Abstract
Purpose of this review
The purpose of this review is to encapsulate our current understanding of the pro-inflammatory cytokines responsible for the inflammation underlying Crohn’s disease and the prospect of using this information to devise therapy for this condition based on inhibition of these cytokines.
Recent Findings
Current research is shedding new light on the role of both Th1 and Th17 responses in the pathogenesis of Crohn’s disease. Initial studies conducted a decade ago highlighted the view that Crohn’s disease inflammation is caused by an IL-12-driven Th1 response which resulted in the generation of IFN-γ which then served as the main inflammatory mediator. In recent years, however, this view has been largely eclipsed by studies, conducted mainly in murine models, showing that a Th17 response is the main cause of Crohn’s disease inflammation through the production of IL-17. Now, a somewhat more balance view is emerging which holds that IFN-γ is still a major pro-inflammatory cytokine in Crohn’s disease although it may arise from both the Th1 and Th17 cell-mediated responses at different phases of the inflammatory process.
Summary
The new findings continue to support the idea that anti-IL-12p40, an antibody that inhibits both the Th1 and Th17 response, is logically the most potent anti-cytokine for the treatment of Crohn’s disease.
Keywords: Crohn’s disease, Th1 Response, Th17 Response, IL-12, IL-23, IFN-γ, IL-17, IL-22, TL1A
Introduction
Perhaps the most decisive advance in our knowledge of the immunopathologic basis of the inflammatory bowel diseases (IBD) has been the elucidation of the inflammatory cytokines driving the two main components IBD, Crohn’s disease (CD) and ulcerative colitis (UC). First because the knowledge already in hand is reasonably complete and second because this knowledge can and is rapidly being harnessed to possible treatment regimens that will allow us to more easily achieve near term disease control. As will become evident in the review of the pro-inflammatory cytokines responsible for CD below, the acquisition of this knowledge has been remarkably enriched by the advent of murine models of mucosal inflammation in the mid-1990’s and, as we approach the end of the second decade of their development and analysis, we find that their usefulness is by no means exhausted [1]. Indeed, in several key instances, insight into the cytokine-basis of IBD originated in the study of murine models and these continue to be the “laboratory” for the development of refinements in our understanding of this area.
Models of Crohn’s disease and the emergence of Th1 mechanisms of disease pathogenesis
With the development of the Th1/Th2 paradigm in the late 1980’s IBD investigators began to seek evidence of where CD falls on the T cell polarization axis. Early studies of patients relevant to this question disclosed that CD patients, but not UC patients, produced elevated amounts of IFN-γ in the inflamed lamina propria, suggesting the presence of a Th1-mediated inflammatory process [2;3]. Somewhat later, studies of two murine models of colitis, the TNBS-induced colitis and the cell transfer colitis, corroborated and extended this view in that in both cases, the colitis was shown to be driven by a cytokine that contained an IL-12p40 chain and that was thus initially identified as IL-12 [4;5]. A number of other murine models were also shown to share this feature and, in addition, increased IL-12 production was shown to characterize the lamina propria cell population of CD patients [6;7]. It was indeed the demonstration of the remarkable reversal of TNBS-colitis by an anti-IL-12p40 that served as a major impetus for the development of a humanized anti-IL-12p40 for evaluation as a therapeutic agent in CD [3]. In due course a clinical trial of this antibody as well as a subsequent clinical trial of a second antibody provided strong initial evidence that anti-IL-12p40 was a viable option for the treatment for CD, with an efficacy on the order of that seen with anti-TNF-α; at the same time, these trials proved that IL-12p40 was in fact a component of a cytokine (thought to be IL-12) that was driving the inflammation of CD [8,9] (See Figure 1).
Figure 1.
Clinical Responses of a Crohn’s Disease Patient Treated with Anti-IL-12p40. In this study a moderate dose of anti-IL-12p40 induced an approximately 50% complete remission rate and a 75% partial remission rate. The efficacy of anti-IL-12p40 can be attributed to the fact that because p40 is a component of both IL-12 and IL-23, it targets both the Th1 and Th17 responses.
The Emergence of Th17
However, the above did not prove to be the end of the story. At about the time the first anti-IL-12p40 antibody was being tested and proven efficacious in humans, a new cytokine, IL-23, made its appearance as a possible driver of CD by virtue of its key role in the development of Th17 T cells mediating murine colitis (see Discussion below). Moreover, since IL-23 and IL-12 are both heterodimers that share the common p40 chain, it became possible that the anti-inflammatory effects of anti-IL-12p40 discovered earlier could, in reality, be due to antagonistic effects on IL-23 (not IL-12) [10].
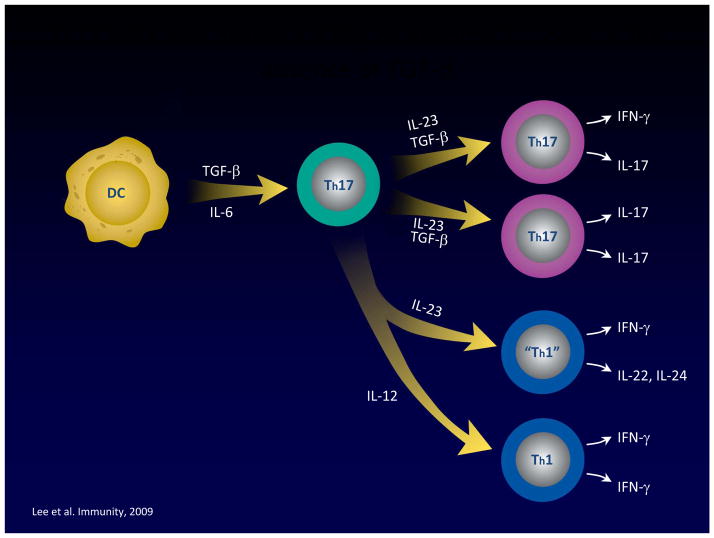
This brings us to discussion of the role of IL-23 and Th17 cells in both murine and human IBD, but before we engage in such discussion, it is important to review certain basic facts about IL-23 and Th17 immunobiology. Whereas IL-12 is a direct inducer of IFN-γ and thus of Th1 T cells, IL-23 is more tangentially (and indirectly) involved in the development of Th17 T cells. The latter cells are in fact initially generated in the absence of IL-23 via the combined activity of TGF-β and IL-6 (or IL-21)[11]. These initial Th17 cells then express an IL-23 receptor and only then come under the influence of IL-23 signaling, which is necessary for the terminal differentiation and persistence of IL-17-producing cells [12]. In addition, Th17 T cells induced by TGF-β and IL-6 in the absence of IL-23 lack the potential to induce inflammation, perhaps because under these circumstances they also produce IL-10, an anti-inflammatory cytokine [13]. Finally, it is important to point out that Th17 development not only gives rise to IL-17-producing T cells, but also to IFN-γ-producing cells and in the absence of TGF-β, both IL-12 and IL-23 suppress IL-17 production and enhance IFN-γ production by cells arising from Th17 differentiation [14] (See Figure 2). An important possible consequence of this is that the subset of IFN-γ producing cells generated during the development of a Th17 response may be necessary for the full expression of the pro-inflammatory potential of this response. This is in fact supported by the finding that anti-IFN-γ administration effectively prevents cell transfer colitis (a murine model thought to be dependent on the Th17 response - see below) as does the absence of T-bet, a transcription factor necessary for IFN-γ production but not IL-17 production [15;16].
Figure 2.
Th17 T cell differentiation results in cells producing IFN-γ. Th17 differentiation leads to cells that produce IFN-γ as well as IL-17; alternatively, Th17 cells may be diverted to mainly IFN-γ producers by IL-12 and IL-23 in environments in which TGF-β concentrations are low. This “Th1-like” component of the Th17 response is probably an important pro-inflammatory component of the Th17 response since anti-IFN-γ administration ameliorates transfer colitis.
The initial studies addressing the possibility that IL-23 and the Th17 response play an important role in gut inflammation were again first conducted in a mouse model, in this case in the cell transfer model in which colitis is induced in immunodeficient mice (RAG-deficient or SCID mice) reconstituted with naïve T cells (CD45RBhigh cells). In the key studies it was shown that RAG-deficient mice also deficient in p19 (i.e., unable to produce IL-23 which is a heterodimer composed of IL-12p40 and p19 chains) did not develop colitis whereas RAG-deficient mice also deficient in p35 (i.e., unable to produce IL-12 which is a heterodimer of IL-12p40 and p35 chains) did develop colitis [17]. This was reflected by the fact that the p19-deficient mice produced greatly reduced amounts of both TNF-α and IFN-γ in their colons, whereas p35-deficient mice produced only moderately reduced amounts of these cytokines. Interestingly, however, systemic inflammation (manifested by splenomegaly and hepatitis) developing in the RAG-deficient mice administered naïve T cells occurs in both p19 and p35-deficient mice, suggesting that systemic inflammation in this model is driven by both IL-12 and IL-23. In addition, whereas p35-deficient mice exhibit highly increased levels of IL-17 in their colons, IL-23p19-deficient mice still exhibit mildly increased levels of IL-17, indicating that IL-17 is down-regulated by the Th1 response and is not entirely under the control of IL-23. In a second study of the cytokines driving cell transfer colitis, inflammation in SCID mice after transfer of antigen-specific T cell lines recognizing a flagellin antigen and producing either IFN-γ or IL-17 was measured [18]. In these studies it was shown that a cell line activated by flagellin and producing IL-17 induced more severe disease than one producing IFN-γ. In addition, anti-IL-23p19 both prevented and treated inflammation induced by cells producing both cytokines, suggesting that the IFN-γ being produced in this model is arising from Th17 cells (under the control of IL-23).
One caveat that needs to be considered in relation to both of these studies arises from recent work which shows that transfer of T cells to IL-23p19-deficient/RAG1-deficient still results in colitis due to a still intact IFN-γ response, provided the mice lack a regulatory T cell response because of an IL-10- or TGF-β-deficiency; furthermore, IL-23p19-deficient mice manifest increased regulatory T cell levels and transfer of T cells into RAG1-deficient/IL-23p19-deficient mice lacking Foxp3 (which cannot develop into regulatory T cells) induces colitis in such mice[19]. These findings indicate that IL-23p19 also contributes to colitis induction by down-regulating regulatory T cell responses and suggests that the lack of colitis development in RAG or SCID recipients deficient in IL-23p19 is not due to the inability of the Th1 response to mediate colitis, but rather to the fact that, in the absence of IL-23p19, the Th1 response is neutralized by a regulatory response. In other words, the Th1 response may indeed contribute to transfer colitis in normal mice mounting a bipartite Th1/Th17 response.
These studies of murine models of inflammation are consonant with studies of CD in that increased production of IL-17 by lamina propria lymphocytes has been shown to occur in CD patients and this production is, as expected, subject to inhibition by the administration of anti-IL-12p40 [6]. More recently, it has been shown that CD patients harbor increased numbers of circulating IL-17- and IFN-γ-producing memory cells which preferentially express CD161(a c-type lectin involved in T cell proliferation) and these cells constitute a high percentage of cells in the gut mucosa [20]. In addition, patients have increased numbers of circulating IL-23R+ T cells which respond to IL-23 with increased production of IL-17, IL-22 (another Th17 cytokine) and IFN-γ, which is further increased by the presence of IL-1β. Finally, these cells express gut homing receptors CCR6 and β7 integrin and are therefore programmed to re-circulate to the lamina propria during an inflammation. These studies thus identify Th17 cells producing both IL-17 and IFN-γ as major players in the CD inflammatory response.
Nevertheless, IFN-γ May Still Be the More Important Effector Cytokine
The above studies in murine models and humans create a persuasive argument that Th17 differentiation (dependent on IL-23) leading to cells producing IL-17 and/or IFN-γ mediate gut inflammation, rather than Th1 differentiation (dependent on IL-12) leading to cells producing only IFN-γ. However, before we can come to this conclusion we need to turn to recent studies of TNBS-colitis, i.e., the murine model of colitis described above which originally was used to identify IL-12-induced Th1 cells as key mediators of inflammation, as well as recent studies of other models of colitis including cell transfer colitis which directly probed the role of IL-17 in colitis. In the TNBS-colitis studies it was shown that, while IL-17 was being produced by T cells in the inflamed tissue (along with IFN-γ), induction of TNBS-colitis in IL-23p19-deficient mice led to a much more intense inflammation than in control mice [21]. Thus, in this model, the presence of a Th17 response was actually associated with a milder form of inflammation. Similarly, deficiency of IL-17 led to increased intensity of dextran sulfate-induced colitis [22]. Finally, and perhaps most importantly, in recent studies of the cell transfer model it was shown that transfer of IL-17-deficient T cells to RAG1-deficient mice led to earlier body weight loss and onset of colitis than transfer of control T cells [23]. Furthermore, such mice manifested higher levels of IFN-γ production and increased expression of other parameters of the Th1 response. The basis of this heightened Th1 response could be traced to the fact that Th1 T cells bear IL-17 receptors and IL-17 signaling via these receptors inhibits T-bet expression, the transcription factor essential for Th1 T cell generation (See Figure 3). This, plus the fact that IL-17 may actually have protective effects on the mucosal epithelium could explain the more severe disease in the deficient mice. These studies suggest that, contrary to the conclusions of the transfer studies described above, the role of the Th17 responses in experimental colitis is, if anything, protective and the tissue destruction accompanying the inflammation is mainly caused by IFN-γ arising from either Th1 cells driven by IL-12 or by Th17 cells supported by IL-23. It also follows that the use of anti-IL-23p19 in the treatment of CD may actually have the contrary effect of intensifying the inflammation, because it eliminates a brake on IFN-γ production.
Figure 3.
IL-17 Inhibits T Cells Producing IFN-γ Via IL-17R. Under conditions in which both T cells producing IFN-g and IL-7 are present, IL-17 signals IFN-γ-producing cells via an IL-17 receptor (IL-17R) and down-regulated IFN-γ production. Thus, in both TNBS-colitis and cell transfer colitis, the absence of a Th17 response actually leads to more severe disease.
A Synthesis: Th1 and Th17 Responses May Subtend Different Phases of Mucosal Inflammation
The above studies showing that Th1 responses may be down-regulated by Th17 responses as well as other studies showing that the reciprocal is also true in that Th1 (and Th2) responses can regulate Th17 responses [24] suggest that the two kinds of responses don’t easily co-exist and may therefore occupy and dominate different phases of the complex inflammation occurring in human CD (as opposed to that in murine models of CD). This idea obtains support from a variant model of TNBS-colitis known as chronic TNBS-colitis that is induced in BALB/c mice (rather than in the SJL/J or B10 used for acute TNBS-colitis) by the weekly intra-rectal administration of relatively low doses of TNBS [25]. The inflammation induced by this regimen is considerably attenuated compared to that in acute TNBS-colitis, but is greatly extended in time. Of particular interest to the present discussion, the cytokine profile characterizing chronic TNBS-colitis is a dynamic one that defines several stages of the inflammation. Initially it consists of a strong but transient “conventional” Th1 response characterized by greatly increased production of both IL-12p40 and IFN-γ which subsides after the first 7 days and is gone by 21 days. This response is then replaced by a Th17 response characterized first by an increase in IL-23p19 production (first noted on day 28), followed quickly by IL-17 production which increases steadily and reaches a plateau which persists for many weeks and finally subside after 70 days. Other cytokines also produced during chronic TNBS-colitis include IL-25, IL-13 and TGF-β which all make their first appearance at 35 days and are involved in the development of fibrosis in this model as well as in its eventual resolution despite continued TNBS administration. This progression of cytokine production is best explained if we assume that: 1) the initial Th1 cytokine first appears first because it requires only dendritic production of IL-12 to be initiated and once initiated it restrains the development of a Th17 response; and 2) as the inflammatory process proceeds, the initial Th1 response wanes, and the cytokines necessary for induction of a Th17 response accumulate, setting the stage for IL-17 production and the actual inhibition of the Th1 response by IL-17, as discussed above. Ultimately, IL-13 and TGF-β produced in association with the Th17 response set in motion a powerful regulatory response that ends the inflammation.
It is attractive to speculate that this progression of cytokine production also occurs in CD inflammation which in this view consists of innumerable inflammatory microenvironments, each moving through a Th1/Th17 cytokine progression. Thus, at any one time the inflammation as a whole consists of localized Th1 and Th17 responses reflecting different stages of a constantly evolving composite inflammation (See Figure 4).
Figure 4.
Crohn’s Disease is a Composite Inflammation Containing Microenvironments in Which Either Th1 or Th17 Responses Dominate. Based on the finding that in chronic TNBS-colitis Th1 and Th17 responses occur sequentially, it is reasonable to postulate that Crohn’s disease is also characterized by evolving inflammatory lesions characterized by sequential dominance of Th1 and Th17 responses. This implies that the mature Crohn’s disease lesion is composed of microenvironments in which one or the other type of response predominate.
What about other pro-Inflammatory Cytokines is Crohn’s disease?
IFN-γ and IL-17 are not, of course, the only cytokines taking part in the CD inflammation. The trio of cytokines “downstream” of IFN-γ and IL-17 (i.e., induced by these cytokines), TNF-α, IL-6 and IL-1β, play pivotal roles as the immediate effectors of inflammation, particularly its tissue destructive aspects. However, they also play upstream roles as well; for example, IL-6 is necessary for initial Th17 differentiation and IL-1β is important in terminal Th17 differentiation; in addition, TNF-α not only enhances IL-12 production, it also plays an anti-apoptotic role, the latter probably accounting for the effectiveness of anti-TNF-α in treating CD.
In addition to these downstream effector cytokines, there are several others that work side by side with IFN-γ and IL-17 in mediating CD inflammation. Two of particular importance are IL-22 and TL1A and we will discuss these briefly to round out our analysis of the cytokines responsible for CD inflammation.
IL-22, IL-17’s “Little Brother”
IL-22, a cytokine generated during the Th17 response along with IL-17A, IL-17F, IL-21 and is considered a member of the IL-10 family of cytokines because it signals through a receptor that contains the IL-10Rβ chain [26; 27]. However, whereas the IL-10 receptor is ubiquitous, the IL-22 receptor is limited to non-hematopoietic cells and IL-22 has no regulatory role (as does IL-10) [28]. IL-22 is increased in both ulcerative colitis and CD which immediately suggests that its activity is not specifically related to either the Th1 or Th17 response [29).
IL-22 plays an ambiguous role in inflammation. Whereas it is clearly a pro-inflammatory cytokine in the context of skin inflammation [30], there are now a number of reports documenting the fact that it has a complex pro- and anti-inflammatory in colonic inflammation. In one study in humans it was shown to target subepithelial myofibroblasts and to induce NF-κB and MAP kinases in these cells, thus accounting for their production of several pro-inflammatory cytokines and matrix metalloproteases [29]. In contrast, in a study of mice with Th2-mediated colitis associated with TCR α-chain deficiency, it was shown that microinjection of IL-22 cDNA induced epithelial cell Stat3 expression and mucus production and thus led to amelioration of the Th2 inflammation; moreover, anti-IL-22 administration intensified DSS-colitis [31].
Two more recent studies have further defined the role of IL-22 in colitis. In one study it was shown that IL-22-deficient T cells induce more severe colitis when transferred to Rag1-deficient/IL-22-deficient recipient mice, indicating that IL-22 ameliorates cell transfer colitis [32]. In addition, IL-22-deficient T cells did not induce more severe colitis in Rag1-deficient/IL-22-sufficient mice because the latter had NK T cells that could produce IL-22 in response to IL-12 or IL-23 stimulation, especially if IL-18 was also present. In a second study it was shown that IL-22 is also secreted by CD11c+ dendritic cells and that such secretion upregulates Stat3 in epithelial cells, as noted earlier [33]. It was also shown that Stat3 regulates the epithelial stress response and wound healing capacity and that mice lacking Stat3 expression in epithelial cells are more susceptible to DSS-colitis. These studies thus indicate that at least one of the protective effects of IL-22 in colitis is through its effects on epithelial cells.
These studies of IL-22 are interesting in their own right as well as by the way they provide additional insight into the nature of the Th17 response during gut inflammation. They indicate quite clearly that at least one component of the Th17 response is anti-inflammatory and lend weight to the idea put forward above that IL-17 itself may also be anti-inflammatory in that it limits the IFN-γ response.
The Role of TL1A in Gut Inflammation
Recent studies have carved out a role for TL1A, a member of the tumor necrosis family also known as TNFSF15, in mucosal inflammation. Studies in humans have shown this cytokine is produced by macrophages of patients with CD and, while it has no effects on lamina propria T cells alone, it enhanced the ability of IL-23 to induce IL-17 and IFN-γ [34]. In addition, studies in mice showed that expression of both TL1A and its receptor, DR3, were increased in the inflamed gut of DSS-colitis and TL1A enhanced both Th1 and Th17 responses by mucosal CD4+ T cells; furthermore, anti-TL1A administration both prevented DSS-colitis and treated established DSS-colitis [35]. Recent as yet unpublished studies corroborate these finding by showing that TL1A appears to act on activated T cells to induce their terminal differentiation and cytokine production. In addition, when constitutively expressed by mice bearing a TL1A transgene, TL1A induces an inflammation limited to the small intestine due to the production of IL-13 [Meylan F and Siegel R, personal communication]. Thus its role in human IBD might be quite complex.
Closing Comments
The profile of cytokines underlying the inflammation of Crohn’s, while reasonably well defined, continues to be a work in progress. We have seen the initial concept that the inflammation is a Th1 inflammation dominated by IL-12 and IFN-γ undergo an eclipse in face of the emergence of the idea that Th17 responses supported by IL-23 are the most salient of the pro-inflammatory cytokines. Very recently, however, the pendulum appears to have swung back with increasing evidence that while the Th17 response may be critical to the maintenance of inflammation, its role is not so much pro-inflammatory as anti-regulatory. Thus, the heavy lifting for inflammation now shifts back to IFN-γ, albeit IFN-γ arising from both Th1 and Th17 cells. It should be noted, however, that this new formulation of cytokine activity in CD does not materially affect the view that anti-IL-12p40 is still the most logical form of anti-pro-inflammatory cytokine therapy for this disease because it has he potential to quell both the Th1 and Th17 response (See Figure 1). Whether other types of anti-cytokine therapy such as anti-TL1A will have an additive role remains to be seen.
References
- 1.Strober W. Why study animal models of IBD? Inflamm Bowel Dis. 2008;14:S129–S131. doi: 10.1002/ibd.20667. [DOI] [PubMed] [Google Scholar]
- 2.Fuss IJ, Neurath M, Boirivant M, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells ma nifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- 3.Breese E, Braegger CP, Corrigan CJ, et al. Interleukin-2 and interferon-gamma-secreting T cells in normal and diseased human intestinal mucosa. Immunology. 1993;78:127–131. [PMC free article] [PubMed] [Google Scholar]
- 4.Neurath MF, Fuss IJ, Kelsall BL, Stüber E, et al. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. “Classic” work first suggesting that anti-IL-12p40 is a powerful method of treating Th1/Th17 T cell-mediated mucosal inflammation in murine models and human Crohn’s disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z, Geboes K, Heremans H, et al. Role of interleukin-12 in the induction of mucosal inflammation and abrogation of regulatory T cell function in chronic experimental colitis. Eur J Immunol. 2001;31:1550–1560. doi: 10.1002/1521-4141(200105)31:5<1550::AID-IMMU1550>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Fuss IJ, Becker C, Yang Z, et al. Both IL-12p70 and IL-23 are synthesized during active Crohn’s disease and are down-regulated by treatment with anti-IL-12 p40 monoclonal antibody. Inflamm Bowel Dis. 2006;12:9–15. doi: 10.1097/01.mib.0000194183.92671.b6. [DOI] [PubMed] [Google Scholar]
- 7.Hart AL, Hafid OA-L, Rigby RJ, et al. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50–65. doi: 10.1053/j.gastro.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Mannon PJ, Fuss IJ, Mayer L, et al. Anti-interleukin-12 antibody for active Crohn’s disease. N Engl J Med. 2004;351:2069–2079. doi: 10.1056/NEJMoa033402. Seminal clinical study providing initial data on the safety and efficacy of anti-IL-12p40 in the treatment of Crohn’s disease. [DOI] [PubMed] [Google Scholar]
- 9.Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135:1130–1141. doi: 10.1053/j.gastro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Oppman B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as w4ell as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 11.Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. The first study to define the cytokines that induce Th17 cells. [DOI] [PubMed] [Google Scholar]
- 12**.McGeachy MJ, Chen Y, Tato CM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. Important study defining the role of IL-23 in the Th17 response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGeachy MJ, Bak-Jensen KS, Chen Y, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 14.Lee YK, Turner H, Maynard CL, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powrie F, Leach MW, Mauze S, et al. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;7:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 16.Neurath MF, Weigmann B, Finotto S, et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J Exp Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hue S, Ahern P, Buonocore S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006:2473–2483. doi: 10.1084/jem.20061099. A study providing the main data establishing the possible role of the Th17 response in experimental intestinal inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elson CO, Cong Y, Weaver CT, et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 19.Izcue A, Hue S, Buonocore S, et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Kleinschek MA, Boniface K, Sadekova S, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009:525–534. doi: 10.1084/jem.20081712. A study mapping the full extent of the Th17 response in Crohn’s disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker C, Domhoff H, Neufert C, et al. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J Immunol. 2006;177:2760–2764. doi: 10.4049/jimmunol.177.5.2760. The initial study defining the counter-regulatory effect of the IL-23 response. [DOI] [PubMed] [Google Scholar]
- 22.Yang XO, Chang SH, Park H, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.O’Connor W, Kamanaka M, Booth CJ, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nature Immunology. 2009;10:603–610. doi: 10.1038/ni.1736. Important study that, along with Ref.21, shows a regulatory role for IL-17 in inflammatory bowel disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunology. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fichtner-Feigl S, Fuss IJ, Young CA, et al. Induction of IL-13 triggers TGF-β1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immmunol. 2007;178:5859–5870. doi: 10.4049/jimmunol.178.9.5859. Study providing initial evidence that the Th1 and Th17 responses may play sequential roles in Crohn’s disease. [DOI] [PubMed] [Google Scholar]
- 26.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Eur Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotenko SV. The family of IL-10-related cytokines and their receptors: related, but to what extent? Cytokine Growth Factor Rev. 2002;13:223–240. doi: 10.1016/s1359-6101(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 28.Wolk K, Kunz S, Witte E, et al. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Andoh A, Zhang Z, Inatomi O, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–984. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 30.Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 31*.Sugimoto K, Ogawa A, Mizoguchi E, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. A study providing evidence that in colitis IL-22 plays a protective role rather than an inflammatory role. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zenewicz L, Yancopoulos GD, Valenzuela DM, et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickert G, Neufert C, Leppkes M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamada N, Hisamatsu T, Honda H, et al. TL1A produced by lamina propria macrophages induces Th1 and Th17 immune responses in cooperation with IL-23 in patients with Crohn’s disease. Inflamm Bowel Dis. 2009 doi: 10.1002/ibd.21124. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Takedatsu H, Michelsen KS, Wei B, et al. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T helper (Th)1 and Th17 activation. Gastroenterology. 2008;135:552–567. doi: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]