Abstract
Purpose.
Glucocorticoids (GCs) effectively reduce retinal edema and induce vascular barrier properties but possess unwanted side effects. Understanding GC induction of barrier properties may lead to more effective and specific therapies. Previous work identified the occludin enhancer element (OEE) as a GC-responsive cis-element in the promoters of multiple junctional genes, including occludin, claudin-5, and cadherin-9. Here, we identify two OEE-binding factors and determine their contribution to GC induction of tight junction (TJ) gene expression and endothelial barrier properties.
Methods.
OEE-binding factors were isolated from human retinal endothelial cells (HREC) using DNA affinity purification followed by MALDI-TOF MS/MS. Chromatin immunoprecipitation (ChIP) assays determined in situ binding. siRNA was used to evaluate the role of trans-acting factors in transcription of TJ genes in response to GC stimulation. Paracellular permeability was determined by quantifying flux through a cell monolayer, whereas transendothelial electrical resistance (TER) was measured using the ECIS system.
Results.
MS/MS analysis of HREC nuclear extracts identified the heterodimer of transcription factors p54/NONO (p54) and polypyrimidine tract-binding protein-associated splicing factor (PSF) as OEE-binding factors, which was confirmed by ChIP assay from GC-treated endothelial cells and rat retina. siRNA knockdown of p54 demonstrated that this factor is necessary for GC induction of occludin and claudin-5 expression. Further, p54 knockdown ablated the pro-barrier effects of GC treatment.
Conclusions.
p54 is essential for GC-mediated expression of occludin, claudin-5, and barrier induction, and the p54/PSF heterodimer may contribute to normal blood-retinal barrier (BRB) induction in vivo. Understanding the mechanism of GC induction of BRB properties may provide novel therapies for macular edema.
Keywords: tight junction, blood-retinal barrier, enhancer, gene expression
We have identified the p54 NONO/PSF heterodimer as a binding partner of the occludin enhancer that is necessary for glucocorticoid induction of barrier properties.
Introduction
Blood-retinal barrier (BRB) breakdown contributes to many ophthalmological diseases, including diabetic macular edema (DME), retinal vein occlusions, and uveitis. Clinical signs of diabetic retinopathy are present in nearly all patients who have had diabetes for more than 20 years.1 As the prevalence of diabetes continues to rise, effective management of eye disease will become more important to improve the quality of life of diabetic patients and limit the increasing economic cost of diabetic ocular diseases.
Recent findings have revealed an important role for elevated cytokines, such as VEGF, and inflammatory cytokines, such as TNF, in the etiology of DME. Anti-VEGF2 therapies can reverse the effects of BRB breakdown, such as macular edema and retinal swelling. Used in conjunction with laser photocoagulation or alone,3 these therapies often prevent further vision loss and improve vision for some patients. Although not tested in large clinical trials, anti-TNF4 therapy may provide an additional therapeutic option. Intravitreal glucocorticoid (GC) injections and fluocinolone acetonide implants5 have also been successfully used to manage ophthalmological diseases, including DME, retinal vein occlusions, and uveitis, and show significant therapeutic benefits in certain populations of patients.6 Numerous trials have shown effective management of diabetic retinopathy with GC treatment7; however, known adverse side effects, including elevated IOP and development of cataracts, have limited GC use.8
GCs exhibit robust therapeutic effectiveness by altering multiple cellular pathways. They exhibit potent anti-inflammatory actions9 while improving endothelial barrier properties through increased tight junction (TJ) integrity.10,11 TJs are specialized cell-cell adhesions that allow the regulation of molecular and ionic flux across endothelial and epithelial cell layers. Vasculature within neural tissue contains abundant TJs, helping to form the blood-brain barrier (BBB) and BRB by controlling entry into the neural environment.12 TJs are significantly reduced and disorganized in the retinal vasculature of diabetic animals, leading to increased permeability.13,14 Occludin and claudin-5 are transmembrane TJ proteins that contribute to barrier properties. Claudin-5 expression is largely restricted to vascular endothelium,15 including the retinal vasculature,16 and gene deletion of claudin-5 leads to permeability of the BBB and death shortly after birth.17,18 The role of occludin in TJs has proven more complex with phosphorylation and ubiquitination of occludin necessary for VEGF-induced endothelial permeability.19,20 Further, occludin content closely matches barrier properties in a number of systems.21–23
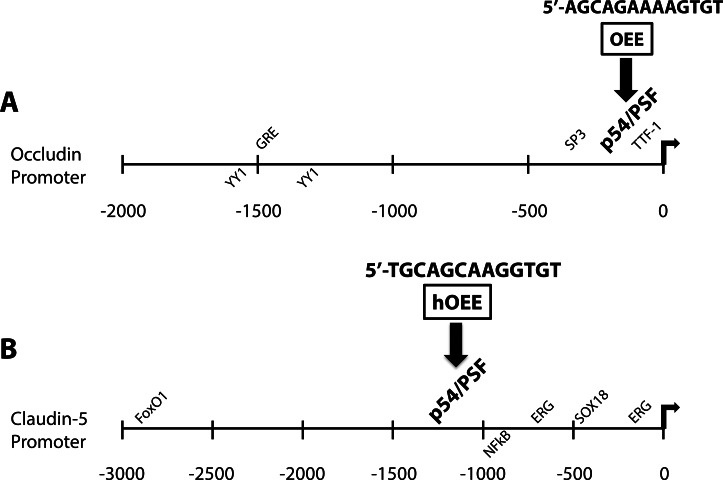
GCs have been used for decades to treat brain edema secondary to tumor development,24,25 acting to reduce VEGF signaling and increase barrier properties. GC treatment leads to increased transcription of occludin26,27 and claudin-528 and decreased occludin phosphorylation, leading to a tighter barrier and less paracellular leakage.26 Mutational analysis of the occludin promoter revealed the presence of an enhancer element that confers GC responsiveness to a separate promoter.28 This enhancer, termed the occludin enhancer element (OEE), demonstrated homology to elements in other steroid-responsive junctional genes, including claudin-5 and cadherin-9. Further, the time frame for steroid responsiveness, 24 to 48 hours, suggested that the OEE was not directly stimulated by activated glucocorticoid receptor (GR), but rather was induced by transactivation. We investigated this process so as to further understand the mechanism behind GC induction of TJ expression and preservation of the BRB, with the hopes of generating new therapeutic strategies to treat diabetic eye disease.
Here we report the identification of a trans-acting factor that functions at the OEE. The multifunctional heterodimer formed by p54/NONO (p54) and polypyrimidine tract-binding protein-associated splicing factor (PSF) was found to form a complex with the OEE in a GC-dependent manner, leading to increased transcription of occludin and claudin-5 genes. The p54/PSF heterodimer has previously been identified as a trans-acting factor that can modulate gene expression as a coactivator. Understanding the mechanism behind the GC induction of coordinated TJ gene transcription may allow the design of therapies to circumvent known adverse side effects of GC therapy while reversing BRB breakdown. Additionally, basic biology behind the development and maintenance of specialized endothelial barriers in the brain and retina may be more completely understood through the identification of the transcriptional elements controlling TJ formation.
Methods
Cell Culture
Primary bovine retinal endothelial cells (BRECs) were isolated from fresh bovine eyes as previously described.29 Human retinal endothelial cells (HRECs) were from Cell Systems (Kirkland, WA). For experimentation, cells grown to confluence were switched to 0% fetal bovine serum (FBS) or 1% FBS and treated with 200 ng/mL dexamethasone where indicated. All experiments were performed with cells between passages four and eight. HRECs were used to increase the chance of trans-acting factors binding to the human OEE sequence.
Western Blotting
Western blots were carried out using previously described protocols,30 using antibodies directed against occludin (Invitrogen, Grand Island, NY; 1:1000), claudin-5 (Invitrogen, 1:1000), p54 (SCBT, 1:1000), PSF (SCBT, Santa Cruz, CA; 1:1000), and GR (SCBT, 1:1000).
Electrophoretic Mobility Shift Assay
DNA oligonucleotides encompassing the 40-bp human OEE (AACAGTTTAATCAAATTCTGGAAGCAGAAAAGTGTCCTGT), or a scrambled control, were annealed, labeled with 32P deoxycytidine triphosphate (dCTP) (Amersham, Piscataway, NJ) and used as a probe in an electrophoretic mobility shift assay (EMSA). The probe was incubated with dialyzed nuclear extract from control or dexamethasone-treated BREC in a binding reaction containing 100 mM NaCl concentration. Unlabeled OEE probe in 500-fold excess was included as a cold competitor where indicated. Complexes were resolved on a 6% nondenaturing acrylamide gel, dried, and exposed to film.
DNA Affinity Purification
The 5′-biotinylated OEE oligonucleotides were annealed and immobilized on a Dynabead M-280 Streptavidin column (Invitrogen) in bind/wash buffer (5 mM Tris-HCL, 0.5 mM EDTA, 1.0 M NaCl, pH 7.5) for 16 hours at 4°C with agitation. Nuclear extract was prepared from dexamethasone-treated HREC using the NE-PER system (Pierce, Rockford, IL) and dialyzed against low-salt buffer (4% glycerol, 20 mM Hepes, 1 mM DTT, 1 mM MgCl2, 20 mM NaCl with protease and phosphatase inhibitors, pH 7.9) at 4°C overnight. Nuclear extract (500 μg) was incubated with the DNA-affinity column in binding buffer (20 mM Hepes, 70 mM NaCl, 10 mM EDTA, 5% glycerol, 1 mM DTT, 5 mM MgCl2, pH 7.9) in the presence of 4 mg/mL poly dI/dC nonspecific competitor at 4°C for 16 hours with agitation. The column was washed four times with binding buffer and bound proteins were eluted with binding buffer containing 500 mM NaCl. Column flow-through, washes, and eluate were separated on a 12.5% SDS-PAGE gel, stained overnight with SYPRO-Ruby (Molecular Probes, Eugene, OR) and bands were visualized on a Typhoon fluorescence scanner (Molecular Dynamics, Sunnyvale, CA). Protein bands enriched in the eluate were excised for mass spectrometry (MS).
Matrix-Assisted Laser Desorption Ionization Time-of-Flight MS/MS
SYPRO-Ruby was removed by washing two times with a 1:1 solution of 200 mM NH4HCO3/Acetonitrile (AcN). Gel slices were reduced with 2 mM TCEP (Tris[2-carboxyethyl]phosphine) in 25 mM ammonium bicarbonate (pH 8.0) for 15 minutes at 37°C. Proteins were alkylated with 20 mM iodoacetamide in 25 mM ammonium bicarbonate (pH 8.0) for 30 minutes at 37°C in the dark. Gel slices were dried and rehydrated with sequencing grade modified trypsin (Promega, Fitchburg, WI) in 10% AcN, 40 mM NH4HCO3, pH 8.0; 0.1% wt/vol n-octylglucoside (1-O-n-Octyl-beta-D-glucopyranoside) for 16 hours at 37°C. Digested peptides were passed over a C18 Ziptip (Millipore, Billerica, MA), spotted on a matrix-assisted laser desorption ionization (MALDI) plate and overlaid with alpha-cyano-4-hydroxycinnamic acid matrix. MS/MS analysis was performed on an Applied Biosystems 4800 Proteomics Analyzer MALDI time-of-flight (MALDI-TOF; Applied Biosystems, Foster City, CA) mass spectrometer. Mass spectra were analyzed using the MASCOT primary peptide sequence database. Factors identified by MS/MS analysis were confirmed to bind the OEE by Western blot following DNA affinity purification.
Chromatin Immunoprecipitation
DNA and trans-acting factors were cross-linked with 1% formaldehyde in basal or dexamethasone-treated HREC. The Millipore Chromatin Immunoprecipitation (ChIP) Assay Kit was used to investigate regions of DNA bound by identified factors p54 and PSF. All antibodies (p54, PSF, and GR) were from Santa Cruz Biotechnology (Santa Cruz, CA). Each antibody was used at 2 μg per ChIP reaction. Indicated primers were used to specifically amplify promoter regions of claudin-5, 5′-GCCAGGTGAGAAGGGAGAGCCTTG and 5′-CTGAGCACACACTCTGCCCCAGGAG, occludin, 5′-ATTTTAACCCCTCTAAGTAATTGTC and 5′-CAGCACTTTGATAGGAAAGGCACG, cadherin-9, 5′-GGCATGTATGTGGTCCCAGCTACTT and 5′-CCTTCTACTCACAACAGTAAAATG, and the distal portion of the claudin-5 promoter, 5′-CTCCCACCTGGTGGGAAGGTGGCA and 5′-CAGAGGCTCCTGCTGACTTCGGAGG. The same protocol was used to demonstrate that p54 and PSF bound to the OEE in rat retina under basal conditions. Indicated primers were used to specifically amplify the promoter regions of occludin, 5′-GAGCTAAACCCCCAACCCCATCTC and 5′-AGGAGGCTGATAAGAACTGAGTCC, and claudin-5, 5′-CTGGGAGCAGACTAGACTTAGGTG and 5′-GGATGGTCAATTGGACCACCTTCT from rat retina.
Small Interfering RNA Transfection
Small interfering (siRNA) duplexes were designed against human and bovine p54 sequences. A smartpool (Dharmacon, Lafayette, CO) against human p54 as well as individual oligos, 10 (GGAUGGGUCAGAUGGCUAU) and 11 (GUCAAUUCUGUGUGGUAUA). were used to verify the specificity of the knockdown in HREC. Oligo 10 was used for the knockdown of p54 in BREC. Cells were transfected with the Lonza Nucleofection System using the HCAEC Nucleofection kit (Lonza, Basel, Switzerland). In short, 5 × 105 cells were resuspended in 100 μL nucleofection solution and transfected with 200 pmol siRNA using program S-005. Cells were resuspended in media and seeded on culture dishes, or directly onto Transwells or ECIS arrays (Applied Biophysics, Troy, NY). After approximately 48 hours, the confluent monolayers were switched to low serum media and treated with 200 ng/mL dexamethasone for 24 hours. Cells were harvested for Western blot analysis or used in functional permeability assays.
Functional Permeability Measures
BRECs were transfected with siRNA and seeded on Transwells (Corning, Corning, NY) or 8W10E+ arrays (Applied Biophysics) at 20k cells/cm2. After approximately 48 hours, confluent monolayers were switched to 1% FBS media and treated with dexamethasone. The resistance of the monolayers was measured using the ECIS Z-theta system at 4000 Hz once every hour.31 Transport assays were performed using 70-kDa Rhodamine B isothiocyanate (RITC)-dextran to mimic paracellular flux of an albumin-sized molecule according to a previously described protocol.32 Briefly, the rate of flux, Po, was calculated over the 4-hour time course from the following formula:
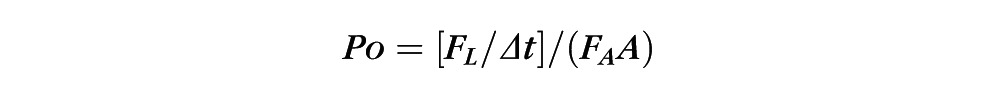 |
where Po is in centimeters per second; FL is basolateral fluorescence; FA is apical fluorescence; Δt is change in time; A is surface area of the filter; and VA is volume of the basolateral chamber.
Results
A Protein Complex Containing p54 and PSF Binds to the OEE cis-Element
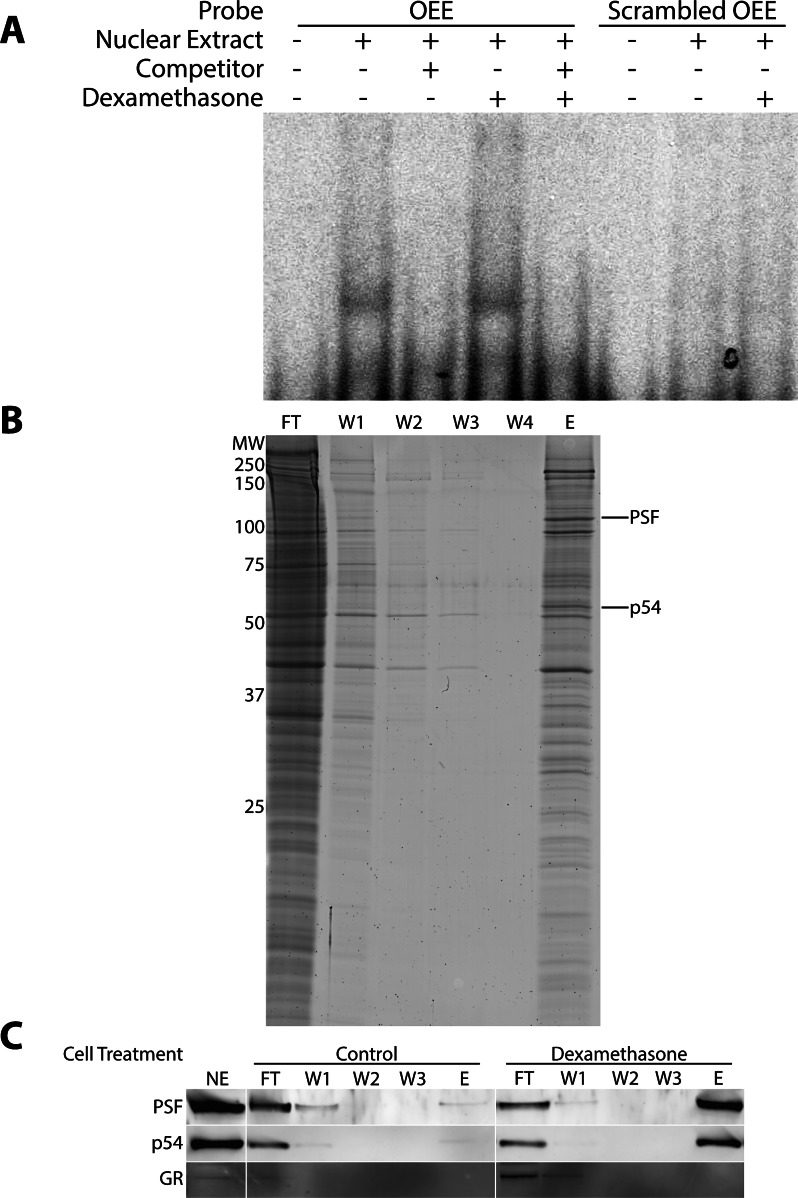
We previously identified the OEE as a DNA element in the promoter region of occludin required for transactivation in response to GC. We hypothesized this transactivation was due to the presence of DNA-binding proteins other than the GR that exhibit increased DNA binding activity following GC treatment. To test this hypothesis, EMSA was used to determine the conditions necessary for trans-acting factor binding to the OEE and to test the effects of GC treatment on the OEE binding activity (Fig. 1A). Endothelial cells were treated with or without dexamethasone for 24 hours and nuclear extracts were prepared and incubated with a 32P-labeled OEE probe. A complex was observed to form and was successfully competed away by the inclusion of an excess of unlabeled probe. A labeled, scrambled OEE probe did not form a complex with nuclear extract, indicating a specific interaction between protein complex and OEE sequence. Thus, specific OEE binding activity was apparent in these cells. In addition, EMSA suggested that the amount of specific OEE binding activity was increased when cells were treated with dexamethasone. Next, the GC-induced trans-acting factors interacting with the OEE cis-element were identified by affinity capture with immobilized OEE followed by MS/MS identification. Biotinylated OEE duplexes were immobilized and incubated with nuclear extract from dexamethasone-treated HREC. Two enriched trans-acting factors that form a heterodimer were identified in the eluate by MS/MS analysis, p54, and PSF (Fig. 1B). DNA affinity purification was again performed, using nuclear extracts from dexamethasone-treated and untreated HREC. The amount of total and OEE-bound p54 and PSF was investigated by Western blot (Fig. 1C). The GR was examined as a negative control for binding to show specific interaction and provide further evidence that the GR does not directly bind the OEE. The analysis demonstrated that dexamethasone treatment strongly increased the amount of both p54 and PSF specifically bound to the OEE without altering the amount of p54 and PSF present in the input samples. Thus, GC treatment increased the specific binding activity of the p54/PSF complex.
Figure 1.
The OEE binds the transcription factors p54/NONO and PSF following dexamethasone treatment. (A) Nuclear extract was prepared from dexamethasone-treated BREC and incubated with 32P-labeled OEE oligos. EMSA analysis showed selective binding of a protein complex to the labeled probe, which was competed away by addition of an excess of cold probe. The scrambled control does not bind the complex. (B) HRECs were treated with dexamethasone for 24 hours and nuclear extract (NE) was incubated with Dynabead-immobilized OEE oligos in a DNA affinity purification protocol. Flow-through (FT), successive washes (W1–W4) and a high salt elution (E) were run on SDS-PAGE to identify bound complexes, demonstrating selective enrichment of certain proteins. Bands were excised from the gel and analyzed by MS/MS following trypsin digest. The bands identified as p54 and PSF are indicated. Confirmation of the MS/MS analysis is shown in (C), as p54 and PSF from dexamethasone-treated nuclear extract bound the immobilized OEE strongly following DNA affinity purification followed by Western blot.
p54 and PSF Bind the OEE in the Promoters of Multiple Junction Genes.
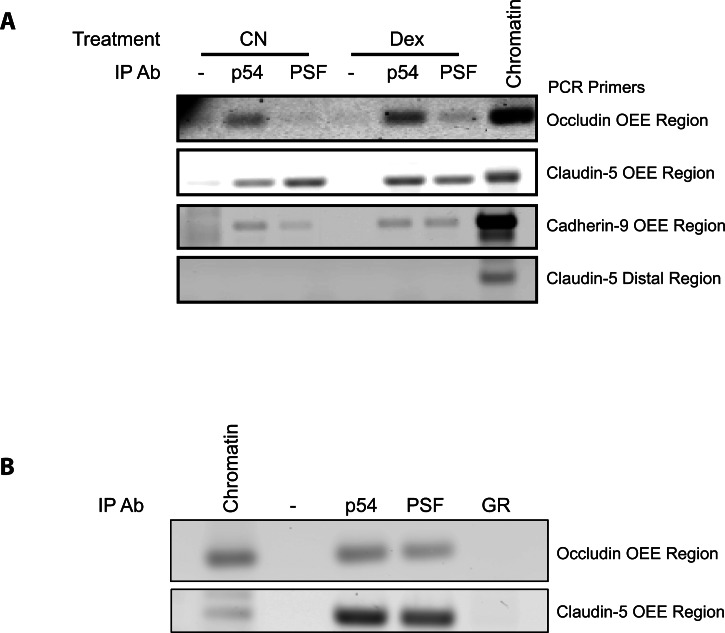
To test in situ binding of p54 and PSF to the OEE, ChIP was performed from basal and dexamethasone-treated HREC (Fig. 2A). Antibodies targeting either p54 or PSF were used to immunoprecipitate the trans-acting factors cross-linked to chromosomal fragments. Regions of DNA that coprecipitated with the trans-acting factors were detected by PCR. As a negative control, ChIP of a distal portion of claudin-5 promoter not containing the OEE was examined. Pull-down with either p54 or PSF antibodies led to coprecipitation of the OEE-containing region of the occludin promoter, as well as regions containing OEE-homologous elements in the claudin-5 and cadherin-9 promoters. Although quantitative ChIP was not performed, binding of both factors was observed in control and dexamethasone-treated samples. No amplification of the distal portion of the claudin-5 promoter occurred following ChIP. To determine if p54 and PSF bound the OEE in the retina, ChIP was performed using nuclear extracts from rat whole retinal tissue as the original input (Fig. 2B). ChIP with p54 and PSF antibodies yielded amplicons for both the occludin and claudin-5 OEE regions in untreated retinas, suggesting these factors bind to the OEE in vivo.
Figure 2.
p54 and PSF bind to the OEE in cells and retina. (A) HRECs, under basal conditions and after dexamethasone treatment, were treated with formaldehyde to cross-link DNA and trans-acting factors. ChIP assays showed the binding of p54 and PSF to the OEE or homologous OEE present in the promoters of occludin, claudin-5, and cadherin-9 using specific primers flanking the OEE sequence. The distal region of the claudin-5 promoter region not containing the OEE sequence served as a negative control. (B) Rats were killed and retinas were flash frozen. The tissue was subjected to the same ChIP protocol to show that p54 and PSF basally bound the OEE in vivo in rodent retina.
p54 Is Essential for the GC Induction of Occludin and Claudin-5 in Endothelial Cells.
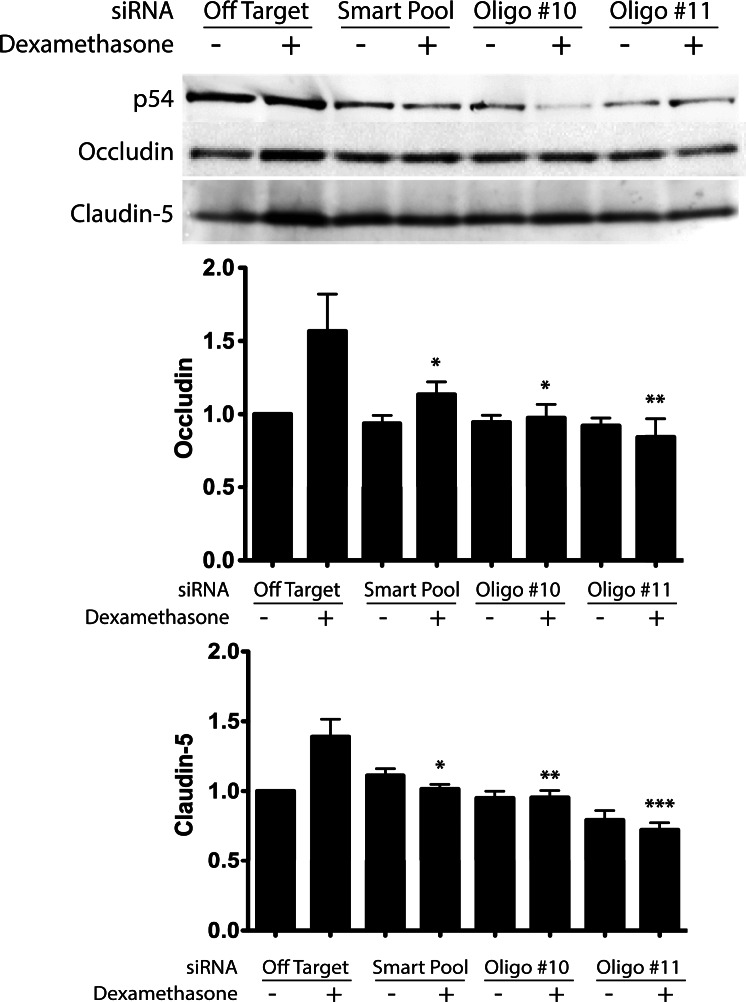
To determine the necessity of p54 in the process of steroid-induced expression of TJ genes in endothelial cells, siRNA was designed to specifically knock down p54 expression. A smartpool of four siRNA duplexes provided a robust knockdown (60%–80%) of p54 in transiently transfected HREC, which was recapitulated with two individual siRNAs from the pool (Fig. 3). Knockdown of PSF with siRNA could not be achieved despite repeat attempts (data not shown). Cells transfected with nontargeting siRNA showed a robust increase of occludin and claudin-5 (∼60% and 40%, respectively) protein content after 24 hours of dexamethasone treatment, as previously reported.28 Knockdown of p54 in these cells using either the smartpool or the individual siRNA duplexes significantly attenuated the GC-induced increase of both occludin and claudin-5 expression. Thus, p54 contributed to the GC-induced transactivation of TJ protein expression.
Figure 3.
p54 knockdown ablates the steroid-induction of occludin and claudin-5. (A) HRECs were transfected with siRNA targeted against p54 and treated with or without dexamethasone in stepdown media. The GC-induced increase of occludin and claudin-5 is prevented by knockdown of p54 using a smartpool of multiple p54-targeted siRNA oligos and individually targeted oligos (#10 and #11). (B) Quantification of protein levels by Western blot showed a significant ablation of dexamethasone-induction of both occludin and claudin-5. *P < 0.05, **P < 0.01, ***P < 0.001.
p54 Knockdown Ablates the GC Induction of Barrier Properties in an Endothelial Monolayer.
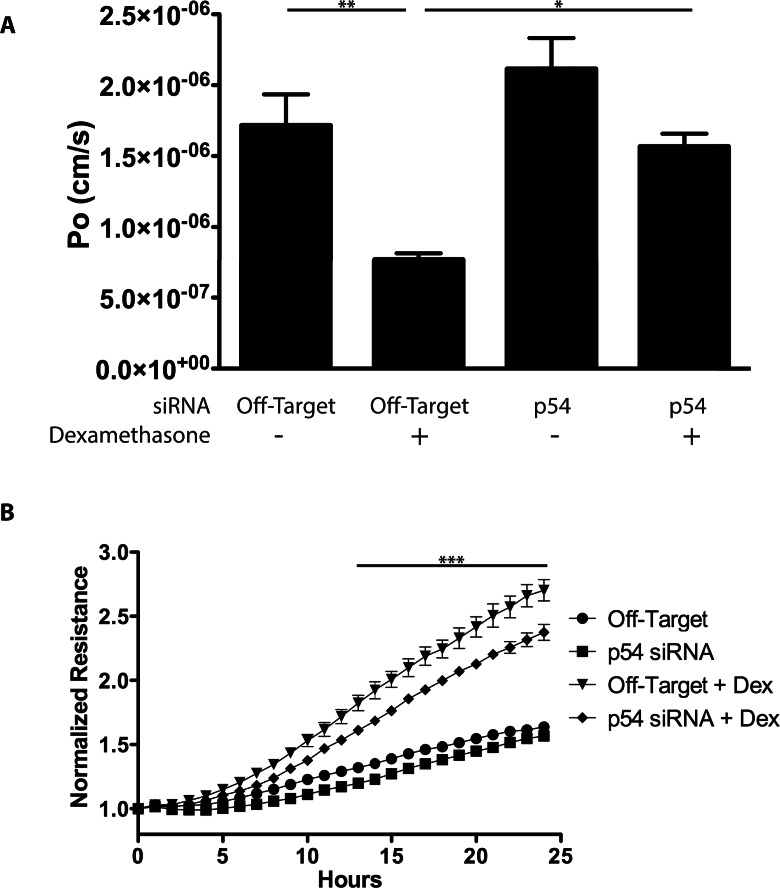
Knockdown with siRNA was further used to investigate the contribution of p54 to the effect of GC on barrier properties of endothelial cells. BREC were transfected with siRNA targeting p54 and seeded on porous 0.4-μm Transwell filters. Confluent monolayers were cultured in 1% FBS media with or without dexamethasone for 24 hours. After treatment, a 70-kDa RITC-dextran tracer was placed in the apical chamber and its accumulation in the basolateral chamber was measured over 4 hours to determine permeability. Dexamethasone treatment in cells transfected with nontargeting siRNA exhibited a significantly reduced permeability of 45% compared with basal conditions (Fig. 4A), similar to the effect observed in previous reports.28 Knockdown of p54 reduced this pro-barrier response to dexamethasone treatment to roughly 91% of basal control cells, significantly higher than dexamethasone treatment alone. p54 knockdown exhibited a permeability 23% greater than the basal control, a difference that was not statistically significant. Barrier induction in BREC was also studied using real-time measures of TER on the ECIS Z-theta system at 4000 Hz once every hour (Fig. 4B). Dexamethasone treatment resulted in a 60% to 70% increase in TER in cells transfected with nontargeting siRNA. Knockdown of p54 attenuated the increase after dexamethasone treatment, resulting in a highly significant 15% decrease compared with dexamethasone-treated cells without p54 knockdown.
Figure 4.
p54 knockdown prevents the barrier induction properties of dexamethasone. (A) BRECs were transfected with siRNA targeting p54 and seeded on 0.4-μm Transwell filters. After 48 hours, cells were switched to stepdown media with or without dexamethasone for 24 hours. Permeability to 70-kDa RITC-dextran tracer over a 4-hour time period was measured. The reduction in endothelial permeability to the dextran after dexamethasone treatment was ablated by p54 knockdown (P < 0.05). (B) BRECs transfected with siRNA against p54 were seeded on 8W10E+ arrays and TER was measured continually on the ECIS Z-theta instrument. GC treatment increased electrical resistance and knockdown of p54 decreased the GC induction of electrical resistance. After 13 hours of dexamethasone treatment and until the end of the experiment, p54 knockdown significantly reduced the glucocorticoid-induction compared with control (P < 0.001). *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
GC treatment is a clinically useful therapy that can effectively reduce macular edema caused by diabetic retinopathy,5 retinal vein occlusions,33 and uveitis.34 GCs may be used solely or in combination with other therapies to prevent macular edema.35 Induction of BRB genes may contribute an important part of this therapeutic effect. Multiple new formulations of GC are being developed and used effectively in the clinic, including dexamethasone36 and fluocinolone acetonide5 implants. Use of dexamethasone in our studies was designed to specifically investigate the role of p54 and the OEE in relation to dexamethasone. It is likely these findings will translate to other clinically used GCs, including triamcinolone acetonide and fluocinolone acetonide due to a shared mechanism of action through the GR. Understanding the mechanisms by which GCs promote barrier tightening may allow development of novel therapeutics that circumvent the adverse side effects of current treatment regimens. We report the identification of the heterodimeric binding partners, p54 and PSF, as part of the complex that binds the OEE in the occludin promoter. MS/MS analysis and Western blotting of the protein complex captured by immobilized OEE revealed the enrichment of both p54 and PSF. Furthermore, other junctional genes that exhibit steroid responsiveness, including claudin-5 and cadherin-9, contain promoter elements homologous to the OEE. ChIP analysis revealed loading of the p54 and PSF proteins onto the homologous OEE in both HREC and in the retina. Finally, knockdown of p54 with siRNA prevented both GC induction of tight junction gene expression and barrier enhancement. Collectively, these data suggest the OEE acts to coordinate junction gene expression in response to GC treatment by increasing transcription of occludin, claudin-5, and cadherin-9. It is important to note that siRNA targeted against p54 completely blocked the GC-induced reduction in permeability to 70-kDa RITC-dextran while ion flux was only partially blocked. The more modest decrease in TER may be due to an incomplete p54 knockdown, alternative mechanisms of barrier induction not affected by p54 knockdown, or differences in the dexamethasone induction of ion versus solute flux. Importantly, large solute flux occurs through broken junctions,37 whereas ion flux occurs through claudin pores.38 Thus, the trans-acting factor p54 may affect barrier properties through gene expression of additional TJ and adherens junction genes as well as proteins involved in junction organization and regulation. Although not statistically different, there was a trend toward increased permeability with p54 knockdown, suggesting a basal role in induction of the barrier separate from steroid treatment.
The heterodimeric p54/PSF transcription factor functions in multiple, diverse cellular roles. Widely conserved and originally identified in Drosophila, both factors have been described to act alone and as part of a heterodimer in transcription,39 RNA splicing, DNA repair, and RNA retention,40 and have been elegantly shown to be involved in the coupling of transcription and splicing activity.41,42 Multiple reports also show that p54/PSF is required as a coactivator43 interacting with other trans-acting factors rather than binding to the DNA cis-element directly. These reports describe transcription of genes of variable functionality, but interestingly, a number of interactions involve genes regulated by nuclear hormone receptors, including the progesterone44 and androgen receptors.45
Transcription of the occludin and claudin-5 genes has been investigated in the interest of understanding the mechanism of barrier induction and to improve therapeutic options for diseases that involve loss of the BBB and BRB. Previous work has identified a number of cis-sequences of the occludin and claudin-5 promoters, as well as transcription factors that regulate their expression (Figs. 5A, 5B). Cytokines, such as TNF-α and IFN-γ reduce expression from the occludin promoter in a luciferase reporter assay.46 Transcription of occludin is inhibited by YY1 binding sites clustered at approximately −1200 to −1700,47 whereas SP3 sites present directly upstream of the transcription start site have been postulated to induce transcription of the gene,47 thus providing a potential model for differential expression of occludin in brain versus lung vascular endothelium. Additionally, TTF-1, an oncogene amplified in lung cancers, interacts with a sequence at −27 to −37 of the occludin promoter to increase expression and paradoxically decrease metastatic potential.48 Our group and others have previously demonstrated GC induction of occludin expression. A distal imperfect glucocorticoid response element (GRE) sequence present in the occludin promoter has been postulated to direct this GC response.49 In the current studies, a smaller promoter was used that did not include the previously mentioned GRE-like sequence. This shorter promoter maintained GC responsiveness and mutations of potential GRE half-sites did not abolish the GC effect.28 Instead, ChIP analysis demonstrated that p54 and PSF transcription factors bind the OEE of occludin and homologous OEEs of claudin-5 and cadherin-9, whereas GR fails to bind. Further, siRNA knockdown experiments revealed that p54 is required for the steroid induction of barrier properties, suggesting p54/PSF binding to the OEE contributes to gene regulation of occludin and claudin-5. However, we did not observe an increase in p54 or PSF protein content in nuclear extracts with steroid treatment, suggesting that GCs do not act by simply increasing p54 expression. Alternatively, GC treatment may increase the specific DNA binding activity of the p54/PSF complex or induce another trans-acting protein that facilitates p54/PSF binding to the OEE.
Figure 5.
Known cis-regulatory sequences of the occludin and claudin-5 promoters. A schematic of occludin and claudin-5 promoters is presented showing activating cis-sequences above the line and inhibitory cis-sequences below the line. (A) The occludin promoter is shown from −2000 to the transcriptional start site, indicating clustered inhibitory YY1 sites,47 an imperfect distal GRE,49 clustered SP3 sites near the transcription start site,47 lung oncogene TTF-1,48 and the RH4 of the OEE, −126 to −114 of the promoter.28 (B) The claudin-5 promoter is positively regulated by FoxO1,54 ERG,55 and SOX18,56 and inhibited by nuclear factor–κB signaling.60 The homologous OEE is shown at position −1159 to −1147 of the promoter.28
Induction of the BBB and BRB has been investigated for many years to gain insight into the factors that drive endothelial barrier formation. This research has revealed a number of mechanisms responsible for regulating expression of some of the necessary barrier genes. Astrocyte50 and pericyte51 interactions with endothelial cells, Wnt signaling,52 and Sonic Hedgehog53 all contribute to the vascular barrier phenotype. FoxO1,54 ERG,55 and SOX1856 have all been implicated as trans-acting factors responsible for control of claudin-5 expression, with cell-cell contact being necessary to relieve inhibition of its expression by Tcf-4/β-catenin.54 Although previous reports have also detailed GC-induced expression of claudin-5 in the BBB57,58 or blood-nerve barrier,59 to our knowledge, description of the OEE/p54/PSF is the first report of a promoter element and trans-acting factor combination responsible for GC induction of claudin-5 gene expression. In addition, ChIP results using extracts from rat retina suggest that the p54/PSF complex is bound to the occludin and claudin-5 promoters in vivo. How the activity of these factors is controlled and the contribution of the OEE to endothelial barrier induction in the brain and retina are areas of future research that may provide insights for treating diseases characterized by loss of the BBB or BRB.
Acknowledgments
The authors thank Edward A. Felinski for his excellent technical skills and Steven Abcouwer for helpful suggestions and critical reading of the manuscript.
Supported by National Institutes of Health Grants R21 EY019392 and R01 EY012021 (DAA), Research to Prevent Blindness (DAA), and the Core Center for Vision Research at the Kellogg Eye Center (P30 EY007003).
Disclosure: J.M. Keil, None; X. Liu, None; D.A. Antonetti, None
References
- 1. Klein R, Klein BE, Moss SE. The Wisconsin epidemiological study of diabetic retinopathy: a review. Diabetes Metab Rev. 1989; 5: 559–570 [DOI] [PubMed] [Google Scholar]
- 2. Virgili G, Parravano M, Menchini F, Brunetti M. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for diabetic macular oedema. Cochrane Database Syst Rev. 2012; 12: CD007419 [DOI] [PubMed] [Google Scholar]
- 3. Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012; 130: 972–979 [DOI] [PubMed] [Google Scholar]
- 4. Tsilimbaris MK, Panagiotoglou TD, Charisis SK, Anastasakis A, Krikonis TS, Christodoulakis E. The use of intravitreal etanercept in diabetic macular oedema. Semin Ophthalmol. 2007; 22: 75–79 [DOI] [PubMed] [Google Scholar]
- 5. Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012; 119: 2125–2132 [DOI] [PubMed] [Google Scholar]
- 6. Gillies MC, Simpson JM, Gaston C, et al. Five-year results of a randomized trial with open-label extension of triamcinolone acetonide for refractory diabetic macular edema. Ophthalmology. 2009; 116: 2182–2187 [DOI] [PubMed] [Google Scholar]
- 7. Stewart MW. Corticosteroid use for diabetic macular edema: old fad or new trend? Curr Diab Rep. 2012; 12: 364–375 [DOI] [PubMed] [Google Scholar]
- 8. Jonas JB, Kreissig I, Degenring R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003; 87: 24–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aveleira CA, Lin CM, Abcouwer SF, Ambrosio AF, Antonetti DA. TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010; 59: 2872–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zettl KS, Sjaastad MD, Riskin PM, Parry G, Machen TE, Firestone GL. Glucocorticoid-induced formation of tight junctions in mouse mammary epithelial cells in vitro. Proc Natl Acad Sci U S A. 1992; 89: 9069–9073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Romero IA, Radewicz K, Jubin E, et al. Changes in cytoskeletal and tight junctional proteins correlate with decreased permeability induced by dexamethasone in cultured rat brain endothelial cells. Neurosci Lett. 2003; 344: 112–116 [DOI] [PubMed] [Google Scholar]
- 12. Aijaz S, Balda M, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol. 2006; 248: 261–298 [DOI] [PubMed] [Google Scholar]
- 13. Antonetti DA, Barber AJ, Khin S, et al. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content. Diabetes. 1998; 47: 1953–1959 [DOI] [PubMed] [Google Scholar]
- 14. Klaassen I, Hughes JM, Vogels IM, Schalkwijk CG, Van Noorden CJ, Schlingemann RO. Altered expression of genes related to blood-retina barrier disruption in streptozotocin-induced diabetes. Exp Eye Res. 2009; 89: 4–15 19284967 [Google Scholar]
- 15. Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999; 147: 185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barber AJ, Antonetti DA. Mapping the blood vessels with paracellular permeability in the retinas of diabetic rats. Invest Ophthalmol Vis Sci. 2003; 44: 5410–5416 [DOI] [PubMed] [Google Scholar]
- 17. Runkle EA, Antonetti DA. The blood-retinal barrier: structure and functional significance. Methods Mol Biol. 2011; 686: 133–148 [DOI] [PubMed] [Google Scholar]
- 18. Nitta T, Hata M, Gotoh S, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003; 161: 653–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murakami T, Felinski EA, Antonetti DA. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J Biol Chem. 2009; 284: 21036–21046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murakami T, Frey T, Lin CM, Antonetti DA. Protein kinase Cb phosphorylates occludin regulating tight junction trafficking in vascular endothelial growth factor–induced permeability in vivo. Diabetes. 2012; 61: 1573–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirase T, Staddon JM, Saitou M, et al. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997; 110: 1603–1613 [DOI] [PubMed] [Google Scholar]
- 22. Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998; 60: 121–142 [DOI] [PubMed] [Google Scholar]
- 23. Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011; 73: 283–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaal ECA, Vecht CJ. The management of brain edema in brain tumors. Curr Opin Oncol. 2004; 16: 593–600 [DOI] [PubMed] [Google Scholar]
- 25. Heiss JD, Papavassiliou E, Merrill MJ, et al. Mechanism of Dexamethasone suppression of brain tumor-associated vascular permeability in rats. J Clin Invest. 1996; 98: 1400–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Antonetti DA, Wolpert EB, DeMaio L, Harhaj NS, Scaduto RCJ. Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J Neurochem. 2002; 80: 667–677 [DOI] [PubMed] [Google Scholar]
- 27. Forster C, Silwedel C, Golenhofen N, et al. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J Physiol. 2005; 565: 475–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Felinski EA, Cox AE, Phillips BE, Antonetti DA. Glucocorticoids induce transactivation of tight junction genes occludin and claudin-5 in retinal endothelial cells via a novel cis-element. Exp Eye Res. 2008; 86: 867–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Antonetti DA, Wolpert EB. Isolation and characterization of retinal endothelial cells. Methods Mol Med. 2003; 89: 365–374 [DOI] [PubMed] [Google Scholar]
- 30. Phillips BE, Cancel L, Tarbell JM, Antonetti DA. Occludin independently regulates permeability under hydrostatic pressure and cell division in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2008; 49: 2568–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chinnaswamy T, Malik AB, Del Vecchio PJ, Keese CR, Giaver I. Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci U S A. 1992; 89: 7919–7923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DeMaio L, Antonetti DA, Scaduto RC Jr, Gardner TW, Tarbell JM. VEGF increases paracellular transport without altering the solvent-drag reflection coefficient. Microvasc Res. 2004; 68: 295–302 [DOI] [PubMed] [Google Scholar]
- 33. Channa R, Smith M, Campochiaro PA. Treatment of macular edema due to retinal vein occlusions. Clin Ophthalmol. 2011; 5: 705–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. LeHoang P. The gold standard of noninfectious uveitis: corticosteroids. Dev Ophthalmol. 2012; 51: 7–28 [DOI] [PubMed] [Google Scholar]
- 35. Wilson CA, Berkowitz BA, Sato Y, Ando N, Handa JT, de Juan E. Treatment with intravitreal steroid reduces blood-retinal barrier breakdown due to retinal photocoagulation. Arch Ophthalmol. 1992; 110: 1155–1159 [DOI] [PubMed] [Google Scholar]
- 36. Zucchiatti I, Lattanzio R, Querques G, et al. Intravitreal dexamethasone implant in patients with persistent diabetic macular edema. Ophthalmologica. 2012; 228: 117–122 [DOI] [PubMed] [Google Scholar]
- 37. Lopez-Quintero SV, Ji XY, Antonetti DA, Tarbell JM. A three-pore model describes transport properties of bovine retinal endothelial cells in normal and elevated glucose. Invest Ophthalmol Vis Sci. 2011; 52: 1171–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Raleigh DR, Boe DM, Yu D, et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol. 2011; 193: 565–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bianconcini A, Lupo A, Capone S, et al. Transcriptional activity of the murine retinol-binding protein gene is regulated by a multiprotein complex containing HMGA1, p54 nrb/NonO, protein-associated splicing factor (PSF) and steroidogenic factor 1 (SF1)/liver receptor homologue 1 (LRH-1). Int J Biochem Cell Biol. 2009; 41: 2189–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Z, Carmichael GG. The fate of dsRNA in the nucleus: a p54nrb-containing complex mediates the nuclear retention of promiscuously A-to-I edited RNAs. Cell. 2001; 106: 465–475 [DOI] [PubMed] [Google Scholar]
- 41. Hata K, Nishimura R, Muramatsu S, et al. Paraspeckle protein p54nrb links Sox9-mediated transcription with RNA processing during chondrogenesis in mice. J Clin Invest. 2008; 118: 3098–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kameoka S, Duque P, Konarska MM. p54nrb associates with the 5′ splice site within large transcription/splicing complexes. EMBO J. 2004; 23: 1782–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amelio AL, Miraglia LJ, Conkright JJ, et al. A coactivator trap identifies NONO (p54nrb) as a component of the cAMP-signaling pathway. Proc Natl Acad Sci U S A. 2007; 104: 20314–20319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dong X, Yu C, Shynlova O, Challis JR, Rennie PS, Lye SJ. p54nrb is a transcriptional corepressor of the progesterone receptor that modulates transcription of the labor-associated gene, connexin 43 (Gja1). Mol Endocrinol. 2009; 23: 1147–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ishitani K, Yoshida T, Kitagawa H, Ohta H, Nozawa S, Kato S. p54nrb acts as a transcriptional coactivator for activation function 1 of the human androgen receptor. Biochem Biophys Res Commun. 2003; 306: 660–665 [DOI] [PubMed] [Google Scholar]
- 46. Mankertz J, Tavalali S, Schmitz H, et al. Expression from the human occludin promoter is affected by tumor necrosis factor α and interferon γ. J Cell Sci. 2000; 113: 2085–2090 [DOI] [PubMed] [Google Scholar]
- 47. Sade H, Holloway K, Romero IA, Male D. Transcriptional control of occludin expression in vascular endothelia: Regulation by Sp3 and YY1. Biochim Biophys Acta. 2009; 1789: 175–184 [DOI] [PubMed] [Google Scholar]
- 48. Runkle EA, Rice SJ, Qi J, et al. Occludin is a direct target of thyroid transcription factor-1 (TTF-1/NKX2-1). J Biol Chem. 2012; 287: 28790–28801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harke N, Leers J, Kietz S, Drenckhahn D, Forster C. Glucocorticoids regulate the human occludin gene through a single imperfect palindromic glucocorticoid response element. Mol Cell Endocrinol. 2008; 295: 39–47 [DOI] [PubMed] [Google Scholar]
- 50. Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006; 7: 41–53 [DOI] [PubMed] [Google Scholar]
- 51. Lai CH, Kuo KH. The critical component to establish in vitro BBB model: pericyte. Brain Res Brain Res Rev. 2005; 50: 258–265 [DOI] [PubMed] [Google Scholar]
- 52. Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008; 322: 1247–1250 [DOI] [PubMed] [Google Scholar]
- 53. Alvarez JI, Dodelet-Devillers A, Kebir H, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011; 334: 1727–1731 [DOI] [PubMed] [Google Scholar]
- 54. Taddei A, Giampietro C, Conti A, et al. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008; 10: 923–934 [DOI] [PubMed] [Google Scholar]
- 55. Yuan L, Le Bras A, Sacharidou A, et al. ETS-related gene (ERG) controls endothelial cell permeability via transcriptional regulation of the claudin 5 (CLDN5) gene. J Biol Chem. 2012; 287: 6582–6591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fontijn RD, Volger OL, Fledderus JO, Reijerkerk A, de Vries HE, Horrevoets AJ. SOX-18 controls endothelial-specific claudin-5 gene expression and barrier function. Am J Physiol Heart Circ Physiol. 2008; 294: H891–H900 [DOI] [PubMed] [Google Scholar]
- 57. Sadowska GB, Malaeb SN, Stonestreet BS. Maternal glucocorticoid exposure alters tight junction protein expression in the brain of fetal sheep. Am J Physiol Heart Circ Physiol. 2010; 298: H179–H188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Burek M, Forster CY. Cloning and characterization of the murine claudin-5 promoter. Mol Cell Endocrinol. 2009; 298: 19–24 [DOI] [PubMed] [Google Scholar]
- 59. Kashiwamura Y, Sano Y, Abe M, et al. Hydrocortisone enhances the function of the blood-nerve barrier through the up-regulation of claudin-5. Neurochem Res. 2011; 36: 849–855 [DOI] [PubMed] [Google Scholar]
- 60. Aslam M, Ahmad N, Srivastava R, Hemmer B. TNF-alpha induced NFkappaB signaling and p65 (RelA) overexpression repress Cldn5 promoter in mouse brain endothelial cells. Cytokine. 2012; 57: 269–275 [DOI] [PubMed] [Google Scholar]