclonal antibodies against CD70 (1:100; Abcam plc, Cambridge, UK). After incubation with the primary antibody, slides were developed using the EnVision™ horseradish peroxidase (HRP) system (DAKO, Glostrum, Denmark), and they were then counterstained with hematoxylin. For the negative controls, the primary antibody was replaced by immunoglobulin G. When more than 10% of the tumor cells demonstrated a moderate to strong staining intensity for CD70, the samples were defined as having a high level of expression; the others were classified as having a low level of expression.
Statistics
The comparisons of five clinicopathological parameters (age, grade, International Federation of Gynecology and Obstetrics stage, achieved cytoreduction level, and cisplatin-based chemotherapy response) between low and high levels of CD70 subgroups were evaluated using the χ2 test or Fisher’s exact test, as appropriate. The association between CD70 expression and chemotherapy response status was assessed using χ2 test and Spearman’s correlation coefficients. Overall survival curves were determined using the Kaplan–Meier method, and the differences in survival rates between the high- and low-CD70 subgroups were compared with a log-rank test. Multivariate analysis included all of the abovementioned clinicopathological features, and CD70 expression status was carried out using the Cox regression model. All statistical analyses were performed using the Statistical Package for the Social Sciences version 16.0 (IBM Corporation, Armonk, NY, USA); P < 0.05 was considered statistically significant.
Results
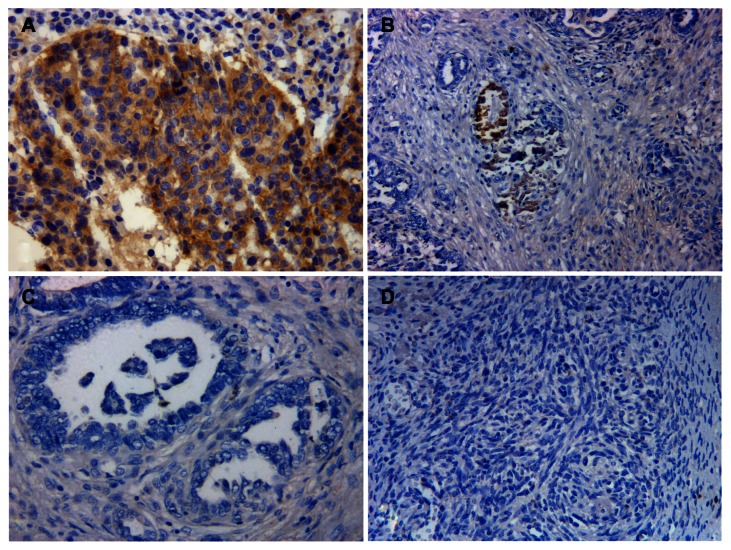
As shown in Figure 1, CD70 staining was accentuated in the tumor cell membrane and in the cytoplasm of ovarian carcinomas with variable intensity. No immunoreactivity was detected in the surrounding stroma. According to the established criteria, 16 out of 92 advanced ovarian carcinoma patients (17.4%) were defined as having high CD70 expression.
Figure 1.
Representative images of CD70 immunostaining in ovarian cancer tissues. (A) high CD70 expression; (B) low CD70 expression having <10% of tumor cells with strong staining intensity; (C) low CD70 expression with totally negative staining; (D) negative control (primary antibody was replaced by immunoglobulin G).
We further evaluated the relationship between CD70 expression and the clinicopathological traits of ovarian carcinomas (Table 1). We found that the “resistance” subgroup had relatively higher frequencies of CD70 expression than the “sensitive” subgroup (68.8% versus 25.0%, respectively; P = 0.001), and no associations with other clinicopathological factors were identified. We found that CD70 overexpression was inversely correlated with cisplatin-based chemotherapy response (Pearson’s correlation coefficient, r = −0.354, P = 0.001).
Table 1.
Correlation between CD70 expression levels with clinicopathological characteristics in ovarian carcinomas
| Total | CD70 expression | P-value | ||
|---|---|---|---|---|
|
|
||||
| Low | High | |||
| Age | ||||
| <51 years | 36 | 30 | 6 | 0.883 |
| ≥51 years | 56 | 46 | 10 | |
| Grade | ||||
| G1 | 16 | 12 | 4 | |
| G2 | 32 | 26 | 6 | 0.572 |
| G3 | 44 | 38 | 6 | |
| FIGO stage | ||||
| 3 | 67 | 52 | 15 | 0.060 |
| 4 | 25 | 24 | 1 | |
| Level of cytoreduction achieved | ||||
| Optimal | 45 | 38 | 7 | 0.649 |
| Suboptimal | 47 | 38 | 9 | |
| Response to cisplatin-based chemotherapy | ||||
| Resistant | 30 | 19 | 11 | 0.001 |
| Sensitive | 62 | 57 | 5 | |
Abbreviation: FIGO, International Federation of Gynecology and Obstetrics.
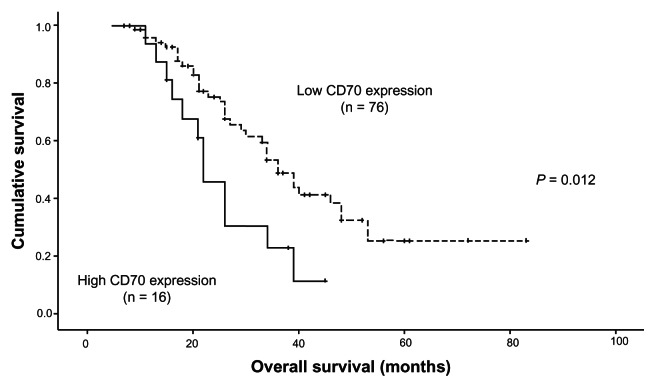
The prognostic significance of CD70 expression was also investigated in this study. Our univariate analysis revealed that ovarian carcinoma patients with high CD70 expression showed a worse overall survival compared to those with low CD70 expression (P = 0.012, Figure 2). All of the clinicopathological features, together with CD70 expression status, were included in a multivariate Cox regression model. As seen Table 2, multivariate analyses revealed that chemotherapy response status and CD70 overexpression levels were independently associated with the poor prognosis of patients with advanced ovarian carcinoma.
Figure 2.
Kaplan–Meier survival curves for overall survival rates of ovarian cancer patients according to CD70 expression.
Note: High CD70 expression subgroup showed a shorter overall survival than the low CD70 expression group (P = 0.012, log-rank test).
Abbreviation: n, number.
Table 2.
Multivariate analysis of overall survival with regard to clinicopathological characteristics and CD70 expression
| Characteristics | Multivariate analysis | |
|---|---|---|
|
|
||
| Hazard ratio (95% CI) | P-value | |
| Age | ||
| (<51 years versus ≥ 51 years) | 0.947 (0.498–1.801) | 0.867 |
| Grade | ||
| (grade 1 versus grade 2 versus grade 3) | 1.155 (0.774–1.723) | 0.480 |
| FIGO stage | ||
| (3 versus 4) | 1.226 (0.566–2.655) | 0.606 |
| Level of cytoreduction achieved (optimal versus suboptimal) | 0.730 (0.405–1.316) | 0.295 |
| Response to cisplatin-based chemotherapy | ||
| (resistant versus sensitive) | 2.164 (1.023–4.578) | 0.043 |
| Level of CD70 expression | ||
| (high versus low) | 3.864 (1.703–8.766) | 0.001 |
Abbreviations: CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics.
Discussion
Despite the progress made in recent years in treating ovarian carcinomas, there is still a considerable proportion of patients demonstrating resistance to the current chemotherapeutic agents.11 Therefore, new prognostic biomarkers and therapeutic targets for ovarian carcinomas are urgently needed.12
Two previous studies have demonstrated that the tumor antigen, CD70, was specifically expressed on neoplastic cells of ovarian carcinoma and not on normal ovarian epithelial cells, making it an attractive target for antitumor drug development; however, the expression levels of CD70 in ovarian carcinoma tissues reported in different studies are not consistent.6,7 In a study by Ryan et al,6 only 15.3% of ovarian carcinoma samples were CD70-positive. Meanwhile, in another study by Aggarwal et al,7 CD70 was expressed in the cancer cells of all ten ovarian cancer tissues at varying levels. Furthermore, the clinicopathological and prognostic significance of CD70 in ovarian carcinomas have not yet been evaluated, and it is still unknown which subgroup of ovarian carcinoma patients would benefit from therapeutic anti-CD70 antibodies or anti-CD70 drug conjugates.
In this study, in a relatively large cohort of ovarian carcinoma specimens, we identified that 17.4% of ovarian carcinomas showed high CD70 expression, which is similar to the results from Ryan et al’s6 study. We further expanded upon the findings from previous studies, which found a positive relationship between CD70 expression and the clinical response to cisplatin-based chemotherapies; we also identified CD70 as a predictor of decreased survival in advanced ovarian carcinoma patients. Aggarwal et al7 demonstrated that CD70 expression was upregulated in a cisplatin-resistant ovarian cancer cell line when compared with its sensitive parent cell line. Most importantly, the study further demonstrated that antibodies against CD70 could inhibit the proliferation of cisplatin-resistant ovarian cancer cells in vitro.7 Our findings, together with other findings, suggest that CD70-targeted therapies might be a treatment choice for advanced ovarian carcinoma (stage 3 and 4), especially for patients who have demonstrated clinical resistance to cisplatin-based combination chemotherapy.
In conclusion, in this study, we found that the high CD70 expression subgroup represented a group of ovarian carcinoma patients that was associated with cisplatin-based chemotherapy resistance and shorter survival times. Since antibodies that target CD70 are currently being explored in early-phase clinical studies,13,14 we propose that future investigations on the effects of CD70-based therapeutic strategies on ovarian cancer tumors are warranted.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.La Vecchia C. Epidemiology of ovarian cancer: a summary review. Eur J Cancer Prev. 2001;10(2):125–129. doi: 10.1097/00008469-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Liu B, Nash J, Runowicz C, Swede H, Stevens R, Li Z. Ovarian cancer immunotherapy: opportunities, progresses and challenges. J Hematol Oncol. 2010;3:7. doi: 10.1186/1756-8722-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boursalian TE, McEarchern JA, Law CL, Grewal IS. Targeting CD70 for human therapeutic use. Adv Exp Med Biol. 2009;647:108–119. doi: 10.1007/978-0-387-89520-8_7. [DOI] [PubMed] [Google Scholar]
- 4.Grewal IS. CD70 as a therapeutic target in human malignancies. Expert Opin Ther Targets. 2008;12(3):341–351. doi: 10.1517/14728222.12.3.341. [DOI] [PubMed] [Google Scholar]
- 5.Junker K, Hindermann W, von Eggeling F, Diegmann J, Haessler K, Schubert J. CD70: a new tumor specific biomarker for renal cell carcinoma. J Urol. 2005;173(6):2150–2153. doi: 10.1097/01.ju.0000158121.49085.ba. [DOI] [PubMed] [Google Scholar]
- 6.Ryan MC, Kostner H, Gordon KA, et al. Targeting pancreatic and ovarian carcinomas using the auristatin-based anti-CD70 antibody-drug conjugate SGN-75. Br J Cancer. 2010;103(5):676–684. doi: 10.1038/sj.bjc.6605816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aggarwal S, He T, Fitzhugh W, et al. Immune modulator CD70 as a potential cisplatin resistance predictive marker in ovarian cancer. Gynecol Oncol. 2009;115(3):430–437. doi: 10.1016/j.ygyno.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 8.Alley SC, Okeley NM, Senter PD. Antibody-drug conjugates: targeted drug delivery for cancer. Curr Opin Chem Biol. 2010;14(4):529–537. doi: 10.1016/j.cbpa.2010.06.170. [DOI] [PubMed] [Google Scholar]
- 9.Law CL, McEarchern JA, Grewal IS. Novel antibody-based therapeutic agents targeting CD70: a potential approach for treating Waldenström’s macroglobulinemia. Clin Lymphoma Myeloma. 2009;9(1):90–93. doi: 10.3816/CLM.2009.n.024. [DOI] [PubMed] [Google Scholar]
- 10.Liu N, Wang X, Sheng X. The clinicopathological characteristics of ‘triple-negative’ epithelial ovarian cancer. J Clin Pathol. 2010;63(3):240–243. doi: 10.1136/jcp.2009.071985. [DOI] [PubMed] [Google Scholar]
- 11.Thigpen T. A rational approach to the management of recurrent or persistent ovarian carcinoma. Clin Obstet Gynecol. 2012;55(1):114–130. doi: 10.1097/GRF.0b013e31824b9bc5. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee S, Kaye S. The role of targeted therapy in ovarian cancer. Eur J Cancer. 2011;47( Suppl 3):S116–S130. doi: 10.1016/S0959-8049(11)70155-1. [DOI] [PubMed] [Google Scholar]
- 13.Oflazoglu E, Stone IJ, Gordon K, et al. Potent anticarcinoma activity of the humanized anti-CD70 antibody h1F6 conjugated to the tubulin inhibitor auristatin via an uncleavable linker. Clin Cancer Res. 2008;14(19):6171–6180. doi: 10.1158/1078-0432.CCR-08-0916. [DOI] [PubMed] [Google Scholar]
- 14.McEarchern JA, Smith LM, McDonagh CF, et al. Preclinical characterization of SGN-70, a humanized antibody directed against CD70. Clin Cancer Res. 2008;14(23):7763–7772. doi: 10.1158/1078-0432.CCR-08-0493. [DOI] [PubMed] [Google Scholar]