Abstract
Parkinson’s disease is associated with emotional changes including depression, apathy, and anxiety. The current study investigated emotional processing in non-demented individuals with Parkinson disease (PD) using an electrophysiological measure, the centro-parietal late positive potential (LPP). Non-demented patients with Parkinson’s disease (n=17) and healthy control participants (n=16) viewed pleasant, neutral, and unpleasant pictures while EEG was recorded from a 64-channel geodesic net. The Parkinson patients did not differ from controls in terms of early electrophysiological components that index perceptual processing (occipital P100, N150, P250). Parkinson patients, however, showed reduced LPP amplitude specifically when viewing unpleasant, compared to pleasant, pictures as well as when compared to controls, consistent with previous studies suggesting a specific difference in aversive processing between PD patients and healthy controls. Importantly, LPP amplitude during unpleasant picture viewing was most attenuated for patients reporting high apathy. The data suggest that apathy in PD may be related to a deficit in defensive activation, and may be indexed cortically using event-related potentials.
Keywords: Parkinson’s disease, ERP, Late positive potential, Apathy, Emotion
1. Introduction
Parkinson’s disease (PD) is a neurodegenerative disease related to the loss of dopamine neurons in the substantia nigra and a degeneration of multiple motor and non-motor basal ganglia circuits (Alexander, DeLong, & Strick, 1986). While the essential characterizing symptom of PD is abnormal motor activity, in the form of tremors, rigidity and bradykinesia, there are also significant impairments in cognitive and emotional functioning (Blonder, Gur, Gur, Saykin, & Hurtig, 1989). For instance, both depression and apathy are significantly higher in Parkinson’s patients than in other medically debilitated populations (Kirsch-Darrow, Fernandez, Marsiske, Okun, & Bowers, 2006; Reijnders JSAM, Lousberg, Aarsland, & Leentjens, 2009), with previous research reporting rates of apathy (without depression) up to 17–30%, suggesting that apathy may be a unique feature of PD (Kirsch-Darrow et al., 2006; Oguru, Tachibana, Toda, Okuda, & Oka, 2010).
Among the possible mechanisms underlying emotional dysfunction in Parkinson’s disease are amygdala abnormalities (Tessitore et al., 2002) and degeneration of non-motor basal ganglia thalamo-cortical loops, caused by dopamine depletion in the ventral striatum, an area known to project to regions of the frontal cortex important in emotional behaviors (i.e., anterior cingulate cortex and lateral oribitofrontal cortex; Alexander et al., 1986; cf. Zgaljardic, Borod, Foldi, & Mattis, 2003). Whether emotional difficulties, particularly apathy, result from an affective disturbance or a behavioral deficit remains unclear (Brown & Pluck, 2000; Levy & Czernecki, 2006). For instance, PD patients report that emotion is experienced as intensely as healthy controls, but rate themselves as less emotionally expressive (Mikos et al., 2009), partially due to reduced emotional facial expressions (Bowers et al., 2006a).
Lang’s motivational theory (Bradley, 2009; Lang & Bradley, 2010) views emotion as reflecting activation in fundamental defensive and appetitive systems that have evolved to mediate attention and action in life-threatening and life-sustaining contexts. According to this view, emotional reactions to environmental cues reflect activation in basic defensive and appetitive neural circuits, indexing the extent of both defensive and appetitive motivation. Pictures depicting threatening and appetitive natural scenes encountered by humans in the world are cues that reliably activate these circuits (Lang & Bradley, 2010); Bowers et al., 2006a, b; Miller, Okun, Marsiske, Fennell, & Bowers, 2009; Zahodne, 2012) have found that individuals with PD showed reduced potentiation of the startle eye blink response, a defensive reflex, when viewing unpleasant pictures, compared to healthy controls.
Reduced defensive activation may be mediated by a disturbance that is driven by amygdala dysfunction, as the amygdala plays a central role in fear potentiated startle circuitry (Davis, 1992; Lang, 1995). While such a deficit might suggest that Parkinson’s patients are generally hypoaroused to emotional stimuli (Miller et al., 2009), in a more recent study we found that pupil dilation, an index of sympathetic arousal, was significantly enhanced in Parkinson’s patients when viewing pleasant or unpleasant pictures, a pattern similar to that found in healthy controls (Dietz, Bradley, Okun, & Bowers, 2011). Electrophysiological indices of brain response during emotional processing could help address these discrepant results. One of the most reliable measures of emotion during picture processing is the amplitude of the late positive potential (LPP), a slow positive deflection over centro-parietal sensors that has been repeatedly found to be enhanced when viewing emotionally arousing pictures (e.g., Cacioppo, Crites, Gardner, & Berntson, 1994; Bradley, 2009; Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Keil et al., 2002). The LPP begins around 300 ms following pictures onset, can last up to 6 s and shows maximal positivity for sensors placed over the centro-parietal area of the brain (Bradley, 2009; Cuthbert et al., 2000). While LPP modulation is correlated with other measures of subjective and autonomic arousal (Cuthbert et al., 2000), it does not completely habituate with stimulus repetition, suggesting it may be a sensitive index of fundamental defensive and appetitive activation (Bradley, 2009).
Thus, in the current study, we measured the late positive potential while Parkinson patients and healthy controls viewed pleasant, neutral, and unpleasant pictures. Based on the pupillary findings of Dietz et al. (2011), one hypothesis is that PD patients will show normal affective modulation of the LPP. An alternative hypothesis, based on findings of blunted startle response in PD patients (Bowers et al., 2006a, b) is that PD patients will show a reduced LPP specifically when viewing unpleasant pictures. Because apathy may be a feature of Parkinson’s disease that significantly affects emotional reactivity, an additional exploratory aim was to examine the relationship between apathy (measured by the apathy scale; Starkstein et al., 1992) and LPP modulation. Marin (1991) has defined apathy as a primary lack of motivation/goal-directed behavior which involves cognitive, affective, and behavioral domains. His proposed diagnostic criteria within the affective domain include “unchanging affect” or “lack of emotional responsivity to positive or negative events,” which suggests that patients high in apathy may show deficits when viewing either pleasant or unpleasant pictures.
Effects of hedonic content on earlier components of the ERP during picture viewing have proved somewhat less reliable, with some studies reporting differences and others not (Olofsson, Nordin, Sequeira, & Polich, 2008). We assessed the magnitude of a number of early ERP components (frontal N250, occipital P100, N150, P250 or EPN) that are involved in picture processing primarily to assess early sensory and perceptual processing in PD patients and controls. If PD patients specifically differ in terms of affective processing, differences in the early ERP components that index initial sensory and perceptual processing between PD patients and controls are not expected. For instance, Wieser et al. (2006) reported that PD patients did not differ from controls on the amplitude of an early occipital component (EPN) found during affective picture viewing.
2. Methods
2.1. Participants
Twenty-four non-demented Parkinson’s patients and 18 healthy older adults participated in the current study. Parkinson’s patients were recruited from the University of Florida Center for Movement Disorders and Neurorestoration and had been previously examined by a movement disorders specialist. All PD participants met UK brain bank criteria for idiopathic Parkinson’s disease (Hughes, Daniel, Kilford, & Lees, 1992). Patients were tested while taking prescribed Parkinson’s medications. The control group was recruited from the community and from spouses of PD patients. Participants were characterized as non-demented (Mini Mental State Exam > 25), free of any self-reported major psychiatric disturbance (e.g., major depression or anxiety, psychotic symptoms, etc.), and had no history of brain surgery, such as deep-brain stimulation for treatment of PD symptoms.
Following data collection, seven patients and two controls were excluded due to having less than seventy percent acceptable trials for EEG analysis, usually due to excessive movements, closing of the eyes, or high electrode impedances that were unable to be reduced to less than 70 k-Ω prior to data acquisition. The final sample consisted of 17 PD patients and 16 healthy controls.
Table 1 lists the demographic and clinical characteristics of the analyzed PD and control groups. Overall, participants were well-educated and predominantly male, as is characteristic of Parkinson’s patients (which affects more males than females). The PD group was significantly younger than the control group (PD mean=59.9 years; control mean=70.6 years, p<.01); thus, age was included as a covariate in subsequent statistical analyses. The groups did not significantly differ in years of education.
Table 1.
Demographic and clinical characteristics of Parkinson’s and healthy older adult groups. Mean (standard deviation) is presented for quantitative variables and ratios are presented for categorical variables.
| Mean (SD) or ratio
|
|||
|---|---|---|---|
| Parkinson (N=17) | Control (N=16) | P-Value | |
| Gender (M/F) | 16/1 | 14/2 | .10 |
| Antidepressant (Y/N) | 6/11 | 0/ss16 | .01 |
| Age | 59.9 (8.4) | 70.6 (9.0) | p<.01 |
| Education | 14.98 (2.7) | 16.3 (3.3) | .19 |
| BDI | 10.1 (4.6) | 3.1 (3.3) | p<.01 |
| Apathy scale | 13.0 (6.2) | 7.4 (2.9) | p<.01 |
| STAI | 38.8 (8.4) | 26.9 (8.9) | p<.01 |
| Disease duration | 6.6 (4.3) | – | |
| UPDRS motor “On” | 39.1 (8.1) | – | |
| “Off” | 27.9 (10.2) | – | |
| Levodopa equivalent dosage | 752.3 | – | |
The PD patients ranged from mild to moderate in disease severity, according to standard staging and severity criteria including the Hoehn–Yahr classification (Hoehn and Yahr, 1967) and the motor score of the Unified Parkinson Disease Rating Scale (UPDRS; Fahn and Elton, 1987). The UPDRS and Hoehn–Yahr staging were performed separately by neurologists at the Center for Movement Disorders and Neurorestoration and took place within 6 months of the experimental protocol, performed at the Cognitive Neuroscience Laboratory, McKnight Brain Institute. All patients exhibited bilateral disease symptoms (Hoehn–Yahr stages 2–3).
Compared to the control group, the PD patients endorsed significantly more symptoms of apathy (apathy scale; Marin, 1991; t(22.1)=3.20, p<.01) and depression (Beck depression inventory-II; Beck, Steer & Carbin, 1988; t(28)=4.69, p<.01). Of the 17 PD patients, six were currently taking antidepressant medications (compared to none for controls). Yate’s continuity corrected X2 for antidepressant usage ratio between the PD and control groups was significant (p=.01), showing that antidepressants were significantly more common among PDs that controls. Thus, patients currently taking antidepressants were compared to those not taking antidepressants in statistical analyses to investigate whether effects might be related to antidepressant usage.
2.2. Stimuli
Seventy-two pictures were selected from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008) that included 24 unpleasant pictures, including mutilations, threatening animals, human violence, etc.; 24 neutral pictures including neutral faces, scenes, household items, etc.; and 24 pleasant pictures, including romantic couples, food, sports activities, etc. Pleasant and unpleasant pictures were comparable in terms of normative arousal ratings. Each picture was shown in two separate blocks, for a total of 48 unpleasant, 48 neutral, and 48 pleasant trials.
2.3. Apparatus
Picture presentation was controlled by a PC running E-Prime software (Psychology Software Tools, Inc., Sharpsburg, PA). Pictures were displayed to participants on a 1024×768 monitor. The EEG was collected using a 64-channel Electrical Geodesics Inc. (EGI; Eugene, Oregon, USA) net and amplifier amplifier system (amplification 20 K, nominal bandpass 0.10–100 Hz). Netstation software was used for continuous recording of EEG data. The electroencephalogram was referenced online to Cz and digitized at 250 Hz.
2.4. Procedures
Each participant signed a consent form and was administered questionnaires prior to engaging in the experimental task. The participant was then moved to the sound/electrical-shielded experimental room and sat in front of a computer screen. The 64-channel EGI net was placed on the head and the participant was given the following instructions: “You will be viewing a series of pictures. Your instructions are simply to look at the pictures. Try not to look away or close your eyes, but rather continue viewing the picture the entire time it is on the screen.” The participant was also asked to stay as still as possible. A fixation point was presented between trials for the duration of the inter-trial interval (ITI).
A single trial consisted of a 3 s presentation of an IAPS picture, followed by a variable ITI of 3, 4, or 5 s. The IAPS pictures were presented in a truly randomized order within each block. In the second block, each IAPS picture from the first block was presented again in a randomized order.
Following picture viewing, the participants were asked to rate how they felt while viewing the picture, in terms of for pleasure and arousal using the Self Assessment Manikin (Bradley & Lang, 1994), a graphic figure with a 9 point scale. For hedonic valence, ratings ranged from unpleasant (1) to neutral (5) to pleasant (9). For arousal, ratings ranged from calm (1) to neutral (5) to very excited (9).
2.5. Data reduction and analysis
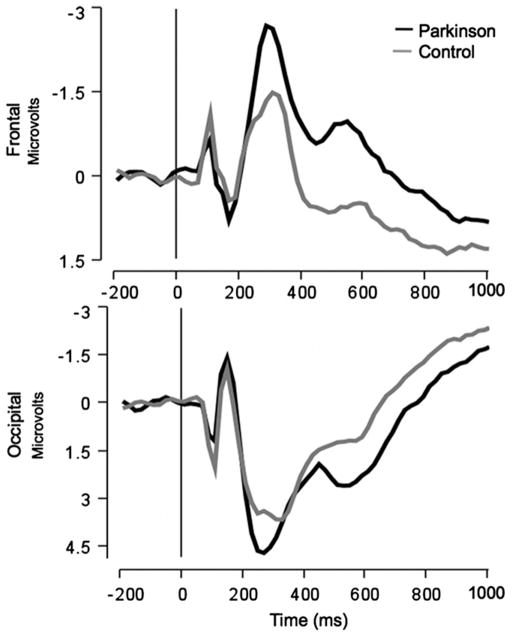
Offline, EEG data was digitally low-pass filtered at 30 Hz, segmented and re-referenced to the average reference. Single trial epochs were rejected if voltages exceeded 150 μV; eye movement and blink artifacts were corrected using a spatial filtering method (Berg & Scherg, 1994) as implemented in Brain Electric Source Analysis (BESA v5.2). Stimulus-locked averages were derived using 200 ms pre-picture baseline and 1200 ms post-picture epoch. Based on the grand-averaged waveforms, the LPP amplitudes for each participant were extracted as the average μV 400–700 ms, at 3 midline sensor clusters1 (frontal, centro-parietal, occipital; see Figs. 1 and 2). A Group (Parkinson, Control)×Content (pleasant, neutral, unpleasant)×Electrode Cluster (frontal, centro-parietal, occipital) ANCOVA revealed that the LPP was maximal at the CPz cluster, which is consistent with the typical scalp distribution of the LPP (F(2.30)=4.09, p<.05). Thus, statistical analyses were conducted using an average over this electrode cluster. The visual P100, N150, and P250 were extracted as the average amplitude between 75–125, 100–200, and 200–300 ms for occipital sensors, and the frontal N250 was extracted as the average amplitude between 250 and 350 ms at frontal sensors.
Fig. 1.
Grand-averaged ERPs averaged over frontal (top) and occipital (bottom) electrode clusters for control and Parkinson groups.
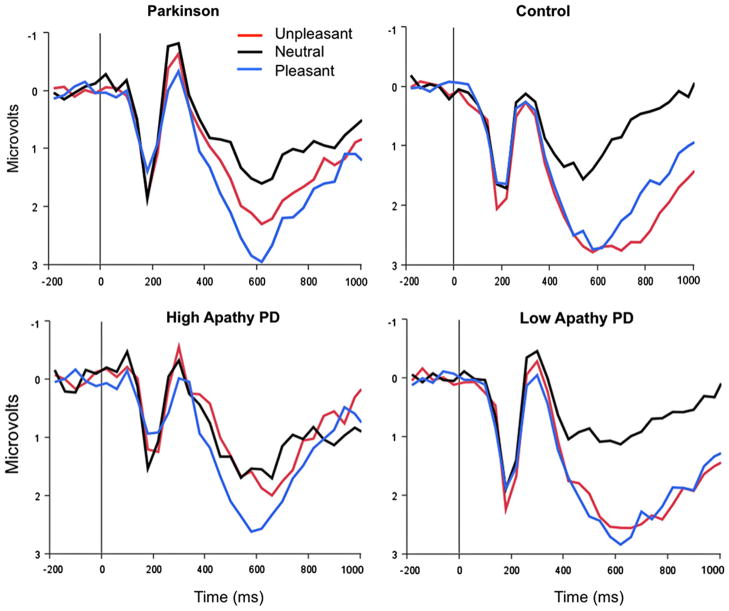
Fig. 2.
Upper panel: Parkinson’s patients (upper right) show smaller centro-parietal LPP amplitude when viewing unpleasant pictures, compared to when viewing pleasant pictures, or when compared to healthy controls (upper left). Lower panel: For descriptive purposes, patients were separated into high and low apathy groups: Whereas LPP amplitude during picture viewing is similar for low-apathy PD patients and controls (lower left), Parkinson patients reporting high apathy show reduced LPPs when viewing unpleasant pictures (lower right).
Group (2)×Content (3) ANCOVA’s were conducted to investigate differences in LPP and P1, N150, and N250 amplitude to emotional and neutral pictures between the groups. Because of a preexisting group difference in age between PD patients and healthy controls (which can effect LPP amplitude and modulation; Wood & Kisley, 2006), age was included as a covariate in the model. Greenhouse–Geisser correction was used when appropriate. Post-hoc tests of individual mean differences are Bonferroni-adjusted for multiple comparisons. Exploratory correlational analyses using mood and disease variables were conducted to investigate how differences were related to group differences in sympomtology. Variables that showed significant relationships were later included as covariates in the analyses. Non-parametric statistics are reported for dependent variables that were not normally distributed.
2.6. Measures
Apathy was assessed using the Marin’s apathy scale (apathy scale; Marin, 1991); state and trait anxiety was assessed by the State-Trait Anxiety Inventory (STAI; Spielberger, Sydeman, Owen, & Marsh, 1999); and depression was assessed by the Beck Depression Inventory II (BDI-II; Beck et al., 1988). All questionnaires are short self report measures.
3. Results
3.1. Early ERP components
Fig. 1 illustrates grand-averaged ERPs measured over frontal and occipital midline electrode clusters for the Parkinson patients and healthy control participants. There were no significant differences between PD patients and healthy controls in the amplitude of early occipital ERP components (i.e., no main effect of Group, nor a group by content interaction on the amplitude of the P100, N150, or P250). On the other hand, overall, the PD group showed a larger frontal N250 than controls (F(1,30)=2.29, p<.05).
3.2. LPP
Fig. 2 shows grand-averaged ERPs when viewing unpleasant, neutral, and pleasant pictures for the Parkinson patients and control participants, averaged over centro-parietal sensors. A significant group by content interaction (F(1.87,58.34)=3.68, p<.05) indicated that PD patients and control individuals differed in LPP amplitude during picture viewing. Simple main effects tests of hedonic content for the PD group along indicated that, whereas the LPP was significantly larger in response when viewing pleasant, compared to neutral, pictures (t(14)=4.35, p<.001), there was no difference in LPP amplitude when viewing unpleasant and neutral pictures (p=.41). Furthermore, for PD patients, LPP amplitude when viewing unpleasant pictures was significantly reduced compared to LPP amplitude when viewing pleasant pictures (t(14)=2.71, p<.05).
On the other hand, healthy control participants showed significantly enhanced LPPs when viewing either unpleasant (t(13)=4.71, p<.001) or pleasant pictures (t(13)=4.35, p<.001), compared to neutral pictures, replicating the pattern of affective modulation normally found in both young adults (e.g., Schupp et al., 2000) and older individuals (Wood & Kisley, 2006).
Between-group simple main effects tests were consistent with the repeated measures analyses in indicating that PD and controls differed in LPP amplitude only when viewing unpleasant pictures (t(30)=2.14, p<.05).
3.3. Apathy and LPP modulation
Correlations between LPP modulation, defined as a difference in amplitude when viewing unpleasant and neutral pictures, with Parkinson disease severity (Hoehn–Yahr stage “on” and “off” medications, UPDRS “on” and “off” medications, LED, side of onset, or disease duration), depressive symptoms (measured by the BDI-II), and anxiety symptoms (STAI) were not significant.
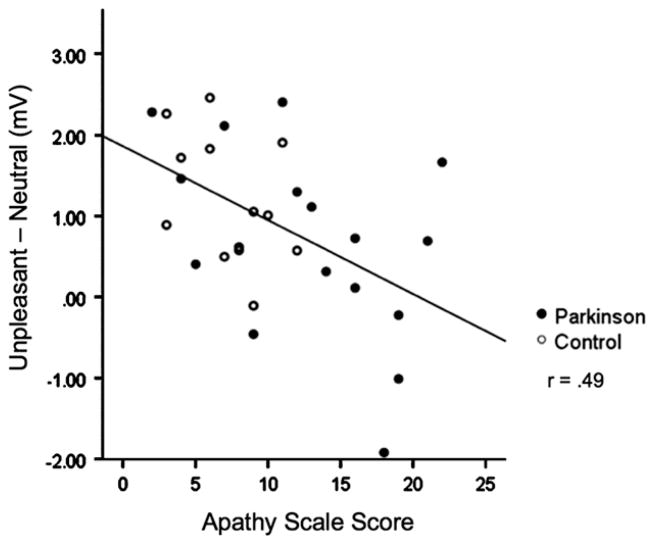
On the other hand, apathy scores were significantly related to reduced LPP modulation when viewing unpleasant pictures, with higher scores on the apathy scale associated with less affective modulation (r=−.49, p<.01), as illustrated in Fig. 3. The relationship between apathy scores and LPP modulation was very similar within each group separately (PD group r=−.46, p=.07; control group r=−.42, p=.16) as the overall correlation. To illustrate the difference, Fig. 2 shows that Parkinson’s patients reporting high apathy2 (apathy scale score ≥14, N=8) those who show a reduced LPP amplitude when viewing unpleasant pictures, whereas those reporting low apathy are much more similar to healthy controls. In fact, when apathy is treated as a covariate in an ANCOVA, the significant difference in LPP amplitude between control and PD patients when viewing unpleasant pictures disappears (i.e., Group by Content interaction is no longer significant), and the removal of apathy-related variance results in a significantly enhanced LPP when viewing unpleasant, compared to neutral, pictures in the PD group, similar to the control group.
Fig. 3.
Higher apathy scores were inversely related to LPP modulation during unpleasant picture viewing.
Because apathy is related to SSRI status, it is possible that these relationships were influenced by PD patients currently taking SSRI medications. However, the mean difference in LPP amplitude when viewing unpleasant versus neutral pictures for PD patients on SSRI medications (n=6) were not different from patients who were not on any SSRI or antidepressant medication (n=10) using a non-parametric Mann Whitney-U test (p=.88).
3.4. Arousal ratings
Table 2 lists pleasure and arousal ratings for Parkinson patients and healthy controls. A significant group by content interaction (F(1.63, 47.15)=5.61, p<.05) indicated that the PD group rated unpleasant pictures as significantly less arousing than pleasant pictures (t(14)=3.04, p<.05), whereas the control group rated unpleasant pictures as significantly more arousing than pleasant pictures (t(12)=2.90, p<.05). This was true for Parkinson’s patients in both the high and low apathy groups. Not surprisingly, both groups rated emotional pictures as significantly more arousing than neutral pictures. There were no significant differences in pleasure ratings between the groups.
Table 2.
Mean picture ratings and early ERP amplitudes and standard deviation when viewing unpleasant, neutral, and pleasant pictures for Parkinson patients (high and low apathya) and healthy controls.
| Unpleasant | Neutral | Pleasant | |
|---|---|---|---|
| Pleasure ratings | |||
| Control | 2.57 (.90) | 4.58 (.93) | 6.68 (1.21) |
| Parkinson | 2.78 (.94) | 4.72 (.65) | 6.86 (.90) |
| High apathy | 2.85 (1.24) | 4.84 (.72) | 6.76 (.94) |
| Low apathy | 2.68 (.70) | 4.54 (.60) | 6.88 (.95) |
| Arousal ratings | |||
| Control | 5.83 (2.08) | 2.64 (1.38) | 4.73 (1.87) |
| Parkinson | 4.42 (2.06) | 2.53 (1.19) | 5.49 (1.4) |
| High apathy | 4.40 (2.16) | 2.23 (1.32) | 4.96 (1.53) |
| Low apathy | 4.11 (2.00) | 2.78 (1.14) | 5.80 (1.14) |
| LPP | |||
| Control | 2.49 (1.69) | 1.22 (1.25) | 2.36 (1.31) |
| Parkinson | 1.77 (1.47) | 1.20 (1.53) | 2.30 (1.47) |
| High apathy | 1.43 (1.89) | 1.39 (1.86) | 2.12 (1.77) |
| Low apathy | 2.07 (1.05) | 0.75 (.98) | 2.38 (1.28) |
| P1 | |||
| Control | 1.48 (1.87) | 1.10 (1.57) | 1.48 (1.87) |
| Parkinson | 0.84 (2.07) | 0.77 (2.76) | 0.84 (2.07) |
| High apathy | 1.62 (2.15) | 2.17 (2.29) | 1.62 (2.15) |
| Low apathy | 0.12 (1.96) | −0.49 (2.81) | 0.12 (1.96) |
| N150 | |||
| Control | 0.49 (2.03) | 0.43 (1.69) | 0.43 (1.78) |
| Parkinson | 0.11 (2.61) | 0.21 (2.84) | −0.17 (3.19) |
| High apathy | 0.32 (2.74) | 0.87 (3.31) | −0.45 (3.73) |
| Low apathy | 0.22 (2.65) | −0.17 (2.47) | 0.51 (2.70) |
| P250 | |||
| Control | 3.45 (3.05) | 3.35 (2.96) | 3.21 (2.65) |
| Parkinson | 4.43 (3.66) | 4.34 (3.72) | 3.38 (4.16) |
| High apathy | 3.19 (3.88) | 3.31 (3.81) | 1.51 (4.20) |
| Low apathy | 5.39 (3.46) | 5.08 (3.81) | 5.06 (3.79) |
| N250 | |||
| Control | −1.66 (1.4) | −1.23 (1.7) | −0.99 (1.5) |
| Parkinson | |||
| All | −2.49 (2.5) | −2.61 (2.4) | −1.91 (3.0) |
| High apathya | −1.67 (3.0) | −1.98 (2.8) | −0.77 (3.3) |
| Low apathy | −3.36 (2.0) | −3.30 (1.98) | −3.17 (2.5) |
High apathy participants scored ≥14 on the apathy scale.
4. Discussion
Emotional processing, indexed by the amplitude of the late positive potential of the ERP during affective picture viewing, was investigated in a Parkinson’s disease and healthy control sample. One hypothesis, based on previous findings of blunted startle potentiation during unpleasant picture viewing, was that PD patients would show an attenuated LPP specifically when viewing unpleasant pictures. This hypothesis was supported. While the LPP was enhanced during pleasant (compared to neutral) picture viewing for Parkinson patients, the LPP elicited during unpleasant picture viewing was not significantly different than the LPP elicited during neutral picture viewing. Importantly, attenuated LPP amplitude to unpleasant pictures was significantly and specifically related to scores on the apathy scale. Higher reported apathy was associated with less modulation of the LPP during unpleasant, compared to neutral, picture viewing. When apathy was controlled in an analysis of covariance, the interaction of group and picture content was no longer significant and adjusted scores indicated normal emotional modulation of the LPP for PD patients and healthy controls.
Reduced modulation of the LPP during unpleasant picture viewing was not due to group differences in early sensory or perceptual processing, as PD patients and healthy controls did not differ on the P100, N150, or P250. A single difference was found, with patients showing overall larger frontal N250 amplitudes, compared to healthy controls. An enhanced N2 in PD during Go-No Go tasks has been reported a number of times previously (Beste, Dziobek, Hielscher, Willemssen, & Falkenstein, 2009; Beste, Willemssen, Saft, & Falkenstein, 2010; Willemssen, Falkenstein, Schwarz, Müller, and Beste, 2011), and interpreted as reflecting inhibition of motor activity (for review, see Folstein & van Petten, 2002). Because Parkinson’s disease causes an imbalance of indirect to direct pathway output from the basal ganglia, overactivity of the inhibitory indirect pathway leading to the motor cortex results (cf. Delong & Wichmann, 2007). Thus, a larger N2 in PD might reflect tonic motor inhibition due to nigrostriatal degeneration, which is also supported by the finding that dopaminergic therapy attenuates N2 amplitude in PD patients (Willemssen et al., 2011).
Parkinson patients rated unpleasant pictures as less arousing than pleasant pictures which is consistent with the finding of an attenuated LPP specifically when viewing unpleasant pictures. This finding is also consistent with Bowers et al. (2006a, b), who reported that PD patients showed blunted startle potentiation during unpleasant picture viewing. And, importantly, blunted startle potentiation was also related to reported apathy in Parkinson patients (Bowers et al., 2008). Given the central role of the amygdala in startle potentiation (see Davis, 1992), Bowers et al. (2006a, b) suggested that differences in aversive processing might be driven by amygdalar dysfunction in the PD group, which is still a viable hypothesis.
A specific deficit in aversive processing in Parkinson patients could also result from dysfunction in other regions in the defense circuit. In a recent combined fMRI-EEG study that investigated BOLD activity and LPP coupling, viewing unpleasant pictures produced significant BOLD-LPP correlations in the insula, ventrolateral prefrontal cortex, and posterior cingulate cortex (Liu, Huanh, McGinnis-Deweese, Keil, & Ding, 2012). These regions were shown in a meta-analysis to be functionally connected to the dorsal striatum, which is significantly affected by PD (Postuma & Dagher, 2006), and could mediate defensive reactions during affective perception.
Specific differences in processing aversive stimuli may also reflect dopamine dysfunction. In one study that investigated the LPP during emotional picture viewing in schizophrenia, patients showed reduced LPP modulation specifically during pleasant picture viewing, compared to controls (Horan, Wynn, Kring, Simons, & Green, 2010). Finding the converse effect in Parkinson patients (i.e., reduced response to unpleasant stimuli) is consistent with the fact that Parkinson’s is a disease of hypodopaminergic transmission (treated with dopaminergic therapy), whereas schizophrenia is a disease of hyper-dopaminergic transmission (treated with dopaminergic antagonists; Mehler-Wex, Riederer, & Gerlach, 2006).
Furthermore, the reduced LPP modulation during unpleasant processing in the current study was significantly related to higher reports of apathy in Parkinson patients, which is consistent with evidence suggesting that apathy may also be related to dopaminergic status (Czernecki et al., 2002; Isella et al., 2002). Although these data provide some support for a dopamine-specific hypothesis, it is not clear whether the deficit reflects disease-related dopamine depletion or effects of dopaminergic therapy, as patients were tested on their dopaminergic medications. Of note, whereas dopamine therapy reduces motor symptoms in PD, emotional symptoms persist, likely due to different optimal dopaminergic dosages for mesolimbic versus nigrostriatal functioning (Cools, 2006). Future studies would do well to assess patients both on and off medications as well as to include more female PD participants, and more closely age-matched controls.
The association between high apathy and an attenuated LPP specifically during the viewing of unpleasant pictures is informative regarding the presentation of apathy in PD. Marin (1991) defined apathy broadly as a primary deficit in motivation, with items on his Apathy Scale (used here) probing general motivation and initiative (e.g., “Do you have motivation?”; “Do you need a push to get started?”; “Do you have plans and goals for the future?”), rather than positive or negative affect. Nonetheless, Marin proposed that lack of motivation and therefore goal-directed behavior results in a “lack of emotional responsivity to positive or negative events”, which is inconsistent with the normal brain response found during pleasant picture viewing for PD patients reporting high apathy in the current study.
Taken together, the current data suggest that apathy in PD does not necessarily involve a loss of pleasure. In this sense it may be separable from depression, in which anhedonia is a core symptom in the current Diagnostic and Statistical Manual of Mental Disorders (loss of pleasure; 4th ed., text rev.; DSM–IV–TR; American Psychiatric Association, 2000). Notably, reported depression did not have any effect on LPP modulation in the current study, whereas reports of apathy did. These data suggest that degree of apathy may have a significant impact on emotional reactivity in PD patients, and could be partly responsible for disparate results obtained in different studies using different measures. To the extent a sample of PD patients do not report high apathy, emotional reactions may seem intact during both aversive and appetitive processing.
4.1. Summary
Parkinson patients showed reduced defensive activation during unpleasant picture viewing as measured by the amplitude of the centro-parietal late positive potential of the ERP. Moreover, reduced LPP modulation during unpleasant picture viewing was significantly and specifically related to reports of high apathy. These effects might reflect dopaminergic dysfunction, selective impairment in brain regions associated with defensive responding, such as the amygdala, insula, cingulate, or vlPFC, or both. Because reported apathy in PD was uniquely related to differences in aversive processing, the current data add to the literature by suggesting that apathy in PD is not identical to anhedonia or depression, but may instead reflect a specific deficit in defensive activation.
Uncited references
Amaral, Price, Pitkanen, and Carmichael (1992), Berridge & Robinson (1998), Catani, Jones, Donato, and Ffytche (2003), Hariri, Tessitore, Mattay, Fera, and Weinberger (2002), Javoy-Agid and Agid (1980), Keil et al. (2009), Levy and Dubois (2006), Liberzon, Phan, Decker and Taylor (2003), Nitschke, Sarinopoulos, Mackiewicz, Schaefer, and Davidson (2006), Ouchi et al. (1999), Phelps and LeDoux (2005), Reijnders JSAM, Lousberg, Aarsland, and Leentjens (2009), Sabatinelli, Bradley, Fitzsimmons, and Lang (2005), Sabatinelli, Lang, Bradley, Costa, and Keil (2009), Sabatinelli, Lang, Keil, and Bradley (2007), Schienle et al. (2002), Schupp, Junghoefer, Weike, and Hamm (2003), Simon et al. (2010), Starkstein and Leentjens (2008), Vrana, Spence, and Lang (1988), Wieser et al. (in press).
Acknowledgments
This work was supported by NINDS (F31NS073331-01[JD]; NS- 079767), the National Parkinson Foundation Center of Excellence, Gaineville, FL.
Footnotes
Sensors included in each cluster, using the 64-channel EGI geodesic sensor net layout, were as follows: (frontal) 7, 8, 3, 4, 9, 58; (centroparietal) 5, 55, Cz, 30, 29, 34, 42; (occipital) 38, 37, & 40.
A score of 14 on the Apathy Scale is the recommended cut-off for clinically significant apathy in Parkinson’s disease (Starkstein et al., 1992).
References
- Alexander G, DeLong M, Strick P. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck depression inventory: 25 years of evaluation. Clinical Psychology Review. 1988:77–100. [Google Scholar]
- Berg P, Scherg M. A multiple source approach to the correction of eye artifacts. Electroencephalography Clinical Neurophysiology. 1994;90:229–241. doi: 10.1016/0013-4694(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Beste C, Dziobek I, Hielscher H, Willemssen R, Falkenstein M. Effects of stimulus–response compatibility on inhibitory processes in Parkinson’s disease. European Journal of Neuroscience. 2009;29(4):855–860. doi: 10.1111/j.1460-9568.2009.06621.x. [DOI] [PubMed] [Google Scholar]
- Beste C, Willemssen R, Saft C, Falkenstein M. Response inhibition subprocesses and dopaminergic pathways: basal ganglia disease effects. Neuropsychologia. 2010;48(2):366–373. doi: 10.1016/j.neuropsychologia.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Blonder XL, Gur RE, Gur RC, Saykin AJ, Hurtig HI. Neuropsychological functioning in hemiparkinsonism. Brain Cognition. 1989;9:244–257. doi: 10.1016/0278-2626(89)90034-1. [DOI] [PubMed] [Google Scholar]
- Bowers D, Kirsch-Darrow L, Mikos A, Springer US, Fernandez HH, Okun MS. Apathy and psychophysiologic blunting in Parkinson disease. Paper presented at the American Academy of Neurology; Chicago. 2008. [Google Scholar]
- Bowers D, Miller K, Bosch W, Gokcay D, Pedraza O, Springer U, et al. Faces of emotion in Parkinsons disease: micro-expressivity and bradykinesia during voluntary facial expressions. Journal of the International Neuropsychological Society. 2006a;12(6):765–773. doi: 10.1017/S135561770606111X. [DOI] [PubMed] [Google Scholar]
- Bowers D, Miller K, Mikos A, Kirsch-Darrow L, Springer U, Fernandez H, et al. Startling facts about emotion in Parkinson’s disease: blunted reactivity to aversive stimuli. Brain. 2006b;129(12):3356–3365. doi: 10.1093/brain/awl301. [DOI] [PubMed] [Google Scholar]
- Bradley M. Natural selective attention: orienting and emotion. Psychophysiology. 2009;46(1):1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Brown R, Pluck G. Negative symptoms: the ‘pathology’ of motivation and goal-directed behaviour. Trends in Neurosciences. 2000;23(9):412–417. doi: 10.1016/s0166-2236(00)01626-x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Crites SL, Gardner WL, Berntson GG. Bioelectrical echoes from evaluative categorization: I. A late positive brain potential that varies as a function of trait negativity and extremity. Journal of Personality and Social Psychology. 1994;67:115–125. doi: 10.1037//0022-3514.67.1.115. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neuroscience Biobehavioral Reviews. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Czernecki V, Pillon B, Houeto J, Pochon J, Levy R, Dubois B. Motivation, reward, and Parkinson’s disease: influences of dopatherapy. Neuropsychologia. 2002;40(13):2257–2267. doi: 10.1016/s0028-3932(02)00108-2. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned fear. In: Aggleton J, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley Publishers; 1992. pp. 255–305. [Google Scholar]
- Dietz J, Bradley M, Okun M, Bowers D. Emotion and ocular responses in Parkinson’s disease. Neuropsychologia. 2011;49(12):3247–3253. doi: 10.1016/j.neuropsychologia.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Archives of Neurology. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL. Members of the UPDRS development committee. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne D, Holstein N, editors. Recent developments in Parkinson’s disease. Vol. 2. Plurham Park, N.J: Macmillian Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- Folstein JR, van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2002;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Horan WP, Wynn JK, Kring AM, Simons RF, Green MF. Electrophysiological correlates of emotional responding in schizophrenia. Journal Abnormal Psychology. 2010;119:18–30. doi: 10.1037/a0017510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. Journal of Neurology, Neurosurgery & Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isella V, Melzi P, Grimaldi M, Iurlaro S, Piolti R, Ferrarese C, et al. Clinical, neuropsychological, and morphometric correlates of apathy in Parkinson’s disease. Movement Disorders. 2002;17(2):366–371. doi: 10.1002/mds.10041. [DOI] [PubMed] [Google Scholar]
- Javoy-Agid F, Agid Y. Is the mesocortical dopaminergic system involved in Parkinson’s disease? Neurology. 1980;30:1326–1330. doi: 10.1212/wnl.30.12.1326. [DOI] [PubMed] [Google Scholar]
- Kaji Y, Hirata K. Apathy and anhedonia in Parkinson’s disease. Neurology. doi: 10.5402/2011/219427. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert TR, Lang PJ. Large-scale neural correlates of affective picture viewing. Psychophysiology. 2002;39:641–649. doi: 10.1017.S0048577202394162. [DOI] [PubMed] [Google Scholar]
- Keil A, Sabatinelli D, Ding M, Lang PJ, Ihssen N, Heim S. Reentrant projections modulate visual cortex in affective perception: directional evidence from granger causality analysis. Human Brain Mapping. 2009;30:532–540. doi: 10.1002/hbm.20521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch-Darrow L, Fernandez HF, Marsiske M, Okun MS, Bowers D. Dissociating apathy and depression in Parkinson disease. Neurology. 2006;67(1):33–38. doi: 10.1212/01.wnl.0000230572.07791.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe. Studies of motivation and attention. American Psychologist. 1995;50(5):372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM. Emotion and the motivational brain. Biological Psychology. 2010;84(3):437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical report. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual; p. A-8. [Google Scholar]
- Levy R, Czernecki C. Apathy and the basal ganglia. Journal of Neurology. 2006;253(7):54–61. doi: 10.1007/s00415-006-7012-5. [DOI] [PubMed] [Google Scholar]
- Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cerebral Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Phan KL, Decker LR, Taylor SF. Extended amygdala and emotional salience: a PET activation study of positive and negative affect. Neuropsychopharmacology. 2003;28:726–733. doi: 10.1038/sj.npp.1300113. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huanh H, McGinnis-Deweese M, Keil A, Ding M. Neural substrate of the late positive potential in emotional processing. The Journal of Neuroscience. 2012;32:14563–14571. doi: 10.1523/JNEUROSCI.3109-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin RS. Apathy: a neuropsychiatric syndrome. Journal of Neuropsychiatry & Clinical Neuroscience. 1991;3:243–254. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Research. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- Mehler-Wex C, Riederer P, Gerlach M. Dopaminergic dysbalance in distinct basal ganglia neurocircuits: implications for the pathophysiology of Parkinson’s disease, schizophrenia and attention deficit hyperactivity disorder. Neurotoxicity Research. 2006;10:167–179. doi: 10.1007/BF03033354. [DOI] [PubMed] [Google Scholar]
- Mikos AE, Springer US, Nisenzon AN, Kellison IL, Fernandez HH, Okun MS, et al. Awareness of expressivity deficits in non-demented Parkinson disease. The Clinical Neuropsychologist. 2009;23(5):805–817. doi: 10.1080/13854040802572434. [DOI] [PubMed] [Google Scholar]
- Miller KM, Okun MS, Marsiske M, Fennell EB, Bowers D. Startle reflex hyporeactivity in Parkinson’s disease: an emotion-specific or arousal-modulated deficit? Neuropsychologia. 2009;47:1917–1927. doi: 10.1016/j.neuropsychologia.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Oguru M, Tachibana H, Toda K, Okuda B, Oka N. Apathy and depression in Parkinson disease. Journal of Geriatric Psychiatry and Neurology. 2010;23(1):35–41. doi: 10.1177/0891988709351834. [DOI] [PubMed] [Google Scholar]
- Oloffson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: an integrative review of ERP findings. Biological Psychology. 2008;77:247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Okada H, Futatsubashi M, Sekine Y, Iyo M, et al. Alterations in binding site density of dopamine transporter in the striatum, orbitofrontal cortex, and amygdala in early Parkinson’s disease: compartment analysis for CFT binding with positron emission tomography. Annals of Neurology. 1999;45:601–610. [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cerebral Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Reijnders JSAMU, Lousberg R, Aarsland D, Leentjens AFG. The association between motor subtypes and psychopathology in Parkinson’s disease. Parkinsonism & Related Disorders. 2009;15(5):379–382. doi: 10.1016/j.parkreldis.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdale and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24:1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Lang P, Bradley M, Costa V, Keil A. The timing of emotional discrimination in human amygdala and ventral visual cortex. The Journal of Neuroscience. 2009;29(47):14864–14868. doi: 10.1523/JNEUROSCI.3278-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Keil A, Bradley MM. Emotional perception: correlation of functional MRI and event-related potentials. Cerebral Cortex. 2007;17(5):1085–1091. doi: 10.1093/cercor/bhl017. [DOI] [PubMed] [Google Scholar]
- Schienle A, Stark R, Walter B, Blecker C, Ott U, Kirsch P, et al. The insula is not specifically involved in disgust processing: an fMRI study. Neuroreport. 2002;13:2023–2026. doi: 10.1097/00001756-200211150-00006. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Schupp HT, Junghoefer M, Weike AI, Hamm AO. Emotional facilitation of sensory processing in the visual cortex. Psycholigical Science. 2003;14:7–13. doi: 10.1111/1467-9280.01411. [DOI] [PubMed] [Google Scholar]
- Simon JJ, Biller A, Walther S, Roesch-Ely D, Stippich C, Weisbrod M, et al. Neural correlates of reward processing in schizophrenia—relationship to apathy and depression. Schizophrenia Research. 2010;118(1–3):154–161. doi: 10.1016/j.schres.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Sydeman SJ, Owen AE, Marsh BJ. Measuring anxiety and anger with the state-trait anxiety inventory (STAI) and the state-trait anger expression inventory (STAXI). In the use of psychological testing for treatment planning and outcomes assessment. 2. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 1999. pp. 993–1021. [Google Scholar]
- Starkstein SE, Leentjens AF. The nosological position of apathy in clinical practice. Journal of Neurology Neurosurgery Psychiatry. 2008;79:1088–1092. doi: 10.1136/jnnp.2007.136895. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. Journal of Neuropsychiatry & Clinical Neurosciences. 1992;4:134–139. doi: 10.1176/jnp.4.2.134. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Hariri A, Fera F, Smith W, Chase T, Hyde T, et al. Dopamine modulates the response of the human amygdala: a study in Parkinson’s disease. The Journal of Neuroscience. 2002;22(20):9099–9103. doi: 10.1523/JNEUROSCI.22-20-09099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: a new measure of emotion? Journal of Abnormal Psychology. 1988;97(4):487–491. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]
- Wieser MJ, Klupp E, Weyers P, Pauli P, Weise D, Zeller D, et al. Reduced early visual emotion discrimination as an index of diminished emotion processing in Parkinson’s disease?—Evidence from event-related brain potentials. Cortex. doi: 10.1016/j.cortex.2011.06.006. In press. [DOI] [PubMed] [Google Scholar]
- Wieser MJ, Muhlberg A, Alpers GW, Macht M, Ellgring H, Pauli P. Emotion processing in Parkinson’s disease: dissociation between early neuronal processing and explicit ratings. Clinical Neurophysiology. 2006;117:94–102. doi: 10.1016/j.clinph.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Willemssen R, Falkenstein M, Schwarz M, Müller T, Beste C. Effects of aging, Parkinson’s disease, and dopaminergic medication on response selection and control. Neurobiology of Aging. 2011;32(2):327–335. doi: 10.1016/j.neurobiolaging.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Wood S, Kisley MA. The negativity bias is eliminated in older adults: age-related reduction in event-related brain potentials associated with evaluative categorization. Psychology and Aging. 2006;21:815–820. doi: 10.1037/0882-7974.21.4.815. [DOI] [PubMed] [Google Scholar]
- Zahodne LB. Depression in Parkinson’s disease: relation to startle eyeblink psychophysiology and affective chronometry. Platform presentation at the 23rd annual meeting of the American Neuropsychiatric Association; New Orleans, LA. 2012. Mar, [Abstract: Journal of Neuropsychiatry and Clinical Neurosciences 24(2)] [Google Scholar]
- Zgaljardic D, Borod J, Foldi N, Mattis P. A review of the cognitive and behavioral sequelae of Parkinson’s disease: relationship to frontostriatal circuitry. Cognitive and Behavioral Neurology. 2003;16(4):193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]