Abstract
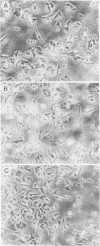
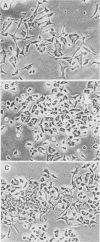
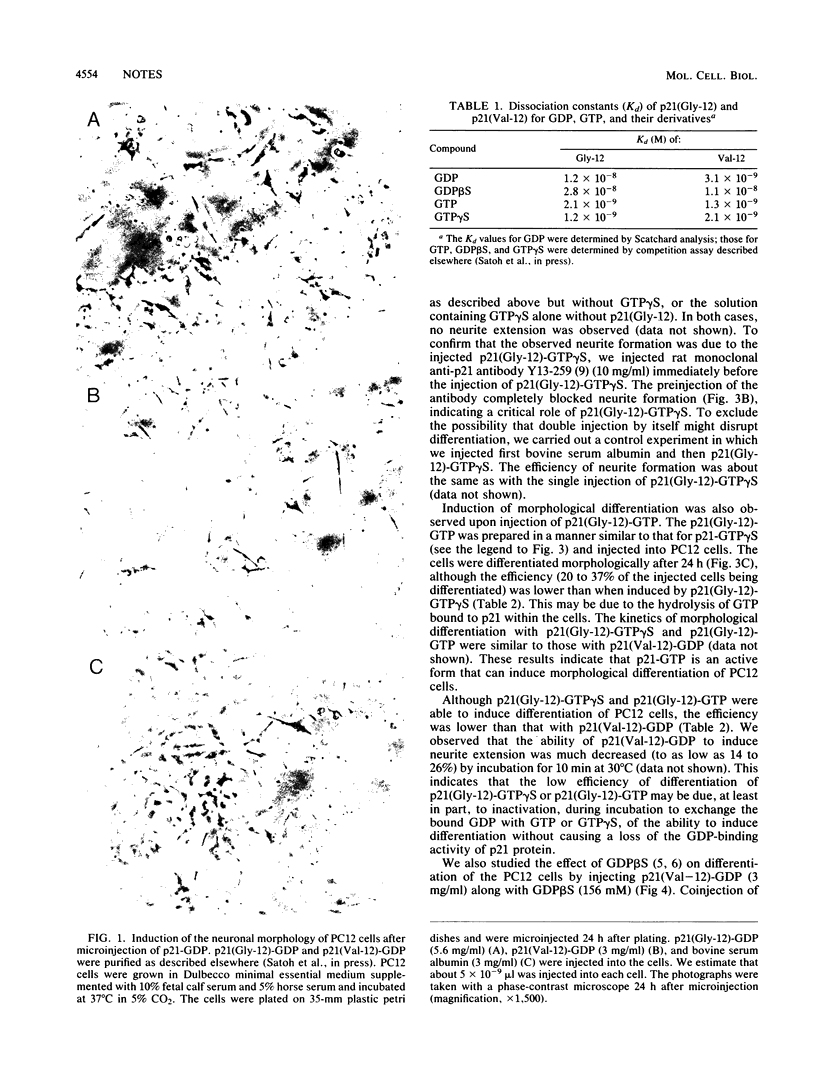
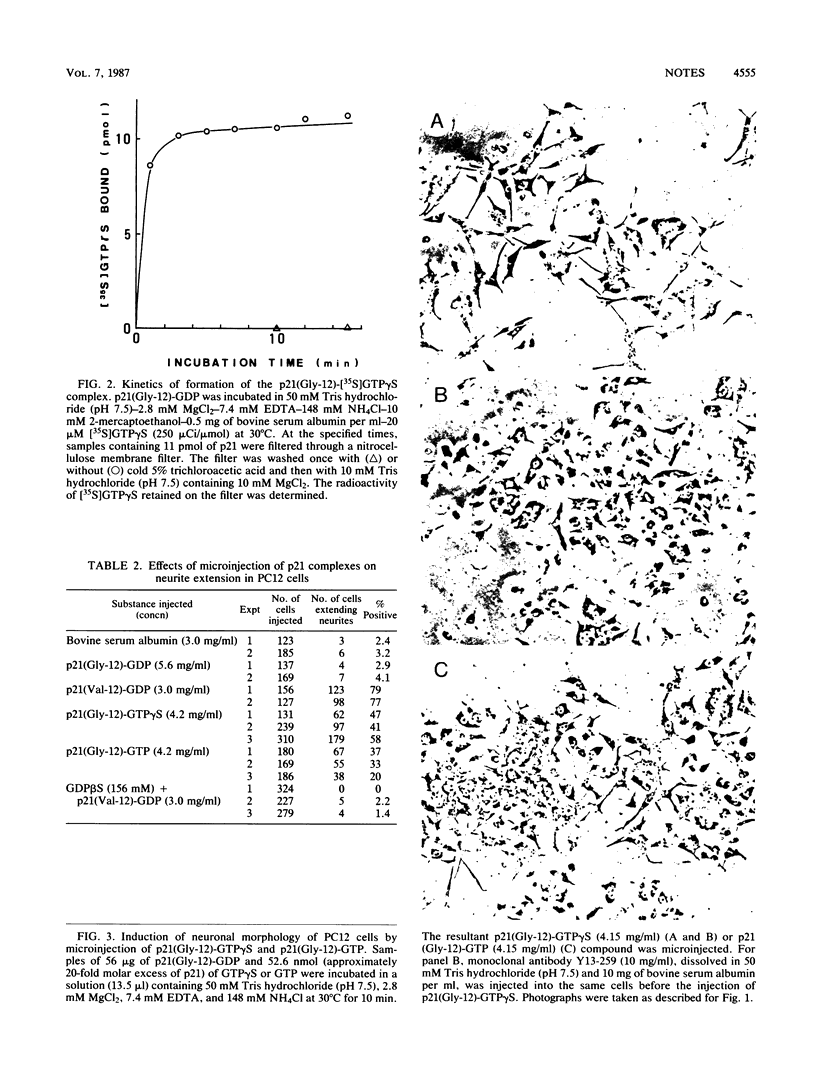
Rat pheochromocytoma (PC12) cells differentiate to neuronal cells in response to nerve growth factor. It has been shown that microinjection of oncogenic but not proto-oncogenic p21 protein induces morphological differentiation in PC12 cells (D. Bar-Sagi and J. R. Feramisco, Cell 42:841-848, 1985). In this paper we describe a recombinant human proto-oncogenic Ha-ras protein which can effectively induce neurite extension of PC12 cells when microinjected as a complex with guanosine-5'-O-(3-thiotriphosphate). The protein was found to be less effective when complexed with GTP. On the other hand, an oncogenic ras protein coinjected with guanosine-5'-O-(2-thiodiphosphate) was entirely inactive. These results indicate that the binary p21-GTP complex, but not the p21-GDP complex, is effective in inducing differentiation in PC12 cells, irrespective of the oncogenic or the proto-oncogenic protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Sagi D., Feramisco J. R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986 Sep 5;233(4768):1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985 Oct;42(3):841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Furth M. E., Scolnick E. M., Lowy D. R. Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus. Nature. 1982 Jun 10;297(5866):479–483. doi: 10.1038/297479a0. [DOI] [PubMed] [Google Scholar]
- DeFeo D., Gonda M. A., Young H. A., Chang E. H., Lowy D. R., Scolnick E. M., Ellis R. W. Analysis of two divergent rat genomic clones homologous to the transforming gene of Harvey murine sarcoma virus. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3328–3332. doi: 10.1073/pnas.78.6.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Fein A. Blockade of visual excitation and adaptation in Limulus photoreceptor by GDP-beta-S. Science. 1986 Jun 20;232(4757):1543–1545. doi: 10.1126/science.3487116. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Clark R., Wong G., Arnheim N., Milley R., McCormick F. Transient reversion of ras oncogene-induced cell transformation by antibodies specific for amino acid 12 of ras protein. Nature. 1985 Apr 18;314(6012):639–642. doi: 10.1038/314639a0. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Gross M., Kamata T., Rosenberg M., Sweet R. W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984 Aug;38(1):109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B., Sigal I. S., Poe M., Scolnick E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero I., Wong H., Pellicer A., Burstein D. E. Activated N-ras gene induces neuronal differentiation of PC12 rat pheochromocytoma cells. J Cell Physiol. 1986 Oct;129(1):71–76. doi: 10.1002/jcp.1041290111. [DOI] [PubMed] [Google Scholar]
- Hagag N., Halegoua S., Viola M. Inhibition of growth factor-induced differentiation of PC12 cells by microinjection of antibody to ras p21. Nature. 1986 Feb 20;319(6055):680–682. doi: 10.1038/319680a0. [DOI] [PubMed] [Google Scholar]
- Kamata T., Feramisco J. R. Epidermal growth factor stimulates guanine nucleotide binding activity and phosphorylation of ras oncogene proteins. Nature. 1984 Jul 12;310(5973):147–150. doi: 10.1038/310147a0. [DOI] [PubMed] [Google Scholar]
- Kaziro Y. The role of guanosine 5'-triphosphate in polypeptide chain elongation. Biochim Biophys Acta. 1978 Sep 21;505(1):95–127. doi: 10.1016/0304-4173(78)90009-5. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Smith M. R., Bekesi E., Manne V., Stacey D. W. Reversal of transformed phenotype by monoclonal antibodies against Ha-ras p21 proteins. Exp Cell Res. 1986 Feb;162(2):363–371. doi: 10.1016/0014-4827(86)90341-1. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Manne V., Bekesi E., Kung H. F. Ha-ras proteins exhibit GTPase activity: point mutations that activate Ha-ras gene products result in decreased GTPase activity. Proc Natl Acad Sci U S A. 1985 Jan;82(2):376–380. doi: 10.1073/pnas.82.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Ko M., Ogura A., Liu D. G., Amano T., Takano T., Ikawa Y. Sarcoma viruses carrying ras oncogenes induce differentiation-associated properties in a neuronal cell line. Nature. 1985 Nov 7;318(6041):73–75. doi: 10.1038/318073a0. [DOI] [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Kung H. F. Transformation of NIH 3T3 cells by microinjection of Ha-ras p21 protein. Nature. 1984 Aug 9;310(5977):508–511. doi: 10.1038/310508a0. [DOI] [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]