Abstract
A network of intracellular signaling pathways and complex intercellular interactions regulate calcium mobilization in vascular cells, arteriolar tone, and blood flow. Different endothelium-derived vasoreactive factors have been identified and the importance of myoendothelial communication in vasoreactivity is now well appreciated. The ability of many vascular networks to conduct signals upstream also is established. This phenomenon is critical for both short-term changes in blood perfusion as well as long-term adaptations of a vascular network. In addition, in a phenomenon termed vasomotion, arterioles often exhibit spontaneous oscillations in diameter. This is thought to improve tissue oxygenation and enhance blood flow. Experimentation has begun to reveal important aspects of the regulatory machinery and the significance of these phenomena for the regulation of local perfusion and oxygenation. Mathematical modeling can assist in elucidating the complex signaling mechanisms that participate in these phenomena. This review highlights some of the important experimental studies and relevant mathematical models that provide the current understanding of these mechanisms in vasoreactivity.
Keywords: vascular tone, smooth muscle, endothelium, vasomotion, spreading responses
I. INTRODUCTION
Calcium (Ca2+) is a universal signaling molecule, and in the microcirculation it plays a central role in important physiological functions including the regulation of vascular tone. An elaborate signaling network exists that regulates Ca2+ concentrations in the vascular wall. This network includes intracellular signaling as well as intercellular communication with paracrine factors or diffusion of species through gap junctions. Signaling between vascular cells affects Ca2+ levels and coordinates vessel function. The importance of myoendothelial communication is now well-appreciated and continuing investigations reveal important aspects of how the 2 cell layers interact. Recent evidence, for example, suggests the presence of localized signaling machinery for Ca2+ control in specialized cellular extensions between the endothelial cell (EC) and the smooth muscle cell (SMC) (often referred to as myoendothelial projections).1 In addition to the local signaling between the 2 cell layers, focal stimulation in some vasculatures leads to spreading Ca2+ and vasomotor responses along the vascular tree, and this ability of vessels to conduct signals is critical for both the rapid and long-term coordination of the vascular network.2 Also, synchronization of Ca2+ oscillations between many cells in the vascular wall causes coordinated rhythmic oscillations in vessel diameter (i.e., vasomotion), which plays an important role in the regulation of tissue perfusion and oxygenation.3 Experiments continuously provide new insights about this elaborate network and the mechanisms that regulate vascular function. Mathematical modeling offers an alternative tool for the analysis of this complex signaling system.
Significant progress has been made in the development of comprehensive models of Ca2+ dynamics for some systems such as in cardiac myocytes.4–8 Often these models are integrated with descriptions for membrane electrophysiology, cell mechanics, and metabolic and signal-transduction pathways, and multicellular/multiscale models have emerged for the heart.9–14 These models are capable of describing function at the tissue level while integrating mechanisms at the subcellular/molecular level and have been utilized to investigate physiological function as well as disease states.15–18 Although Ca2+ signaling in various cell types shows many common features and motifs,19 there are significant differences in Ca2+ mobilization between cardiac and vascular cells.20–25 Recently, similar attempts have been made in the modeling of the vasculature and cellular models of Ca2+ dynamics have emerged for vascular cells. These single-cell models can form the basis for integrated models of the vasculature.
This review focuses on recent efforts in the development of mathematical models to describe vascular Ca2+ dynamics in single cells and in multicellular vascular segments. These models investigate intercellular communication in the vessel wall and the mechanisms that regulate vasoreactivity in the microcirculation.
II. VASCULAR CELL MODELS
A series of Ca2+ dynamics models for vascular cells have been presented and recently reviewed.26 The level of detail in these models ranges from minimal models with phenomenologic descriptions to models with detailed descriptions of transmembrane currents. Models exist that incorporate biophysical descriptions for the signaling pathways leading to or affected by Ca2+ mobilization. Published models also differ in the level of detail in spatial organization, from models with a single cytosolic compartment with single or multiple internal stores to models with cytosolic subcompartments and spatial gradients.
SMCs differ from the electrically nonexcitable ECs in channel and receptor composition and in their contribution to the various Ca2+ mobilization pathways. Though L-type Ca2+ channels and calcium-induced calcium release (CICR) from ryanodine receptor (RyR)–sensitive stores play a predominant role in myocytes, the phosphatidylinositol pathway and capacitative calcium entry (CCE) have a significant role in ECs. Furthermore, the type and density of membrane ion channels exhibit significant differences among species and tissues and probably change with the size of the vessel even within the same vascular bed. This heterogeneity suggests a need for cell- and vessel-specific models of Ca2+ signaling and dynamics in the vasculature.
II.A. Endothelial Cell Models
The first endothelial cell models were based on earlier generic models of calcium dynamics in electrically nonexcitable cells.27–30 Early models started with simple descriptions of Ca2+ handling components and few transmembrane currents. Winston and coworkers31 studied the effects of mechanical strain on cultured bovine pulmonary artery endothelial cells. Ca2+ dynamics and electrical activity of vascular ECs first were examined in a model introduced by Wong and Klassen.32,33
The first in-depth model of calcium dynamics was presented by Wiesner and coworkers34 for human umbilical vein endothelial cells. Their model incorporated detailed thrombin receptor kinetics, as described by Vu et al.35 The model included most of the major calcium mobilization pathways including CICR, CCE, inositol (1,4,5)-trisphosphate receptor (IP3R)–dependent store Ca2+ release, and Ca2+ buffering. The model described IP3 and Ca2+ dynamics and included separate mass balances for cytosolic, store, and buffered Ca2+. Schuster and coworkers36 developed a model to simulate changes in membrane electrical activity following bradykinin stimulation of coronary artery ECs. Experimental data provided empirical correlations for the cytosolic calcium and IP3 changes after stimulation. The focus of the model was to predict K+ currents and membrane potential (Vm) changes in the presence and absence of extracellular Ca2+.
In a recent study, we presented a detailed EC model that integrates both EC Ca2+ dynamics and plasmalemmal electrical activity to investigate EC responses to various stimulatory conditions and the relationship between Ca2+ and Vm.37 The model describes most of the major membrane channels and pumps present in EC of rat mesenteric arteries (RMAs). The plasma membrane includes kinetic descriptions for nonselective cation (NSC) channels, store-operated cation channels, small (SKCa) and intermediate (IKCa) conductance calcium-activated K+ channels, inward rectifier K+ channels (Kir), volume regulated anion channels (VRAC), calcium-activated Cl channels, Na+-Ca2+ exchanger (NCX), and Na+-K+-Cl cotransporter and Na+-K+-adenosine triphosphatase (ATPase) pump. It also includes intracellular Ca2+ handling components such as IP3 receptor, sarco/endoplasmic reticulum, Ca2+-ATPase (SERCA) and plasma membrane Ca2+-ATPase pumps. For the first time, we integrated balances for the major ionic species (i.e., K+, Cl−, Na+) in addition to the balances for cytosolic, store, and buffered Ca2+ and IP3 and the Hodgkin-Huxley–type formalism for the Vm. The model reproduces experimentally observed Ca2+ transients during agonist stimulation.
To date, the majority of the EC models were compartmental in nature. Recently, Hong et al.38 developed a 2-dimensional finite element model that incorporated intracellular resolution and spatial concentration gradients. This study highlighted the need for subcellular resolution in future models of EC Ca2+ dynamics and signaling.
II.B. Smooth Muscle Cell Models
Modeling of Ca2+ dynamics in SMCs has received more attention than in ECs. Early attempts include the generic models of Wong and Klassen,39,40 the oscillatory model of Gonzalez-Fernandez and Ermentrout,41 the IP3-dependent Ca2+ release model in A7r5 cells of Fink and coworkers,42 and the norepinephrine (NE) diffusion model of Bennett and coworkers.43 The complexity of Ca2+ mobilization and the many components affecting a membrane’s electrical activity prompted Parthimos and coworkers44 to develop a minimal model of an SMC. The reduced number of parameters made their model attractive for subsequent studies of vascular signaling.45–47 Model simulations showed chaotic behavior in Ca2+ levels as a result of the nonlinear interaction between a membrane oscillator and an intracellular store calcium oscillator. The model was able to reproduce behavior seen under various experimental conditions and pharmacological interventions.48,49
A different approach was followed by Yang and coworkers.50 Their aim was to develop a detailed model that will incorporate all the known significant components of the plasma membrane in SMCs for rat cerebrovascular arteries. Their study presents perhaps the first attempt at a detailed, tissue-specific vascular model of Ca2+ dynamics. The ability of this study to simulate macroscale responses prompted the development of subsequent models that followed a similar, detailed, tissue-specific modeling approach with significant effort to obtain current descriptions based on electrophysiological recordings.51,52 Combined, these detailed models include descriptions for important transmembrane currents such as voltage-operated Ca2+ channels (VOCCs), voltage-dependent K+ channels (Kv), large conductance Ca2+ activated K+ channels (BKCa), Kir channels, stress-activated NSC channels, Cl channels, NCX, Na+–K+-ATPase, and Ca2+-ATPase pumps. Descriptions for Ca2+, Na+, Cl−, K+, and IP3 balance have been presented. IP3- and Ca2+-sensitive intracellular stores also have been implemented that exhibit CICR and Ca2+ sequestrations through SERCA. Descriptions for the α1-adrenoceptor and nitric oxide (NO)/cyclic guanosine monophosphate (cGMP) signaling pathways have been utilized as well as formulations for the diacylglycerol (DAG) dependence of NSC activation as a mechanism for sustained cell depolarization following adrenoceptor stimulation.52,53 The Ca2+ models have been integrated with biomechanics models capable of simulating diameter responses to Ca2+ mobilization.54,55 In one of the models,51 an intracellular subcompartment accounts for subcellular heterogeneity and the presence of subplasmalemmal microdomains. Elements from the minimal model of Parthimos44 and the detailed approach of Yang50 have been incorporated in an SMC model presented by Jacobsen and coworkers.47 A significant feature of this model is that it abandons the compartmental approach of the previous modeling attempts and provides a description for cytosolic Ca2+ with spatial heterogeneity. This was accomplished by incorporating diffusion of Ca2+ within the cytosol in the longitudinal direction of the cell.
Through this process, it has become obvious that detailed models of Ca2+ dynamics offer many advantages in investigations of Ca2+ signaling. These models, however, have to deal with significant obstacles/limitations in the form of a considerable number of unknown parameters, the absence of tissue-specific quantitative data (particularly electrophysiological recordings for transmembrane currents), the structure and function of the intracellular stores, and the spatial distribution of receptors, channels, and pumps, to name a few. ECs and SMCs regulate Ca2+ entry and Vm by expressing an abundant and diverse collection of ion channels. In addition, considering the balance of the other major intracellular ionic species (i.e., Na+, K+, Cl−) is essential to modeling both single-cell electrophysiology and cell-to-cell electrochemical coupling and communication. Finally, the models need to integrate relevant signal transduction pathways. Consequently, there is still a need to build upon previous modeling work to develop cellular models that will assist in the investigations of vascular tone regulation in health and disease.
III. MYOENDOTHELIAL COMMUNICATION
Myoendothelial communication plays an important role in modulating arterial tone and blood flow. Complex bidirectional pathways between the EC and SMC form tightly controlled feedback loops that regulate vascular Ca2+ dynamics and vasoreactivity. These pathways are often found disrupted in diseases related to altered vasoreactivity such as in hypertension.56–59 Many experimental studies have been conducted to elucidate myoendothelial signaling pathways, but only few theoretical models have addressed this subject.
III.A. Endothelium Derived Signals
The mechanisms of EC control of SMC tone has been a topic of intense investigation and a number of endothelium derived factors have been identified. The endothelium-derived relaxing factor (EDRF), now recognized as NO, has been well-characterized,60,61 whereas other pathways such as the endothelium-derived hyperpolarizing factor (EDHF), identified around the same time,62 still remain controversial. Ca2+ increase in ECs often leads to the activation of many of these endothelium-derived vasoreactive species. NO signaling seems to play a major role in vasoreactivity of larger arteries whereas EDHF-related responses are more dominant in small resistance arteries.63,64 Several factors have been proposed for the role of EDHF, including electrical signaling through myoendothelial gap junctions (MEGJs), extracellular accumulation of K+ ions, epoxyeicosatrienoic acids, hydrogen peroxide (H2O2), and C-type natriuretic peptide, among others, and have been reviewed by Sandow65 and Edwards et al.66 In most preparations the initial step for EDHF action depends on the opening of IKCa and SKCa channels.1,67–73
MEGJs often play a key role in EDHF vasorelaxation. MEGJ channels are composed of proteins called connexins that physically connect the cytoplasm of the EC and SMC. This allows for the electrical and chemical coupling between the 2 cell layers, as shown by membrane potential and dye transfer studies.74,75 Their presence has been documented in many tissues that exhibit EDHF responses.59,76,77 Blockade or the absence of MEGJs significantly reduces and in some cases abolishes EDHF-dependent SMC hyperpolarization in many arterial beds.68,78–84 This evidence suggests that Ca2+ elevation in ECs and the resulting hyperpolarization through the activation of IKCa/SKCa channels may be transmitted through the gap junction to the smooth muscle to induce arteriolar relaxation.
The reduction or abolishment of EDHF action seen after blockade of IKCa and SKCa channels also could be attributed to the role for K+ as an EDHF.68,72,85 Stimulation of the endothelium can increase K+ eflux from IKCa and SKCa channels and increase extracellular concentration of K+ in the interstitial space.72 This K+ accumulation might be sufficient to activate certain isoforms of Na+/K+ ATPases and/or Kir channels on the SMC membrane leading to SMC hyperpolarization.72,86 Mathematical modeling can make predictions for the contribution of electrical coupling through gap junctions and/or extracellular K+ accumulation in vasoreactivity and elucidate the conditions under which these mechanisms become important.
III.B. Myoendothelial Feedback
Endothelium-derived signals also can be evoked during SMC stimulation. This allows the vessel to generate a feedback response that modulates arterial constriction. SMCs can be stimulated by a range of vasoconstrictors that activate the phospholipase C (PLC) pathway, causing an increase in SMC Ca2+and IP3 along with SMC depolarization. This depolarization has been observed to spread to the EC via gap junctions.74 EC depolarization will tend to reduce cytosolic Ca2+ levels by reducing electrochemical gradient for Ca2+ entry, but Ca2+ mobilization in EC after SMC stimulation has been reported.87 Induced by SMC stimulation, elevated EC Ca2+ can activate EDHF and EDRF pathways and form feedback loops that moderate SMC constriction. This myoendothelial feedback may play an important role in the regulation of vasoreactivity, but many aspects of this mechanism remain unresolved.
Some studies have reported global EC Ca2+ events after SMC stimulation87–89 that are inhibited by gap junction uncouplers, whereas others have provided evidence for discrete and confined Ca2+ mobilization in EC.90–92 Some authors have suggested Ca2+ diffusion through MEGJs as an important mediator of the EC feedback response.87,89 Because gap junctions allow for the transport of small, second-messenger molecules such as IP3 in addition to Ca2+, it is possible for IP3 to travel down its concentration gradient into the EC and cause Ca2+ release from Ca2+ stores in the endothelium. Certain studies have provided evidence that IP3 diffusion plays an important role in generating the feedback.88,93,94 Ca2+ movement inside the cell is restricted by extensive buffering. IP3, on the other hand, is rapidly degraded by phosphatases present in the cytoplasm.42,93,95 At this stage, the relative contribution of the 2 second messengers in this feedback response has not been resolved. Furthermore, stimulating the endothelium to induce Ca2+ transients does not yield a corresponding increase in SMC Ca2+. Differences in IP3 kinetics and IP3R expression between the 2 cell types may explain the observed unidirectionality of IP3 signaling between the 2 cell layers.93
III.C. Localized Signals
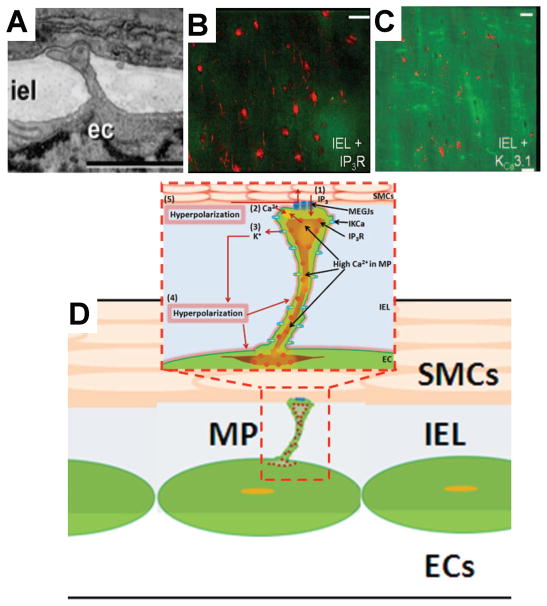
Local EC Ca2+ mobilization after SMC stimulation has been shown to be present inside and in the vicinity of structures called myoendothelial projections or microprojections (MPs). MPs are cellular extensions from EC and/or SMC that come in close contact in the internal elastic lamina96,97 (Fig. 1A). Recent information regarding the functional characteristics of these structures suggests that they can play an important role as a local regulatory module in myoendothelial communication. The presence of MPs has been documented in many rat arteries such as the saphenous, mesenteric, femoral, and caudal and mouse cremaster arterioles using electron microscopy techniques.59,78,82,83,97–99 MPs are known to occur in different numbers and sizes, with variation in vascular beds, age, species, sex, and disease states. However, a general observation is an increase in occurrence of MPs with a decrease in vessel diameter. Highest numbers of MPs often appear in small arteries and arterioles, which are also the main site of EDHF activity.97,98 Most MEGJs are located on top of these projections, although almost 50% of the projections do not have gap junctions on them.59,98,99 Immunohistochemical labeling studies show a heavy localization of IP3R (Fig. 1B) and IKCa channels (Fig. 1C) in MPs.1,68,90,94,100 Colocalization of Na+/K+-ATPases with IKCa channels around the MP has been shown.68 This sort of arrangement creates high IP3R density in a small volume (<1% of EC volume), which may be activated even with a small amount of IP3 entering during SMC stimulation and cause local increase in EC Ca2+ by release from ER stores (Fig. 1D, 1 and 2). Ca2+ increases can cause nearby IKCa channels to open, leading to EC hyperpolarization, which spreads to SMC via MEGJs (Fig. 1D, 3 through 5). The localization of IP3Rs and IKCa channels in the MP suggests that a local Ca2+ transient in MP rather than a global Ca2+ increase may be sufficient to provide feedback to SMC. In addition, these structures may provide confined, small-volume extracellular space needed for K+ accumulation in EDHF. Current experimental efforts are directed toward characterizing local Ca2+ events and the amount of hyperpolarizing feedback, as well as the localization of receptors and channels in the vicinity of these structures. Mathematical models can assist in the investigations of these mechanisms and test under what conditions some of the proposed hypotheses can have a significant impact in vascular Ca2+ regulation.
FIGURE 1.
Experimental characterization of microprojections (MP) and their functional role in myoendothelial feedback. (A) Electron microscopy images of an endothelial microprojection in rat saphenous arteries. Reproduced with permission from Sandow et al.78. Copyright 2004, John Wiley & Sons, Inc. (B, C) Immunohistochemical labeling studies in rat mesenteric artery showing localization of IKCa channels and IP3R receptors on the projections. Reproduced with permission from Ledoux et al.90. Copyright 2008, National Academy of Sciences. (D) Proposed mechanism of myoendothelial feedback involves IP3 diffusion from smooth muscle cell (SMC) to endothelial cell (EC) MP (1), activation of IP3R and increase of Ca2+ in MP (2), activation of IKCa channels (3), and hyperpolarizing current in EC (4) and SMC (5).
FIGURE 3.
Computational model of spreading responses. (A) Assumed arrangement of discrete endothelial cells (ECs) and smooth muscle cells (SMCs) into an isolated vessel segment. (B) Predicted EC Ca2+ elevation induced by focal acetylcholine (Ach) stimulation at x=0 had limited spread from the local site. (C) Local Ach-induced EC hyperpolarization was conducted to distant ECs through endothelial gap junctions. (D) SMC Ca2+ was reduced at local and distant sites by hyperpolarizing current transmitted from ECs through myoendothelial gap juctions. Reproduced with permission from Kapela et al.106 Copyright 2010, The American Physiological Society.
III.C. Models
Most electrophysiological models of vascular ECs34,36,37 and SMCs44,47,51,52,101,102 have examined Vm and Ca2+ dynamics in isolated cells. Brace and Anderson103 presented a model of membrane potential changes in vascular SMCs to examine the effect of extracellular K+ concentration. Model simulations showed that extracellular K+ has the potential to act as a hyperpolarizing factor through activation of Na+-K+-ATPase. On the other hand, Schuster et al.36 made theoretical calculations of extracellular K+ changes based on the EC IKCa and SKCa channel currents during bradykinin stimulation of cultured ECs. The model predicted that a significant increase in extracellular K+ is possible (10–70 mM), depending on the assumed thickness of the internal elastic lamina (1–6 μm). More recent studies have incorporated myoendothelial coupling and accounted for the effect of EC-SMC interaction in vasomotion,104 conducted responses,105,106 and myoendothelial feedback.107
We have recently presented a theoretical analysis of myoendothelial communication107 by integrating an isolated EC37 and an SMC52 model. The cells were coupled by nonselective gap junctions (permeable to Ca2+, K+, Na+ and Cl− ions and to IP3) and the diffusion of NO (Fig. 2A). SMC was agonist-activated through α1-adrenoceptors and NSC channels that cause membrane depolarization, opening of VOCC channels, and increase in intracellular Ca2+. EC agonist generated IP3 that activates IP3Rs and increases cytosolic Ca2+, which opens SKCa and IKCa channels and hyperpolarizes EC. The model was able to capture many of the known features of EC-SMC interaction. EC stimulation increased EC Ca2+ but reduced SMC Ca2+ through the EDHF and EDRF mechanisms. The EDHF action was caused by hyperpolarizing current flowing through MEGJs and was abolished in a synergistic manner by inhibition of EC SKCa and IKCa channels. Following stimulation of either cell, the predicted myoendothelial Ca2+ fluxes were too small to affect bulk Ca2+ in the other cell. Significant gap junction permeability to IP3 and EC IP3R currents had to be assumed to capture the feedback response to SMC stimulation in the model.
FIGURE 2.
(A) Schematic diagram of compartmental endothelial cell (EC)-smooth muscle cell (SMC) model with endothelial MPs showing membrane and cytosolic components and their localization as implemented by Kapela et al.108 (B) Two-dimensional mesh of the finite element method model with endothelial MPs and extracellular cleft. (C) Predicted Ca2+ transients in the MP during norepinephrine stimulation of SMC.109
This model was recently extended to include subcompartments accounting for MPs in the EC and an extracellular cleft to quantify the accumulation of extracellular K+ (Fig. 2A).108 K+ accumulation was observed in the cleft during acetylcholine (Ach) stimulation of the EC. However, the extracellular K+ concentration is limited by the diffusion of K+ toward the bulk extracellular space and by the inhibition of the eflux of K+ through the EC KCa channels as concentration increases. In addition, the hyperpolarizing effect of K+ accumulation on SMC Vm through Na+-K+-ATPase is reduced by a depolarizing effect of the changing K+ Nernst potential in both EC and SMC. Thus, K+-mediated SMC hyperpolarization was rather small and transient under most of the examined scenarios. Further studies and model simulations are required to investigate the role of extracellular K+ in the EDHF pathway.
Compartmental (Fig. 2A) as well as continuum (Fig. 2B) models have been developed to account for the effect of projections and localization of channels on myoendothelial communication. Finite element method simulations, for example, allow us to capture Ca2+ and IP3 concentration gradients in the MPs and predict the levels of Ca2+ accumulation.109 Simulations predict that, after SMC stimulation, IP3-mediated Ca2+ transients are present in the MP (Fig. 2C). The potential of these transients to spread significantly into the bulk of the EC and generate a global response is rather limited. These Ca2+ transients can activate IKCa channels localized on/near the MP and generate hyperpolarizing current that can spread back to the SMC. Hyperpolarizing feedback of a few millivolts can be generated, which translates in a significant modulation of arterial tone. Interestingly, feedback is abolished in the absence of MPs. Thus, simulations suggest that MPs are essential structures for the EC feedback response to SMC stimulation.
IV. CONDUCTED RESPONSES
Some vasoactive agents, when applied focally to microvessels, may cause significant vasomotor responses both locally and at relatively distant sites. Such spreading responses can be conducted by intrinsic signal transduction mechanisms within the vascular wall, independently of the diffusion of the stimulating agent, hemodynamic effects, or innervations. Conducted responses have been reported in small arterioles and as far as small resistance arteries with 3 to 5 layers of SMCs from many vascular beds and species.110–115 The distance of spreading responses depends on vessel type, stimulation, and experimental conditions, but the mechanical length constant describing the decay of diameter changes is typically between 1 and 2 mm.113
Spreading relaxation may play a central role in the hemodymanics of microcirculation because in its absence local relaxation would not significantly increase blood flow restricted by other sections of the vessel. Thus, vasodilatation initiated locally by increased metabolic demand may be conducted upstream to feed arteries to allow adequate increase in perfusion.116–118 Conducted vasoconstrictions also were demonstrated experimentally and were implicated in the tubuloglomerular feedback mechanism of renal autoregulation.119 Theoretical simulations suggest that axial communication in the vasculature is also required to suppress the generation of large proximal shunts during long-term structural adaptation of microvascular networks.120
IV.A. Signal Transduction At The Local Site
Spreading relaxation can be initiated by various vasoactive substances acting through different receptors and signal transduction pathways, but the mechanisms that link local metabolic state or oxygen saturation level with spreading responses remain unclear. One possibility is ATP released by erythrocytes in response to changes in the oxygen saturation level, and by perivascular nerves or parenchymal tissue.121–123 ATP acting on P2Y receptors can then produce local and spreading relaxation.123–125 Local changes in oxygen level also can induce conducted responses independently from the effect of erythrocytes on ATP or NO.126 Low (or high) oxygen tension induces conducted vasodilatation (or vasoconstriction) that is inhibited by glibenclamide, a blocker of ATP-sensitive K+ channels.127 This suggests that oxygen level may modulate KATP channels in the SM through its effect on cytosolic ATP levels.
A common feature of stimuli that are effective in initiating conducted vasodilatation is their ability to produce local hyperpolarization.115 Having strong hyperpolarizing effect, Ach is the agonist used most often to induce conducted dilations in a variety of preparations. Ach increases EC Ca2+, activates endothelial IKCa and SKCa channels, and produces both endothelial and smooth muscle hyperpolarization.128–130
IV.B. Mechanisms of Signal Conduction
Gap junctions are central elements for the ability of a vessel to produce conducted responses.131–133 Homocellular gap junctions are present in the endothelium (Cx40 and Cx37) and smooth muscle (Cx43 and Cx45) but usually are more prevalent in the endothelium.76,99,113,134,135 MEGJs, connecting SMCs with ECs, are present in many but not all vessels.80,99,134 The homo- and heterocellular gap junctions are thought to be nonselective and permeable to small ions and molecules such as Ca2+ and IP3, respectively.136,137 Electrotonic conduction of membrane potential changes through the gap junctions is considered to be the major mechanism of spreading responses in most vascular beds.113,133,134 The length constant of the electrical spread is similar to the mechanical length constant and in the range of 1 to 2 mm.113,128,138,139
Experiments with disrupted endothelial or smooth muscle layers and genetically engineered mice with cell-specific connexin deficiencies indicate that the endothelium, rather than the smooth muscle, plays the leading role in the propagation of spreading responses in most vascular networks.115,123,128,133 Local Vm changes usually spread axially through the endothelial gap junctions and then radially to the SMCs through the MEGJs. In some vessels, like in RMAs, the endothelium was identified as the major conducting pathway. Vm changes, however, initiated either in the endothelium or in the smooth muscle, spread similarly, suggesting strong myoendothelial coupling and that endothelium with smooth muscle acts as an electrical syncytium.128 In other vessels, however, SM may provide a separate pathway from the endothelium with different conduction properties.140 In the mouse cremaster muscle, Ach induces vasodilatation that spreads through the endothelium, whereas adenosine activates SM KATP channels and induces distant vasodilatations through the SM layer.129
IV.C. Facilitation of Spreading Responses
In addition to passive electrotonic coupling through gap junctions, conducted responses may involve different facilitating mechanisms, depending on the type of stimulation and the vascular network.115 For instance, in hamster feed arteries, the conduction of hyperpolarization was augmented during Ach stimulation compared with electric current stimulation, as indicated by an increase in the electrical length constant from 1.2 mm to 1.9 mm.139 Membrane hyperpolarizations potentially can be enhanced by inwardly rectifying potassium channels and/or the sodium-potassium pump.141 Kir channels acting as amplifiers in conducted dilations were demonstrated in coronary microcirculation,142 hamster retractor muscle feed arteries,143 and mouse cremaster muscles.129 Kir also may amplify conducted responses in RMAs according to some, but not all, studies.128,144
In many vessels, Ach produces distant relaxations without significant endothelial Ca2+ spread.113,114,134,145 For example, in RMA a noticeable EC Ca2+ increase appears only within few hundred micrometers from the Ach stimulation site.128 NO has negligible effect on conducted responses in RMAs,125 mouse cremaster microcirculation,126 and rat cerebral penetrating arterioles,123 presumably because of the limited EC Ca2+ spread that is required for its release at distant sites. However, some studies reported significant NO- and EDHF-dependent components of the conducted response and suggested an EC Ca2+ increase at remote sites.116,146–148 In some ECs, hyperpolarization itself can increase intracellular Ca2+ by increasing the driving force for Ca2+ entry.21,149 It was speculated that conducted hyperpolarization could trigger Ca2+ transients and activate NO release at distant sites to dilate the vessel.147 However, there is no direct evidence for such mechanism in spreading responses, and it is not clear if such hyperpolarization-induced Ca2+ transient could be significant.145
Recently distant EC Ca2+ waves were observed in hamster feed arteries150,151 and cremaster muscle arterioles in transgenic mice.2 The mechanism behind the propagating Ca2+ wave along the endothelium remains unclear. Experimental data suggest that direct Ca2+ communication via homocellular gap junctions is not essential for Ca2+ waves.152 In mice cremaster muscle arterioles, Ca2+ waves were significantly faster than can be accounted for by the diffusion of IP3 or Ca2+, suggesting an underlying active/regenerative mechanism (i.e., a regenerative release of IP3 triggered by Ach).2 A regenerative mechanism based on the activation of endothelial voltage-dependent sodium (Nav) and calcium (Cav) channels also has been suggested,148 but mepivacaine, a blocker of Nav channels, had no effect in another study.129 The role of Ca2+ waves in conducted responses also has been questioned by Welsh et al.153
Spreading vasodilatations can be influenced significantly by the strength of vessel preconstriction. Haug and Segal154 reported that activation of SMC α1- and α2-adrenoceptors in feed arteries of the hamster retractor muscle inhibits conducted vasodilatation, possibly by decreased SMC membrane resistance or increased myoendothelial resistance. Gustafsson and Holstein-Rathlou155 showed experimentally that angiotensin II increased the electrical length constant in RMA and hypothesized that this was a result of increased cell-to-cell coupling or membrane resistance. Experimentation with hamster feed arteries led Uhrenholt et al.150 to suggest that SM tone could increase basal EC [Ca2+]i and sensitize the endothelium for Ca2+ wave propagation. Conducted responses also were reported to change under pathological conditions. In cerebral arterioles, ischemia augmented conducted vasodilatation, presumably to improve cerebral perfusion.156 Conducted responses in RMAs were significantly attenuated in spontaneous hypertensive rats compared with Wistar Kyoto rats, although the rate of decay with distance did not change.144
IV.D. Theoretical Models
A number of theoretical models described the electrical aspect of spreading responses. Hirst and Neild138 modeled a vessel segment as a continuous wire with uniform axial resistance and applied traditional cable theory to determine its electrical properties and cable length constant. A general equation for steady-state spread of Vm changes in a short segment of blood vessel stimulated with current source has been derived by Crane and Neild.157 The effect of branching on the passive spread of electrical signals was examined by Segal and Neild158 and Crane et al.159 The simulation results suggested that a thick smooth muscle layer could favor electrical conduction, but passive conduction was insufficient to explain the experimental recordings. Haug and Segal154 used a passive cable model to demonstrate that the experimentally observed inhibition of conducted vasodilation by activation of SM α1- and α2-adrenoceptors can be explained by decreased smooth muscle cell membrane resistance or increased myoendothelial resistance.
Diep et al.105 developed a computational model of skeletal muscle resistance artery with discrete SMCs and ECs. Each cell was represented by a nonlinear resistor and a capacitor equivalent to the whole-cell membrane conductance and capacitance, respectively. The gap junctions were assumed to be ohmic resistors with 3 MΩ between ECs, 90 MΩ between SMCs, and 900 MΩ per SMC between ECs and SMCs. In the simulations, the vessel wall was not a syncytium and electrical stimulations of ECs and SMCs conducted differentially. The axial orientation of ECs and low EC-to-EC resistance, as opposed to circumferential arrangement of SMCs and higher SMC-SMC resistance, were the major factors for the preferential conduction of Vm changes along the endothelium. In a later study, Jantzi and coworkers143 modified the model of Diep et al.105 to examine the role of Kir current in the facilitation of conducted responses. A Kir-like current was added (with minimal activation at −40 mV, maximal activation at −60 mV [1.5 pA], and reversal at −80 mV), as well as a second layer of SMCs. The model demonstrated that, because of the negative slope conductance of the Kir current, it can enhance hyperpolarization of SM induced by local current injection and reduce hyperpolarization decay along the artery compared with the responses with blocked Kir and in the agreement with corresponding experimental data.
Previous theoretical studies of spreading responses focused on the electrical behavior of the vessel wall.105,138,154,159 In a recent study106 we developed a computational model of spreading responses that integrates detailed cellular models with tissue-specific descriptions of Ca2+ and ionic dynamics and plasma membrane electrophysiology (Fig. 3A). Discrete ECs and SMCs were coupled in a proper arrangement by homocellular and heterocellular gap junctions permeable to Ca2+, K+, Na+, and Cl− ions and IP3. ECs released NO, in a Ca2+-dependent manner, which could reduce cytosolic Ca2+ in their overlapping SMCs. Representative responses are presented in Fig. 3B–D following focal application of Ach in a row of ECs at x=0. Local Ca2+ transients do not propagate significantly along the vessel (Fig. 3B). This limited spread of EC Ca2+ to neighboring ECs was mediated by diffusion of IP3. Hyperpolarization, however, can spread significantly through the endothelial layer (Fig. 3C), and through MEGJs this distant hyperpolarization is transmitted to the SMCs to reduced SMC Ca2+ (Fig. 3D) and subsequently arteriolar tone.
When the assumed myoendothelial resistance was small (below 70 MΩ), EC and SMC agonists could produce similar spreading responses that were conducted primarily through the endothelium. Larger myoendothelial resistances compromised spreading responses initiated in SMCs but increased sensitivity of SMCs to local stimuli. The IP3-mediated limited Ca2+ spread to neighboring ECs can amplify the total current generated at the local site by the stimulated ECs and thus affect distant dilations. The amplitude and distance of spreading responses also depended nonlinearly on local stimulus strength and SM prestimulation levels. The study did not identify any facilitating/regenerative mechanisms that presumably exist in some vessels. Nevertheless, it proposes a theoretical framework for developing other tissue-specific models that can account for such effects.
The electrical resistances and permeabilities of the homocellular and heterocellular gap junctions are likely to have a major effect on the robustness and differential properties of spreading responses, but they remain unknown for most vessels. Therefore, tissue-specific measurements of the gap junction properties would greatly enhance the understanding and modeling of spreading responses because the incidence of MEGJs may vary significantly between vascular beds and may even be smaller in proximal than distal vessels.99 The physiological justification for the differential response to endothelial and smooth muscle agonists in some but not all vessels is unclear. According to theoretical analysis, as myoendothelial coupling decreases, the ability of the SMCs to generate a distant signal decreases, but the sensitivity of these cells to agonist stimulation locally increases. The hemodynamic consequences of a strong local constriction instead of a weaker spreading constriction over a larger vascular segment could be studied in the future models that integrate fluid, Ca2+, and Vm dynamics. Detailed mathematical models integrating tissue-specific Ca2+ dynamics and membrane channels may assist experimentalists in the elucidation of the controversial role of Ca2+ waves and regenerative mechanisms in conducted responses.
V. VASOMOTION
Many small blood vessels, under appropriate conditions, exhibit spontaneous diameter oscillations called vasomotion. Vasomotion results from coordinated contractile activity of many SMCs within the vessel wall and is independent from pulsatile blood flow or other periodic stimuli. The period of diameter oscillations varies from seconds to minutes, depending on the vessel and experimental conditions.3,160,161
Vasomotion has been reported in intact animals as well as isolated vessels in various species and tissues including RMA162–165; various skeletal muscles166; basilar artery167; cerebral microcirculation of humans, rats, rabbits, and cats168–171; human umbilical and placental veins172; and human skin.173 Vasomotion has been observed directly through microscopic techniques and indirectly through methods like laser Doppler flowmetry, pressure measurement techniques, near infrared spectroscopy, reflected and scattered light, optical imaging spectroscopy, electrophysiology, and cellular Ca2+ imaging techniques.174 Though most studies associate vasomotion with normal physiological conditions, some suggest that it occurs only under pathological conditions such as reduced perfusion.166,175,176 Hypertensive animals also show enhanced vasomotion in most of the studies.177 e physiologic or pathophysiologic roles of vasomotion are under investigation, and several hypotheses have been proposed.3 Diameter oscillations may increase flow conductance and, under certain conditions, oscillations of oxygen tension may improve oxygenation compared with steady flow through the same average diameter.178 Vasomotion can enhance tissue perfusion179 and may also contribute to maternal-fetus blood flow and fetal nutrition and development.172 Desynchronized vasomotion and fiber activation have been shown to enhance oxygenation in a model of skeletal muscles.180 However, vasomotion may be best understood as a network phenomenon modulated by information transfer between various vascular segments to ensure optimal tissue perfusion.174
V.A. Mechanisms of Oscillations
In most vessels, the oscillations of the vascular tone underlying vasomotion originate from synchronization of the spontaneous activity in the majority of SMCs. The small remaining fraction of SMCs may exhibit oscillatory behavior as a result of coupling with the other cells, but distinct pacemaker activity driving the oscillations has not been identified to date. The variation of SMC tone results from intracellular Ca2+ changes, which can be generated through different mechanisms depending on vessel type and experimental conditions.
Vasomotion may originate from cytosolic oscillators and membrane oscillators.163,181,182 The most common mechanism giving rise to self-sustained regular Ca2+ oscillations comprises internal Ca2+ stores and RyRs or IP3Rs.3,161 Cytosolic oscillators have been suggested in many vascular beds, including human umbilical and placental veins.172 In rat mesenteric arterioles, a cytosolic oscillator is the predominant mechanism for oscillations, but inhibition of SERCA gives rise to SR-independent vasomotion with a significantly longer period (~1 min) and larger amplitude.183 It has been suggested that in this case, Ca2+ oscillations are generated by a membrane oscillator comprised of VOCC and BKCa channels.3,161 This may hold true for some vessels (e.g., rat basilar arteries) under control conditions as well.184
V.B. Synchronization Mechanisms
For the initiation of vasomotion a synchronization mechanism is required in addition to the spontaneous ability of SMCs for oscillations. This mechanism needs to compensate for the biological variability of the individual Ca2+ oscillators and their intrinsic frequencies and for noise-induced phase fluctuations. Gap junctions are thought to play a key role for the synchronization because gap junction uncouplers consistently inhibit vasomotion.133,172 However, the identity, origin, and mechanism of the synchronizing signal remain uncertain.
1. Electrical Coupling
Electrical current has been suggested as the only signal fast enough (>1 cm/s) to synchronize several millimeters-long vessels and to mediate long-range coordination in gastric and lymphatic smooth muscle.160,185,186 Experimental data now suggest that electrical coupling directly through SMC-SMC gap junctions and/or indirectly through the endothelium may also mediate Ca2+ synchronization in vascular SMCs. Several experiments have demonstrated that Vm oscillates during vasomotion with the amplitude of a few millivolts, and these oscillations are highly correlated to the Ca2+, diameter, and force oscillations.3,184,187 In vessels where vasomotion is driven by the membrane oscillators, Ca2+ and Vm oscillations are in phase and both have significant amplitudes. Thus, electrical coupling through gap junctions can have a direct synchronizing effect. However, Ca2+ oscillations produced by cytosolic oscillators do not require Vm oscillations. Secondary Vm oscillations are generated by the dependence of Vm on membrane channels regulated directly or indirectly by cytosolic Ca2+. Thus, Vm oscillations may have an arbitrary phase shift and amplitude depending on the Ca2+-Vm coupling mechanism, conductance, and type of the channels. In this scenario, electrical coupling through gap junctions can mediate both synchronizing and desynchronizing effect.
In rat mesenteric arterioles, vasomotion seems to depend on Ca2+-activated Cl− channels. ClCa channels depolarize Vm in phase with local Ca2+ and thus can mediate synchronization by modulating VOCCs in coupled SMCs (Fig. 4). Initially, only cGMP-dependent ClCa channels were implicated in the synchronization because application of a membrane-permeable analogue of cGMP, 8Br-cGMP, induced vasomotion.163,188 Later studies showed that cGMP-independent ClCa channels may also be involved, but the absence of highly specific ClCa inhibitors makes it difficult to assess their relative contributions.189 On the other hand, activation of BKCa channels has a hyperpoarizing effect opposite to the ClCa effect. Indeed, blocking BKCa with IbTx or Kv with 4-AP did not eliminate vasomotion in Mauban and Weir.190 Furthermore, blockade of K+ channels promotes vasomotion in some vessels. The exact mechanism is unclear, but reduced membrane conductance or tetraethylammonium-induced formation of gap junctions have been suggested as potential explanations.3
FIGURE 4.

Schematic diagram of possible Ca2+ synchronization pathways in vascular smooth muscle cells (SMCs). Ca2+ increase in first cell (left) can activate directly ClCa and BKCa channels, and indirectly nonselective cation (NSC)channels through Ca2+-sensitive phospholipase C (PLC) and diacylglycerol (DAG). The activation of NSC and/or ClCa depolarizes Vm (solid green lines), although their effect may be moderated by BKCa channels (solid red line). The depolarization spreads through gap junctions to neighboring cells (right), activates voltage-operated Ca2+ channels and increases in-phase cytosolic Ca2+. Ca2+ elevation in one cell can increase Ca2+ in neighboring cells by direct diffusion of Ca2+ through the gap junctions. Oscillatory IP3, generated by Ca2+-dependent PLC and diffusing to other cells, may also have synchronizing effect by acting on IP3 receptors.
2. Ca2+ Coupling
Ca2+ diffusion through nonselective SMC-SMC gap junctions could be the most direct way to coordinate both cytosolic and membrane Ca2+ oscillators (Fig. 4). In a series of theoretical studies of vasomotion, Ca2+ fluxes proportional to intercellular concentration differences are the only significant synchronizing signals.45,46,102,104 However, there is no strong experimental evidence to support such a role for Ca2+, and a number of studies have argued that Ca2+ is not the major synchronizing agent. Sell et al.191 estimated Ca2+ propagation velocity in single SMCs of 25 μm/s and concluded that is too slow for intercellular Ca2+ synchronization. Jacobsen et al.101 also argued that the diffusion of Ca2+ or other second messengers is too slow and the quantitative movement of Ca2+ is too small to have a major effect.
3. IP3 Coupling
Movement of IP3, rather than Ca2+, between cells seems to play more significant role in Ca2+ wave propagation, and IP3 diffuses faster than Ca2+.192 IP3 diffusing to other cells could influence their Ca2+ oscillations by modulating activity of IP3Rs. IP3-mediated synchronization also would require that IP3 concentration oscillates together with Ca2+ (Fig. 4). Ca2+ oscillations are accompanied by IP3 oscillations in many cell types, including airway SMCs.192 In many, but not all, instances, IP3 oscillations are generated by Ca2+ dependence of agonist-activated PLC and/or the agonist-independent isoform PLC-δ. PLC-δ was identified experimentally in rat mesenteric arteriole SMCs.88,193 Although there is no direct evidence for IP3 oscillations in RMAs, EC Ca2+ oscillations in phase with SM Ca2+ oscillations during vasomotion is most likely attributed to SMC-to-EC communication mediated by IP3.88,89
V.C. Role of Endothelium
The role of endothelium in vasomotion remains controversial because endothelium can both promote and inhibit vasomotion.3,163,165,188,190,191 The differential effect of endothelium can be explained in part by the modulatory role of the endothelium-derived factors on mean SM Ca2+ and transitions between oscillatory and nonoscillatory domains.102,104,187,194 However, the inconsistent role of endothelium may also result from multiple competing effects on the synchronization, with their net result being sensitive to experimental conditions.
cGMP produces only partial synchronization in endothelium-denuded RMA, whereas intact endothelium allowed full synchronization even with L-NAME and ODQ inhibition.188 Thus, a synchronizing effect of the endothelium, through some unidentified mechanism, has been suggested. Endothelium has relatively low electrical resistance and thus can provide global coupling for SMCs through MEGJs. This, however, can either enhance or attenuate synchronization depending on the type of Ca2+ oscillators and the phase delay between SMC Vm and Ca2+.
A modulatory role for vasomotion was assigned to NO and cGMP by Mauban and Weir.190 NO/cGMP may affect vasomotion, however, in a complex way because of their potential effects on many cellular components, including components that can induce depolarizing and hyperpolarizing currents. In cerebral rat arteries, asynchronous propagating Ca2+ waves were recorded under control conditions, and synchronous Ca2+ oscillations and vasomotion were observed after NO inhibition.187 In this study, the inhibitory role of NO for vasomotion was attributed to soluble guanylyl cyclase–independent effects on RyRs and VOCCs. Some studies report that during vasomotion EC Ca2+ is irregular and unsynchronized with SM Ca2+, whereas others suggest that they oscillate effectively in phase and synchrony.88,89 If NO also oscillates (i.e., because of the Ca2+ dependence of endothelial NO synthase), then its effect on synchronization will be even more complex.
V.D. Mathematical Models
1. Single-Cell Models of Oscillations
Gonzalez-Fernandez and Ermentrout41 presented a model of vasomotion where oscillations originating from a membrane oscillator. In this model the interaction of VOCC and BKCa channels gives rise to regular Ca2+ oscillations. It also incorporated Ca2+-dependent myosin light chains phosphorylation, crossbridges formation, and the mechanics of a thick-walled cylinder. Changes in transmural pressure were assumed to modulate VOCC channels and influence the rate and amplitude of vessel oscillations. Model results were similar to the vessel pacemaker activity reported by Meyer et al.195 e minimal model of Parthimos and coworkers44 investigated arterial chaos generated by coupled cytosolic and membrane oscillators. Nonlinear interactions between the 2 oscillators produced chaotic behavior, similar to irregular arteriolar contractions in rabbit ear arteries. Subsequent work investigated observed changes in histamine-induced chaotic vasomotion after inhibition of endothelial NO.48 The effect of L-NAME was simulated by reducing the maximum rate of Ca2+ uptake into the store. The original model was also modified to study the effect of various pharmacological interventions on vasomotion in isolated rat cerebral arteries.49 The membrane oscillator was eliminated in the new implementation, and regular Ca2+ oscillations were generated only by CICR via RyR or IP3Rs. Careful phase-space and parametric analyses were performed and compared with experiments. Experimental data could be simulated by the model by using a low-order 3-variable system that incorporates nonlinear interactions between dynamical control variables. Their model predicted that inhibition of PLC by U73122 terminates vasomotion mostly by preventing DAG production and activation of DAG-dependent NSC channels, which leads to Vm hyperpolarization and Ca2+ reduction below oscillatory domain. This prediction was confirmed by a recent study that suggests that the NSC channels are encoded by PLC-activated TRPC1 channels, which, by passing Na+ and Ca2+ currents, depolarize Vm, activate VOCC, and initiate vasomotion.196 Spontaneous Ca2+ oscillations also appeared in our isolated SMC model.52 The oscillations were generated by CICR through RyRs and the slow refilling of the stores whenever average Ca2+ levels shifted within a concentration window. The Ca2+ levels could be increased by agonist-activated NSC channels or elevated extracellular K+ and reduced by exogenous NO. In the integrated EC-SMC model, the endothelium-derived NO and EDHF desensitized SMC to agonist stimulation and increased the range of agonist concentration, inducing Ca2+ oscillations.197 Analogous stabilizing effect of the endothelium was reported in experiments.194 The concentrations of NE and extracellular K+ producing Ca2+ oscillations in the model and vasomotion in isolated RMA were comparable.164
2. Multicellular Models of Synchronization
Individual SMC Ca2+ oscillations or waves need to be synchronized for the occurrence of macroscopic diameter changes; hence, multicellular models are needed to account for intercellular interactions and examine the mechanisms that enable coordination of SMCs in vasomotion.
Koenigsberger et al.45,46 proposed a multicellular 2-dimensional model to study recruitment and synchronization of SMCs in rat mesenteric arterial segments. The description of each individual cell was based on the single cell model of Parthimos et al.,44 but was modified to account for Ca2+ oscillations only from the stores, agonist and Ca2+-dependent IP3 formation, and differences in channel conductances. Variability between SMCs was introduced by Gaussian noise added to membrane conductances. The cells were coupled by electrical current proportional to intercellular Vm difference and Ca2+ and IP3 fluxes proportional to concentration differences. It was argued that the electrical coupling has a desynchronizing effect caused by the hyperpolarizing action of BKCa. Ca2+ diffusion was necessary to override the influence of noise and BKCa, whereas oscillatory IP3 generated by Ca2+-dependent PLC-δ did not play a role.
The effect endothelium and wall stress on vasomotion was examined in subsequent publications.102,104 A layer of ECs was coupled to the SMCs by electrical current and IP3 diffusion. The dynamics of Ca2+, IP3, and Vm in each EC was taken from the earlier EC model of Schuster et al.36 Agonist stimulation of the smooth muscle layer increased SMC Ca2+ and EC Ca2+ through diffusion of IP3 from SMCs to ECs and/or opening of stretch-activated EC Ca2+ channels. Endothelial KCa channels activated by the indirect EC Ca2+ elevation generated EDHF, which fed back on the smooth muscle and moderated its Ca2+ elevation. The controversial effect of endothelium and wall stress on vasomotion was ascribed mostly to their modulatory effect on the Ca2+ levels in the smooth muscle. Thus, removal of endothelium or increase in pressure can move the average smooth muscle Ca2+ into or out of the oscillatory window and induce or terminate vasomotion depending on the initial conditions. A synchronizing mechanism through active stress modulating stretch-activated Ca2+ channels in neighboring SMCs through the vessel wall was identified, but it was too weak to synchronize Ca2+ oscillations in the absence of gap junctions.
The SMC model of Jacobsen and coworkers47,101,198 examines the role of cGMP-sensitive ClCa in the transition of intracellular Ca2+ waves to whole-cell oscillations and in the intercellular coordination of the oscillations. A spindle-shaped SMC was modeled and discretized along its longitudinal axis. A cGMP-sensitive ClCa current was formulated based on experimental data from rat mesenteric small arteries.185 Other membrane and intracellular components were taken from earlier models. Each segment could give rise to Ca2+ oscillations through IP3Rs, but Ca2+ waves were generated by the segment with the highest intrinsic frequency determined by random perturbation of parameters along the cell. Activation of ClCa by cGMP increased coupling between Ca2+ dynamics and Vm, leading to Vm oscillations and transition from Ca2+ waves to whole-cell oscillations. To examine intercellular synchronization, 15 cells were arranged into a tube-shaped layer. The cells were coupled by electrical current and Ca2+ flux proportional to concentration and potential difference. It was concluded that cGMP promotes vasomotion in RMAs through activation of ClCa channels and the coupling of Vm depolarization with Ca2+ elevation. The depolarization is transmitted to neighboring cells by the gap junctions, activates VOCC, and coordinates Ca2+ elevation. The intercellular Ca2+ diffusion had no effect on the synchronization.
Inhibition or activation of various cellular components often has an inconsistent effect on vasomotion. Different mechanisms may participate in the initiation of vasomotion in different vascular beds and species. Multiple mechanisms (competing or compensating) also may be involved even within a particular vascular bed. Thus, the importance of a specific signaling pathway for vasomotion may be difficult to assess and can be sensitive to experimental conditions, such as the presence and concentration of various agonists and blockers. The 2 published multicellular models of vasomotion predict fundamentally different synchronization pathways (intercellular Ca2+ diffusion vs. electrical coupling) and can account for different subsets of experimental data from rat mesenteric arterioles.45,101
Future theoretical studies of vasomotion that will be sufficiently comprehensive and will integrate tissue-specific, relevant cellular components have the potential to identify alternative mechanisms and investigate their relative contributions at different experimental conditions. Figure 4 shows selected signaling pathways that may influence Ca2+ synchronization based on experimental data, mathematical models, and our preliminary simulations. DAG-activated NSC channels may be involved in vasomotion, not only through their depolarizing effect necessary for the transition from resting to oscillatory domain, as reported from experiments196 and theoretical models,49 but also by their synchronizing effect similar to ClCa channels (Fig. 4, solid green lines). In our model, an oscillatory activity of NSC channels results from Ca2+ dependence of PLC, which modulates concentrations of DAG and IP3. DAG-activated transient receptor potential channel–like nonselective channels are expressed in RMA SMCs,53 and synchronous oscillations of DAG, IP3 and Ca2+ were measured in Chinese hamster ovary cell culture.199 The oscillatory IP3 also could mediate Ca2+ coordination, given sufficient permeability of gap junctions and/or density of IP3Rs (Fig. 4, dashed line). More detailed models potentially can account for the spatial distribution of RyRs and IP3Rs and their differential compartmentalization with membrane channels,161 a stochastic origin for the Ca2+ oscillations,200 the transition of unsynchronized intracellular Ca2+ waves to global oscillations at the onset of vasomotion,163 and for the intercellular Ca2+ waves associated with propagation of vasomotion.201
VI. SUMMARY
Experimentation has revealed a complex network of intra- and intercellular signaling pathways that regulate Ca2+ levels in the vascular wall. Recently, mathematical models have emerged that can integrate these complex pathways and describe cellular behavior and how vessel function emerges from coordination of responses in many cells. The first models of Ca2+ dynamics offer many advantages in investigations of Ca2+ signaling. However, further development is needed to identify tissue-specific parameters and incorporate recently acquired knowledge about the function and spatial distribution of cellular components (i.e., stores, receptors, channels, or pumps). Integration of these cellular models enables us to elucidate important aspects of vessel function. Some of the early attempts that were reviewed in this article outline a methodology for cell integration and show how multicellular models can provide important physiological insights and assist in elucidation of experimental findings.
Acknowledgments
N. M. Tsoukias has been supported by the National Institutes of Health (grant No. HL095101).
References
- 1.Sandow SL, Haddock RE, Hill CE, Chadha PS, Kerr PM, Welsh DG, et al. What’s where and why at a vascular myoendothelial microdomain signalling complex. Clin Exp Pharmacol Physiol. 2009;36(1):67–76. doi: 10.1111/j.1440-1681.2008.05076.x. [DOI] [PubMed] [Google Scholar]
- 2.Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, et al. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: measurements in Cx40BAC GCaMP2 transgenic mice. Circ Res. 2007;101(12):1300–9. doi: 10.1161/CIRCRESAHA.107.149484. [DOI] [PubMed] [Google Scholar]
- 3.Aalkjaer C, Nilsson H. Vasomotion: cellular background for the oscillator and for the synchronization of smooth muscle cells. Br J Pharmacol. 2005;144(5):605–16. doi: 10.1038/sj.bjp.0706084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ Res. 1994;74(6):1071–96. doi: 10.1161/01.res.74.6.1071. [DOI] [PubMed] [Google Scholar]
- 5.Jafri MS, Rice JJ, Winslow RL. Cardiac Ca2+ dynamics: the roles of ryanodine receptor adaptation and sarcoplasmic reticulum load. Biophys J. 1998;74(3):1149–68. doi: 10.1016/S0006-3495(98)77832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saucerman JJ, McCulloch AD. Mechanistic systems models of cell signaling networks: a case study of myocyte adrenergic regulation. Prog Biophys Mol Biol. 2004;85(2–3):261–78. doi: 10.1016/j.pbiomolbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Tusscher KH, Noble D, Noble PJ, Panfllov AV. A model for human ventricular tissue. Am J Physiol Heart Circ Physiol. 2004;286(4):H1573–89. doi: 10.1152/ajpheart.00794.2003. [DOI] [PubMed] [Google Scholar]
- 8.Shannon TR, Wang F, Puglisi J, Weber C, Bers DM. A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophys J. 2004;87(5):3351–71. doi: 10.1529/biophysj.104.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nickerson D, Smith N, Hunter P. New developments in a strongly coupled cardiac electromechanical model. Europace. 2005;7(Suppl 2):118–27. doi: 10.1016/j.eupc.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Noble D. Modeling the heart--from genes to cells to the whole organ. Science. 2002;295(5560):1678–82. doi: 10.1126/science.1069881. [DOI] [PubMed] [Google Scholar]
- 11.Hunter P, Nielsen P. A strategy for integrative computational physiology. Physiology (Bethesda) 2005;20:316–25. doi: 10.1152/physiol.00022.2005. [DOI] [PubMed] [Google Scholar]
- 12.Hunter PJ, Pullan AJ, Smaill BH. Modeling total heart function. Annu Rev Biomed Eng. 2003;5:147–77. doi: 10.1146/annurev.bioeng.5.040202.121537. [DOI] [PubMed] [Google Scholar]
- 13.McCulloch AD. Modeling the human cardiome in silico. J Nucl Cardiol. 2000;7(5):496–9. doi: 10.1067/mnc.2000.109682. [DOI] [PubMed] [Google Scholar]
- 14.Winslow RL, Scollan DF, Holmes A, Yung CK, Zhang J, Jafri MS. Electrophysiological modeling of cardiac ventricular function: from cell to organ. Annu Rev Biomed Eng. 2000;2:119–55. doi: 10.1146/annurev.bioeng.2.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudy Y. From genome to physiome: integrative models of cardiac excitation. Ann Biomed Eng. 2000;28(8):945–50. doi: 10.1114/1.1308484. [DOI] [PubMed] [Google Scholar]
- 16.Saucerman JJ, Healy SN, Belik ME, Puglisi JL, McCulloch AD. Proarrhythmic consequences of a KCNQ1 AKAP-binding domain mutation: computational models of whole cells and heterogeneous tissue. Circ Res. 2004;95(12):1216–24. doi: 10.1161/01.RES.0000150055.06226.4e. [DOI] [PubMed] [Google Scholar]
- 17.Kapela A, Tsoukias N, Bezerianos A. New aspects of vulnerability in heterogeneous models of ventricular wall and its modulation by loss of cardiac sodium channel function. Med Biol Eng Comput. 2005;43(3):387–94. doi: 10.1007/BF02345817. [DOI] [PubMed] [Google Scholar]
- 18.Noble D. Modeling the heart. Physiology (Bethesda) 2004;19:191–7. doi: 10.1152/physiol.00004.2004. [DOI] [PubMed] [Google Scholar]
- 19.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 20.van Breemen C, Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–29. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]
- 21.Nilius B, Viana F, Droogmans G. Ion channels in vascular endothelium. Annu Rev Physiol. 1997;59:145–70. doi: 10.1146/annurev.physiol.59.1.145. [DOI] [PubMed] [Google Scholar]
- 22.Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci U S A. 2002;99(3):1115–22. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81(4):1415–59. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 24.Sanders KM. Invited review: mechanisms of calcium handling in smooth muscles. J Appl Physiol. 2001;91(3):1438–49. doi: 10.1152/jappl.2001.91.3.1438. [DOI] [PubMed] [Google Scholar]
- 25.Berridge MJ. Smooth muscle cell calcium activation mechanisms. TJ Physiol. 2008;586(Pt 21):5047–61. doi: 10.1113/jphysiol.2008.160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsoukias NM. Calcium dynamics and signaling in vascular regulation: computational models. Wiley interdisciplinary reviews. 2011;3(1):93–106. doi: 10.1002/wsbm.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korngreen A, Gold’shtein V, Priel Z. A realistic model of biphasic calcium transients in electrically nonexcitable cells. Biophys J. 1997;73(2):659–73. doi: 10.1016/S0006-3495(97)78101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldbeter A, Dupont G, Berridge MJ. Minimal model for signal-induced Ca2+ oscillations and for their frequency encoding through protein phosphorylation. Proc Natl Acad Sci U S A. 1990;87(4):1461–5. doi: 10.1073/pnas.87.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Young GW, Keizer J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc Natl Acad Sci U S A. 1992;89(20):9895–9. doi: 10.1073/pnas.89.20.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jafri MS, Vajda S, Pasik P, Gillo B. A membrane model for cytosolic calcium oscillations. A study using Xenopus oocytes. Biophys J. 1992;63(1):235–46. doi: 10.1016/S0006-3495(92)81583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winston FK, Thibault LE, Macarak EJ. An analysis of the time-dependent changes in intracellular calcium concentration in endothelial cells in culture induced by mechanical stimulation. J Biomech Eng. 1993;115(2):160–8. doi: 10.1115/1.2894116. [DOI] [PubMed] [Google Scholar]
- 32.Wong AY, Klassen GA. A model of cytosolic calcium regulation and autacoids production in vascular endothelial cell. Basic Res Cardiol. 1992;87:317–32. doi: 10.1007/BF00796518. [DOI] [PubMed] [Google Scholar]
- 33.Wong AY, Klassen GA. A model of electrical activity and cytosolic calcium dynamics in vascular endothelial cells in response to fluid shear stress. Ann Biomed Eng. 1995;23(6):822–32. doi: 10.1007/BF02584481. [DOI] [PubMed] [Google Scholar]
- 34.Wiesner TF, Berk BC, Nerem RM. A mathematical model of cytosolic calcium dynamics in human umbilical vein endothelial cells. Am J Physiol. 1996;270(5 Pt 1):C1556–69. doi: 10.1152/ajpcell.1996.270.5.C1556. [DOI] [PubMed] [Google Scholar]
- 35.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64(6):1057–68. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 36.Schuster A, Beny JL, Meister JJ. Modelling the electrophysiological endothelial cell response to bradykinin. Eur Biophys J. 2003;32(4):370–80. doi: 10.1007/s00249-003-0279-x. [DOI] [PubMed] [Google Scholar]
- 37.Silva HS, Kapela A, Tsoukias NM. A mathematical model of plasma membrane electrophysiology and calcium dynamics in vascular endothelial cells. Am J Physiol Cell Physiol. 2007;293(1):C277–93. doi: 10.1152/ajpcell.00542.2006. [DOI] [PubMed] [Google Scholar]
- 38.Hong D, Jaron D, Buerk DG, Barbee KA. Transport-dependent calcium signaling in spatially segregated cellular caveolar domains. Am J Physiol Cell Physiol. 2008;294(3):C856–66. doi: 10.1152/ajpcell.00278.2007. [DOI] [PubMed] [Google Scholar]
- 39.Wong AY, Klassen GA. A model of calcium regulation in smooth muscle cell. Cell Calcium. 1993;14(3):227–43. doi: 10.1016/0143-4160(93)90070-m. [DOI] [PubMed] [Google Scholar]
- 40.Wong AY, Klassen GA. Endothelin-induced electrical activity and calcium dynamics in vascular smooth muscle cells: a model study. Ann Biomed Eng. 1996;24(5):547–60. doi: 10.1007/BF02684224. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez-Fernandez JM, Ermentrout B. On the origin and dynamics of the vasomotion of small arteries. Math Biosci. 1994;119(2):127–67. doi: 10.1016/0025-5564(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 42.Fink CC, Slepchenko B, Loew LM. Determination of time-dependent inositol-1,4,5-trisphosphate concentrations during calcium release in a smooth muscle cell. Biophys J. 1999;77(1):617–28. doi: 10.1016/S0006-3495(99)76918-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bennett MR, Farnell L, Gibson WG. A quantitative description of the contraction of blood vessels following the release of noradrenaline from sympathetic varicosities. Journal of theoretical biology. 2005;234(1):107–22. doi: 10.1016/j.jtbi.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 44.Parthimos D, Edwards DH, Griffith TM. Minimal model of arterial chaos generated by coupled intracellular and membrane Ca2+ oscillators. The American journal of physiology. 1999;277(3 Pt 2):H1119–44. doi: 10.1152/ajpheart.1999.277.3.H1119. [DOI] [PubMed] [Google Scholar]
- 45.Koenigsberger M, Sauser R, Lamboley M, Beny JL, Meister JJ. Ca2+ dynamics in a population of smooth muscle cells: modeling the recruitment and synchronization. Biophys J. 2004;87(1):92–104. doi: 10.1529/biophysj.103.037853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koenigsberger M, Sauser R, Meister JJ. Emergent properties of electrically coupled smooth muscle cells. Bull Math Biol. 2005;67(6):1253–72. doi: 10.1016/j.bulm.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Jacobsen JC, Aalkjaer C, Nilsson H, Matchkov VV, Freiberg J, Holstein-Rathlou NH. Activation of a cGMP-sensitive calcium-dependent chloride channel may cause transition from calcium waves to whole cell oscillations in smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;293(1):H215–28. doi: 10.1152/ajpheart.00726.2006. [DOI] [PubMed] [Google Scholar]
- 48.Parthimos D, Edwards DH, Griffith TM. Shil’nikov homoclinic chaos is intimately related to type-III intermittency in isolated rabbit arteries: role of nitric oxide. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;67(5 Pt 1):051922. doi: 10.1103/PhysRevE.67.051922. [DOI] [PubMed] [Google Scholar]
- 49.Parthimos D, Haddock RE, Hill CE, Griffith TM. Dynamics of a three-variable nonlinear model of vasomotion: comparison of theory and experiment. Biophys J. 2007;93(5):1534–56. doi: 10.1529/biophysj.107.106278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang J, Clark JW, Jr, Bryan RM, Robertson C. The myogenic response in isolated rat cerebrovascular arteries: smooth muscle cell model. Med Eng Phys. 2003;25(8):691–709. doi: 10.1016/s1350-4533(03)00100-0. [DOI] [PubMed] [Google Scholar]
- 51.Edwards A, Pallone TL. Modification of cytosolic calcium signaling by subplasmalemmal microdomains. Am J Physiol Renal Physiol. 2007;292(6):F1827–45. doi: 10.1152/ajprenal.00387.2006. [DOI] [PubMed] [Google Scholar]
- 52.Kapela A, Bezerianos A, Tsoukias NM. A mathematical model of Ca2+ dynamics in rat mesenteric smooth muscle cell: agonist and NO stimulation. J Theor Biol. 2008;253(2):238–60. doi: 10.1016/j.jtbi.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Hill AJ, Hinton JM, Cheng H, Gao Z, Bates DO, Hancox JC, et al. A TRPC-like non-selective cation current activated by alpha 1-adrenoceptors in rat mesenteric artery smooth muscle cells. Cell Calcium. 2006;40(1):29–40. doi: 10.1016/j.ceca.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Yang J, Clark JW, Jr, Bryan RM, Robertson CS. The myogenic response in isolated rat cerebrovascular arteries: vessel model. Med Eng Phys. 2003;25(8):711–7. doi: 10.1016/s1350-4533(03)00101-2. [DOI] [PubMed] [Google Scholar]
- 55.Yang J, Clark JW, Bryan RM, Robertson CS. Mathematical modeling of the nitric oxide/cGMP pathway in the vascular smooth muscle cell. Am J Physiol Heart Circ Physiol. 2005;289(2):H886–97. doi: 10.1152/ajpheart.00216.2004. [DOI] [PubMed] [Google Scholar]
- 56.Fujii K, Tominaga M, Ohmori S, Kobayashi K, Koga T, Takata Y, et al. Decreased endothelium-dependent hyperpolarization to acetylcholine in smooth muscle of the mesenteric artery of spontaneously hypertensive rats. Circ Res. 1992;70(4):660–9. doi: 10.1161/01.res.70.4.660. [DOI] [PubMed] [Google Scholar]
- 57.Goto K, Fujii K, Kansui Y, Iida M. Changes in endothelium-derived hyperpolarizing factor in hypertension and ageing: response to chronic treatment with renin-angiotensin system inhibitors. Clin Exp Pharmacol Physiol. 2004;31(9):650–5. doi: 10.1111/j.1440-1681.2004.04054.x. [DOI] [PubMed] [Google Scholar]
- 58.Kansui Y, Fujii K, Goto K, Abe I, Iida M. Effects of fluvastatin on endothelium-derived hyperpolarizing factor- and nitric oxide-mediated relaxations in arteries of hypertensive rats. Clin Exp Pharmacol Physiol. 2004;31(5–6):354–9. doi: 10.1111/j.1440-1681.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- 59.Sandow SL, Bramich NJ, Bandi HP, Rummery NM, Hill CE. Structure, function, and endothelium-derived hyperpolarizing factor in the caudal artery of the SHR and WKY rat. Arterioscler Thromb Vasc Biol. 2003;23(5):822–8. doi: 10.1161/01.ATV.0000067425.06019.D7. [DOI] [PubMed] [Google Scholar]
- 60.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327(6122):524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 61.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 62.Chen G, Suzuki H, Weston AH. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol. 1988;95(4):1165–74. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, et al. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol. 1996;28(5):703–11. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 64.Hwa JJ, Ghibaudi L, Williams P, Chatterjee M. Comparison of acetylcholine-dependent relaxation in large and small arteries of rat mesenteric vascular bed. Am J Physiol. 1994;266(3 Pt 2):H952–8. doi: 10.1152/ajpheart.1994.266.3.H952. [DOI] [PubMed] [Google Scholar]
- 65.Sandow SL. Factors, fiction and endothelium-derived hyperpolarizing factor. Clin Exp Pharmacol Physiol. 2004;31(9):563–70. doi: 10.1111/j.1440-1681.2004.04048.x. [DOI] [PubMed] [Google Scholar]
- 66.Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. European Journal of Physiology. 2010;459(6):863–79. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 67.Chen G, Cheung DW. Effect of K(+)-channel blockers on ACh-induced hyperpolarization and relaxation in mesenteric arteries. The American journal of physiology. 1997;272(5 Pt 2):H2306–12. doi: 10.1152/ajpheart.1997.272.5.H2306. [DOI] [PubMed] [Google Scholar]
- 68.Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res. 2008;102(10):1247–55. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doughty JM, Plane F, Langton PD. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium. Am J Physiol. 1999;276(3 Pt 2):H1107–12. doi: 10.1152/ajpheart.1999.276.3.H1107. [DOI] [PubMed] [Google Scholar]
- 70.Eichler I, Wibawa J, Grgic I, Knorr A, Brakemeier S, Pries AR, et al. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br J Pharmacol. 2003;138(4):594–601. doi: 10.1038/sj.bjp.0705075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coleman HA, Tare M, Parkington HC. K+ currents underlying the action of endothelium-derived hyperpolarizing factor in guinea-pig, rat and human blood vessels. J Physiol. 2001;531(Pt 2):359–73. doi: 10.1111/j.1469-7793.2001.0359i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396(6708):269–72. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 73.Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol. 2003;553(Pt 1):183–9. doi: 10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beny J-L. Electrical coupling between smooth muscle cells and endothelial cells in pig coronary arteries. European Journal of Physiology. 1997;433:364–7. doi: 10.1007/s004240050289. [DOI] [PubMed] [Google Scholar]
- 75.Nicholson BJ, Weber PA, Cao F, Chang H, Lampe P, Goldberg G. The molecular basis of selective permeability of connexins is complex and includes both size and charge. Braz J Med Biol Res. 2000;33(4):369–78. doi: 10.1590/s0100-879x2000000400002. [DOI] [PubMed] [Google Scholar]
- 76.Figueroa XF, Isakson BE, Duling BR. Connexins: gaps in our knowledge of vascular function. Physiology (Bethesda) 2004;19:277–84. doi: 10.1152/physiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- 77.Isakson BE, Best AK, Duling BR. Incidence of protein on actin bridges between endothelium and smooth muscle in arterioles demonstrates heterogeneous connexin expression and phosphorylation. Am J Physiol Heart Circ Physiol. 2008;294(6):H2898–904. doi: 10.1152/ajpheart.91488.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sandow SL, Goto K, Rummery NM, Hill CE. Developmental changes in myoendothelial gap junction mediated vasodilator activity in the rat saphenous artery. J Physiol. 2004;556(Pt 3):875–86. doi: 10.1113/jphysiol.2003.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goto K, Fujii K, Kansui Y, Abe I, Iida M. Critical role of gap junctions in endothelium-dependent hyperpolarization in rat mesenteric arteries. Clin Exp Pharmacol Physiol. 2002;29(7):595–602. doi: 10.1046/j.1440-1681.2002.03689.x. [DOI] [PubMed] [Google Scholar]
- 80.Hill CE, Hickey H, Sandow SL. Role of gap junctions in acetylcholine-induced vasodilation of proximal and distal arteries of the rat mesentery. J Auton Nerv Syst. 2000;81(1–3):122–7. doi: 10.1016/s0165-1838(00)00113-2. [DOI] [PubMed] [Google Scholar]
- 81.Sokoya EM, Burns AR, Setiawan CT, Coleman HA, Parkington HC, Tare M. Evidence for the involvement of myoendothelial gap junctions in EDHF-mediated relaxation in the rat middle cerebral artery. Am J Physiol Heart Circ Physiol. 2006;291(1):H385–93. doi: 10.1152/ajpheart.01047.2005. [DOI] [PubMed] [Google Scholar]
- 82.Dora KA, Sandow SL, Gallagher NT, Takano H, Rummery NM, Hill CE, et al. Myoendothelial gap junctions may provide the pathway for EDHF in mouse mesenteric artery. J Vasc Res. 2003;40(5):480–90. doi: 10.1159/000074549. [DOI] [PubMed] [Google Scholar]
- 83.Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ Res. 2002;90(10):1108–13. doi: 10.1161/01.res.0000019756.88731.83. [DOI] [PubMed] [Google Scholar]
- 84.Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res. 2005;97(4):399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- 85.Dora KA, Garland CJ. Properties of smooth muscle hyperpolarization and relaxation to K+ in the rat isolated mesenteric artery. Am J Physiol Heart Circ Physiol. 2001;280(6):H2424–9. doi: 10.1152/ajpheart.2001.280.6.H2424. [DOI] [PubMed] [Google Scholar]
- 86.Weston AH, Richards GR, Burnham MP, Feletou M, Vanhoutte PM, Edwards G. K+-induced hyperpolarization in rat mesenteric artery: identification, localization and role of Na+/K+-ATPases. Br J Pharmacol. 2002;136(6):918–26. doi: 10.1038/sj.bjp.0704787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci U S A. 1997;94(12):6529–34. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lamboley M, Pittet P, Koenigsberger M, Sauser R, Beny JL, Meister JJ. Evidence for signaling via gap junctions from smooth muscle to endothelial cells in rat mesenteric arteries: possible implication of a second messenger. Cell Calcium. 2005;37(4):311–20. doi: 10.1016/j.ceca.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 89.Schuster A, Oishi H, Beny JL, Stergiopulos N, Meister JJ. Simultaneous arterial calcium dynamics and diameter measurements: application to myoendothelial communication. Am J Physiol Heart Circ Physiol. 2001;280(3):H1088–96. doi: 10.1152/ajpheart.2001.280.3.H1088. [DOI] [PubMed] [Google Scholar]
- 90.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, et al. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci U S A. 2008;105(28):9627–32. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Tallini YN, Kotlikoff MI, Mark T, Nelson MT. Ca2+ pulsars: spatially restricted, IP3R-mediated Ca2+ release important for endothelial function. FASEB J. 2008;22:1181. [Google Scholar]
- 92.Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca2+ events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium. 2008;44(2):135–46. doi: 10.1016/j.ceca.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res. 2007;100(2):246–54. doi: 10.1161/01.RES.0000257744.23795.93. [DOI] [PubMed] [Google Scholar]
- 94.Isakson BE. Localized expression of an Ins(1,4,5) P3 receptor at the myoendothelial junction selectively regulates heterocellular Ca2+ communication. J Cell Sci. 2008;121(Pt 21):3664–73. doi: 10.1242/jcs.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sims CE, Allbritton NL. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate by the oocytes of Xenopus laevis. J Biol Chem. 1998;273(7):4052–8. doi: 10.1074/jbc.273.7.4052. [DOI] [PubMed] [Google Scholar]
- 96.Moore DH, Ruska H. The fine structure of capillaries and small arteries. J Biophys Biochem Cytol. 1957;3(3):457–62. doi: 10.1083/jcb.3.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heberlein KR, Straub AC, Isakson BE. The myoendothelial junction: breaking through the matrix? Microcirculation. 2009;16(4):307–22. doi: 10.1080/10739680902744404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sandow SL, Gzik DJ, Lee RM. Arterial internal elastic lamina holes: relationship to function? J Anat. 2009;214(2):258–66. doi: 10.1111/j.1469-7580.2008.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86(3):341–6. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- 100.Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function? J Anat. 2006;209(5):689–98. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jacobsen JC, Aalkjaer C, Nilsson H, Matchkov VV, Freiberg J, Holstein-Rathlou NH. A model of smooth muscle cell synchronization in the arterial wall. Am J Physiol Heart Circ Physiol. 2007;293(1):H229–37. doi: 10.1152/ajpheart.00727.2006. [DOI] [PubMed] [Google Scholar]
- 102.Koenigsberger M, Sauser R, Beny JL, Meister JJ. Effects of arterial wall stress on vasomotion. Biophys J. 2006;91(5):1663–74. doi: 10.1529/biophysj.106.083311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brace RA, Anderson DK. Predicting transient and steady-state changes in resting membrane potential. J Appl Physiol. 1973;35(1):90–4. doi: 10.1152/jappl.1973.35.1.90. [DOI] [PubMed] [Google Scholar]
- 104.Koenigsberger M, Sauser R, Beny JL, Meister JJ. Role of the endothelium on arterial vasomotion. Biophys J. 2005;88(6):3845–54. doi: 10.1529/biophysj.104.054965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Diep HK, Vigmond EJ, Segal SS, Welsh DG. Defining electrical communication in skeletal muscle resistance arteries: a computational approach. J Physiol. 2005;568(Pt 1):267–81. doi: 10.1113/jphysiol.2005.090233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kapela A, Nagaraja S, Tsoukias NM. A mathematical model of vasoreactivity in rat mesenteric arterioles. II. Conducted vasoreactivity. Am J Physiol Heart Circ Physiol. 2010;298(1):H52–65. doi: 10.1152/ajpheart.00546.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kapela A, Bezerianos A, Tsoukias NM. A mathematical model of vasoreactivity in rat mesenteric arterioles: I. Myoendothelial communication. Microcirculation. 2009;16:1–20. doi: 10.3109/10739680903177539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kapela A, Nagaraja S, Tsoukias N. A theoretical study of myoendothelial communication: K+-mediated EDHF signaling and the role of myoendothelial projections. FASEB J. 2009;23:672.12. [Google Scholar]
- 109.Nagaraja S, Kapela A, Tsoukias N. Microprojections amplify EC response to SMC stimulation: A theoretical study. FASEB J. 2010;24:976.14. [Google Scholar]
- 110.Krogh A, Harrop GA, Rehberg PB. Studies on the physiology of capillaries: III. The innervation of the blood vessels in the hind legs of the frog. J Physiol. 1922;56(3–4):179–89. doi: 10.1113/jphysiol.1922.sp002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Duling BR, Berne RM. Propagated vasodilation in the microcirculation of the hamster cheek pouch. Circ Res. 1970;26(2):163–70. doi: 10.1161/01.res.26.2.163. [DOI] [PubMed] [Google Scholar]
- 112.Segal SS. Microvascular recruitment in hamster striated muscle: role for conducted vasodilation. Am J Physiol. 1991;261(1 Pt 2):H181–9. doi: 10.1152/ajpheart.1991.261.1.H181. [DOI] [PubMed] [Google Scholar]
- 113.Gustafsson F, Holstein-Rathlou N. Conducted vasomotor responses in arterioles: characteristics, mechanisms and physiological significance. Acta Physiol Scand. 1999;167(1):11–21. doi: 10.1046/j.1365-201x.1999.00603.x. [DOI] [PubMed] [Google Scholar]
- 114.Dora KA, Xia J, Duling BR. Endothelial cell signaling during conducted vasomotor responses. Am J Physiol Heart Circ Physiol. 2003;285(1):H119–26. doi: 10.1152/ajpheart.00643.2002. [DOI] [PubMed] [Google Scholar]
- 115.Dora KA. Coordination of vasomotor responses by the endothelium. Circ J. 2010;74(2):226–32. doi: 10.1253/circj.cj-09-0879. [DOI] [PubMed] [Google Scholar]
- 116.Segal SS, Welsh DG, Kurjiaka DT. Spread of vasodilatation and vasoconstriction along feed arteries and arterioles of hamster skeletal muscle. J Physiol. 1999;516(Pt 1):283–91. doi: 10.1111/j.1469-7793.1999.283aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12(1):33–45. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 118.Arciero JC, Carlson BE, Secomb TW. Theoretical model of metabolic blood flow regulation: roles of ATP release by red blood cells and conducted responses. Am J Physiol Heart Circ Physiol. 2008;295(4):H1562–71. doi: 10.1152/ajpheart.00261.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen YM, Yip KP, Marsh DJ, Holstein-Rathlou NH. Magnitude of TGF-initiated nephron-nephron interactions is increased in SHR. Am J Physiol. 1995;269(2 Pt 2):F198–204. doi: 10.1152/ajprenal.1995.269.2.F198. [DOI] [PubMed] [Google Scholar]
- 120.Pries AR, Secomb TW, Gaehtgens P. Structural adaptation and stability of microvascular networks: theory and simulations. Am J Physiol. 1998;275(2 Pt 2):H349–60. doi: 10.1152/ajpheart.1998.275.2.H349. [DOI] [PubMed] [Google Scholar]
- 121.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269(6 Pt 2):H2155–61. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 122.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26(1):40–7. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- 123.Dietrich HH, Horiuchi T, Xiang C, Hongo K, Falck JR, Dacey RG., Jr Mechanism of ATP-induced local and conducted vasomotor responses in isolated rat cerebral penetrating arterioles. J Vasc Res. 2009;46(3):253–64. doi: 10.1159/000167273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Collins DM, McCullough WT, Ellsworth ML. Conducted vascular responses: communication across the capillary bed. Microvasc Res. 1998;56(1):43–53. doi: 10.1006/mvre.1998.2076. [DOI] [PubMed] [Google Scholar]
- 125.Winter P, Dora KA. Spreading dilatation to luminal perfusion of ATP and UTP in rat isolated small mesenteric arteries. J Physiol. 2007;582(Pt 1):335–47. doi: 10.1113/jphysiol.2007.135202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Riemann M, Rai A, Ngo AT, Dziegiel MH, Holstein-Rathlou NH, Torp-Pedersen C. Oxygen-dependent vasomotor responses are conducted upstream in the mouse cremaster microcirculation. J Vasc Res. 2011;48(1):79–89. doi: 10.1159/000318777. [DOI] [PubMed] [Google Scholar]
- 127.Ngo AT, Jensen LJ, Riemann M, Holstein-Rathlou NH, Torp-Pedersen C. Oxygen sensing and conducted vasomotor responses in mouse cremaster arterioles in situ. Pflugers Arch. 2010;460(1):41–53. doi: 10.1007/s00424-010-0837-x. [DOI] [PubMed] [Google Scholar]
- 128.Takano H, Dora KA, Spitaler MM, Garland CJ. Spreading dilatation in rat mesenteric arteries associated with calcium-independent endothelial cell hyperpolarization. J Physiol. 2004;556(Pt 3):887–903. doi: 10.1113/jphysiol.2003.060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.de Wit C. Different pathways with distinct properties conduct dilations in the microcirculation in vivo. Cardiovasc Res. 2010;85(3):604–13. doi: 10.1093/cvr/cvp340. [DOI] [PubMed] [Google Scholar]
- 130.Wolfle SE, Schmidt VJ, Hoyer J, Kohler R, de Wit C. Prominent role of KCa3.1 in endothelium-derived hyperpolarizing factor-type dilations and conducted responses in the microcirculation in vivo. Cardiovasc Res. 2009;82(3):476–83. doi: 10.1093/cvr/cvp060. [DOI] [PubMed] [Google Scholar]
- 131.Figueroa XF, Paul DL, Simon AM, Goodenough DA, Day KH, Damon DN, et al. Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ Res. 2003;92(7):793–800. doi: 10.1161/01.RES.0000065918.90271.9A. [DOI] [PubMed] [Google Scholar]
- 132.Wolfle SE, Schmidt VJ, Hoepfl B, Gebert A, Alcolea S, Gros D, et al. Connexin45 cannot replace the function of connexin40 in conducting endothelium-dependent dilations along arterioles. Circ Res. 2007;101(12):1292–9. doi: 10.1161/CIRCRESAHA.107.163279. [DOI] [PubMed] [Google Scholar]
- 133.de Wit C, Griffith TM. Connexins and gap junctions in the EDHF phenomenon and conducted vasomotor responses. Pflugers Arch. 2010;459(6):897–914. doi: 10.1007/s00424-010-0830-4. [DOI] [PubMed] [Google Scholar]
- 134.Takano H, Dora KA, Garland CJ. Spreading vasodilatation in resistance arteries. J Smooth Muscle Res. 2005;41(6):303–11. doi: 10.1540/jsmr.41.303. [DOI] [PubMed] [Google Scholar]
- 135.Schmidt VJ, Wolfle SE, Boettcher M, de Wit C. Gap junctions synchronize vascular tone within the microcirculation. Pharmacol Rep. 2008;60(1):68–74. [PubMed] [Google Scholar]
- 136.Christ GJ, Spray DC, el-Sabban M, Moore LK, Brink PR. Gap junctions in vascular tissues. Evaluating the role of intercellular communication in the modulation of vasomotor tone. Circ Res. 1996;79(4):631–46. doi: 10.1161/01.res.79.4.631. [DOI] [PubMed] [Google Scholar]
- 137.Brink PR, Ricotta J, Christ GJ. Biophysical characteristics of gap junctions in vascular wall cells: implications for vascular biology and disease. Braz J Med Biol Res. 2000;33:415–22. doi: 10.1590/s0100-879x2000000400007. [DOI] [PubMed] [Google Scholar]
- 138.Hirst GD, Neild TO. An analysis of excitatory junctional potentials recorded from arterioles. J Physiol. 1978;280:87–104. doi: 10.1113/jphysiol.1978.sp012374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Emerson GG, Neild TO, Segal SS. Conduction of hyperpolarization along hamster feed arteries: augmentation by acetylcholine. Am J Physiol Heart Circ Physiol. 2002;283(1):H102–9. doi: 10.1152/ajpheart.00038.2002. [DOI] [PubMed] [Google Scholar]
- 140.Figueroa XF, Duling BR. Dissection of two Cx37-independent conducted vasodilator mechanisms by deletion of Cx40: electrotonic versus regenerative conduction. Am J Physiol Heart Circ Physiol. 2008;295(5):H2001–7. doi: 10.1152/ajpheart.00063.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Crane GJ, Neild TO, Segal SS. Contribution of active membrane processes to conducted hyperpolarization in arterioles of hamster cheek pouch. Microcirculation. 2004;11(5):425–33. doi: 10.1080/10739680490457836. [DOI] [PubMed] [Google Scholar]
- 142.Rivers RJ, Hein TW, Zhang C, Kuo L. Activation of barium-sensitive inward rectifier potassium channels mediates remote dilation of coronary arterioles. Circulation. 2001;104(15):1749–53. doi: 10.1161/hc4001.098053. [DOI] [PubMed] [Google Scholar]
- 143.Jantzi MC, Brett SE, Jackson WF, Corteling R, Vigmond EJ, Welsh DG. Inward rectifying potassium channels facilitate cell-to-cell communication in hamster retractor muscle feed arteries. Am J Physiol Heart Circ Physiol. 2006;291(3):H1319–28. doi: 10.1152/ajpheart.00217.2006. [DOI] [PubMed] [Google Scholar]
- 144.Goto K, Rummery NM, Grayson TH, Hill CE. Attenuation of conducted vasodilatation in rat mesenteric arteries during hypertension: role of inwardly rectifying potassium channels. J Physiol. 2004;561(Pt 1):215–31. doi: 10.1113/jphysiol.2004.070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fleming I. Bobbing along on the crest of a wave: NO ascends hamster cheek pouch arterioles. Circ Res. 2003;93(1):9–11. doi: 10.1161/01.RES.0000082769.87973.B9. [DOI] [PubMed] [Google Scholar]
- 146.Doyle MP, Duling BR. Acetylcholine induces conducted vasodilation by nitric oxide-dependent and -independent mechanisms. Am J Physiol. 1997;272(3 Pt 2):H1364–71. doi: 10.1152/ajpheart.1997.272.3.H1364. [DOI] [PubMed] [Google Scholar]
- 147.Budel S, Bartlett IS, Segal SS. Homocellular conduction along endothelium and smooth muscle of arterioles in hamster cheek pouch: unmasking an NO wave. Circ Res. 2003;93(1):61–8. doi: 10.1161/01.RES.0000080318.81205.FD. [DOI] [PubMed] [Google Scholar]
- 148.Figueroa X, Chen CC, Campbell KP, Damon DN, Day KH, Ramos S, et al. Are voltage-dependent ion channels involved in the endothelial cell control of vasomotor tone? Am J Physiol Heart Circ Physiol. 2007;293:H1371–83. doi: 10.1152/ajpheart.01368.2006. [DOI] [PubMed] [Google Scholar]
- 149.Luckhoff A, Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Arch. 1990;416(3):305–11. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- 150.Uhrenholt TR, Domeier TL, Segal SS. Propagation of calcium waves along endothelium of hamster feed arteries. Am J Physiol Heart Circ Physiol. 2007;292(3):H1634–40. doi: 10.1152/ajpheart.00605.2006. [DOI] [PubMed] [Google Scholar]
- 151.Domeier TL, Segal SS. Electromechanical and pharmacomechanical signalling pathways for conducted vasodilatation along endothelium of hamster feed arteries. J Physiol. 2007;579(Pt 1):175–86. doi: 10.1113/jphysiol.2006.124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sanderson MJ, Charles AC, Boitano S, Dirksen ER. Mechanisms and function of intercellular calcium signaling. Mol Cell Endocrinol. 1994;98(2):173–87. doi: 10.1016/0303-7207(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 153.Welsh DG, Tran NN, Plane F, Sandow S. Letter to the Editor: “Are voltage-dependent ion channels involved in the endothelial cell control of vasomotor tone?”. Am J Physiol Heart Circ Physiol. 2007;293:H2007. doi: 10.1152/ajpheart.00722.2007. [DOI] [PubMed] [Google Scholar]
- 154.Haug SJ, Segal SS. Sympathetic neural inhibition of conducted vasodilatation along hamster feed arteries: complementary effects of alpha1- and alpha2-adrenoreceptor activation. J Physiol. 2005;563(Pt 2):541–55. doi: 10.1113/jphysiol.2004.072900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gustafsson F, Holstein-Rathlou NH. Angiotensin II modulates conducted vasoconstriction to norepinephrine and local electrical stimulation in rat mesenteric arterioles. Cardiovasc Res. 1999;44(1):176–84. doi: 10.1016/s0008-6363(99)00174-1. [DOI] [PubMed] [Google Scholar]
- 156.Ngai AC, Nguyen TS, Meno JR, Britz GW. Post-ischemic augmentation of conducted dilation in cerebral arterioles. Stroke. 2007;38(1):124–30. doi: 10.1161/01.STR.0000252157.93998.47. [DOI] [PubMed] [Google Scholar]
- 157.Crane GJ, Neild TO. An equation describing spread of membrane potential changes in a short segment of blood vessel. Phys Med Biol. 1999;44(10):N217–21. doi: 10.1088/0031-9155/44/10/402. [DOI] [PubMed] [Google Scholar]
- 158.Segal SS, Neild TO. Conducted depolarization in arteriole networks of the guinea-pig small intestine: effect of branching of signal dissipation. J Physiol. 1996;496(Pt 1):229–44. doi: 10.1113/jphysiol.1996.sp021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Crane GJ, Hines ML, Neild TO. Simulating the spread of membrane potential changes in arteriolar networks. Microcirculation. 2001;8(1):33–43. [PubMed] [Google Scholar]
- 160.Nilsson H, Aalkjaer C. Vasomotion: mechanisms and physiological importance. Mol Interv. 2003;3(2):79–89. 51. doi: 10.1124/mi.3.2.79. [DOI] [PubMed] [Google Scholar]
- 161.Haddock RE, Hill CE. Rhythmicity in arterial smooth muscle. J Physiol. 2005;566(Pt 3):645–56. doi: 10.1113/jphysiol.2005.086405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gustafsson H. Vasomotion and underlying mechanisms in small arteries. An in vitro study of rat blood vessels. Acta Physiol Scand Suppl. 1993;614:1–44. [PubMed] [Google Scholar]
- 163.Peng H, Matchkov V, Ivarsen A, Aalkjaer C, Nilsson H. Hypothesis for the initiation of vasomotion. Circ Res. 2001;88(8):810–5. doi: 10.1161/hh0801.089603. [DOI] [PubMed] [Google Scholar]
- 164.Schuster A, Lamboley M, Grange C, Oishi H, Beny JL, Stergiopulos N, et al. Calcium dynamics and vasomotion in rat mesenteric arteries. J Cardiovasc Pharmacol. 2004;43(4):539–48. doi: 10.1097/00005344-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 165.Lamboley M, Schuster A, Beny JL, Meister JJ. Recruitment of smooth muscle cells and arterial vasomotion. Am J Physiol Heart Circ Physiol. 2003;285(2):H562–9. doi: 10.1152/ajpheart.00526.2002. [DOI] [PubMed] [Google Scholar]
- 166.Faber JE, Harris PD, Wiegman DL. Anesthetic depression of microcirculation, central hemodynamics, and respiration in decerebrate rats. Am J Physiol. 1982;243(6):H837–43. doi: 10.1152/ajpheart.1982.243.6.H837. [DOI] [PubMed] [Google Scholar]
- 167.Fujii K, Heistad DD, Faraci FM. Ionic mechanisms in spontaneous vasomotion of the rat basilar artery in vivo. J Physiol. 1990;430:389–98. doi: 10.1113/jphysiol.1990.sp018297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gokina NI, Bevan RD, Walters CL, Bevan JA. Electrical activity underlying rhythmic contraction in human pial arteries. Circ Res. 1996;78(1):148–53. doi: 10.1161/01.res.78.1.148. [DOI] [PubMed] [Google Scholar]
- 169.Auer LM, Gallhofer B. Rhythmic activity of cat pial vessels in vivo. Eur Neurol. 1981;20(6):448–68. doi: 10.1159/000115278. [DOI] [PubMed] [Google Scholar]
- 170.Gotoh F, Muramatsu F, Fukuuchi Y, Okayasu H, Tanaka K, Suzuki N, et al. Video camera method for simultaneous measurement of blood flow velocity and pial vessel diameter. J Cereb Blood Flow Metab. 1982;2(4):421–8. doi: 10.1038/jcbfm.1982.48. [DOI] [PubMed] [Google Scholar]
- 171.Hundley WG, Renaldo GJ, Levasseur JE, Kontos HA. Vasomotion in cerebral microcirculation of awake rabbits. Am J Physiol. 1988;254(1 Pt 2):H67–71. doi: 10.1152/ajpheart.1988.254.1.H67. [DOI] [PubMed] [Google Scholar]
- 172.Garcia-Huidobro DN, Garcia-Huidobro MT, Huidobro-Toro JP. Vasomotion in human umbilical and placental veins: role of gap junctions and intracellular calcium reservoirs in their synchronous propagation. Placenta. 2007;28(4):328–38. doi: 10.1016/j.placenta.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 173.Kastrup J, Bulow J, Lassen NA. Vasomotion in human skin before and after local heating recorded with laser Doppler flowmetry. A method for induction of vasomotion. Int J Microcirc Clin Exp. 1989;8(2):205–15. [PubMed] [Google Scholar]
- 174.Pradhan RK, Chakravarthy VS. Informational dynamics of vasomotion in microvascular networks: a review. Acta Physiol (Oxf) 2011;201:193–218. doi: 10.1111/j.1748-1716.2010.02198.x. [DOI] [PubMed] [Google Scholar]
- 175.Schmidt JA, Intaglietta M, Borgstrom P. Periodic hemodynamics in skeletal muscle during local arterial pressure reduction. J Appl Physiol. 1992;73(3):1077–83. doi: 10.1152/jappl.1992.73.3.1077. [DOI] [PubMed] [Google Scholar]
- 176.Schmidt-Lucke C, Borgstrom P, Schmidt-Lucke JA. Low frequency flowmotion/(vasomotion) during pathophysiological conditions. Life Sci. 2002;71(23):2713–28. doi: 10.1016/s0024-3205(02)02110-0. [DOI] [PubMed] [Google Scholar]
- 177.Myers JH, Lamb FS, Webb RC. Norepinephrine-induced phasic activity in tail arteries from genetically hypertensive rats. Am J Physiol. 1985;248(3 Pt 2):H419–23. doi: 10.1152/ajpheart.1985.248.3.H419. [DOI] [PubMed] [Google Scholar]
- 178.Goldman D, Popel AS. A computational study of the effect of vasomotion on oxygen transport from capillary networks. J Theoret Bio. 2001;209(2):189–99. doi: 10.1006/jtbi.2000.2254. [DOI] [PubMed] [Google Scholar]
- 179.Sakurai T, Terui N. Effects of sympathetically induced vasomotion on tissue-capillary fluid exchange. Am J Physiol Heart Circ Physiol. 2006;291(4):H1761–7. doi: 10.1152/ajpheart.00280.2006. [DOI] [PubMed] [Google Scholar]
- 180.Pradhan RK, Chakravarthy VS. Desynchronized vasomotion and desynchronized fiber activation pattern enhance oxygenation in a model of skeletal muscle. J Theoret Biol. 2009;259(2):242–52. doi: 10.1016/j.jtbi.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 181.Lee CH, Poburko D, Sahota P, Sandhu J, Ruehlmann DO, van Breemen C. The mechanism of phenylephrine-mediated [Ca(2+)](i) oscillations underlying tonic contraction in the rabbit inferior vena cava. J Physiol. 2001;534(Pt 3):641–50. doi: 10.1111/j.1469-7793.2001.t01-1-00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Omote M, Kajimoto N, Mizusawa H. The ionic mechanism of phenylephrine-induced rhythmic contractions in rabbit mesenteric arteries treated with ryanodine. Acta Physiol Scand. 1993;147(1):9–13. doi: 10.1111/j.1748-1716.1993.tb09467.x. [DOI] [PubMed] [Google Scholar]
- 183.Rahman A, Hughes A, Matchkov V, Nilsson H, Aalkjaer C. Antiphase oscillations of endothelium and smooth muscle [Ca2+]i in vasomotion of rat mesenteric small arteries. Cell Calcium. 2007;42(6):536–47. doi: 10.1016/j.ceca.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 184.Haddock RE, Hill CE. Differential activation of ion channels by inositol 1,4,5-trisphosphate (IP3)- and ryanodine-sensitive calcium stores in rat basilar artery vasomotion. J Physiol. 2002;545(Pt 2):615–27. doi: 10.1113/jphysiol.2002.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Matchkov VV, Aalkjaer C, Nilsson H. A cyclic GMP-dependent calcium-activated chloride current in smooth-muscle cells from rat mesenteric resistance arteries. J Gen Physiol. 2004;123(2):121–34. doi: 10.1085/jgp.200308972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Imtiaz MS, von der Weid PY, van Helden DF. Synchronization of Ca2+ oscillations: a coupled oscillator-based mechanism in smooth muscle. Febs J. 2009;277(2):278–85. doi: 10.1111/j.1742-4658.2009.07437.x. [DOI] [PubMed] [Google Scholar]
- 187.Yuill KH, McNeish AJ, Kansui Y, Garland CJ, Dora KA. Nitric oxide suppresses cerebral vasomotion by sGC-independent effects on ryanodine receptors and voltage-gated calcium channels. J Vasc Res. 2010;47(2):93–107. doi: 10.1159/000235964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rahman A, Matchkov V, Nilsson H, Aalkjaer C. Effects of cGMP on coordination of vascular smooth muscle cells of rat mesenteric small arteries. J Vasc Res. 2005;42(4):301–11. doi: 10.1159/000086002. [DOI] [PubMed] [Google Scholar]
- 189.Boedtkjer DM, Matchkov VV, Boedtkjer E, Nilsson H, Aalkjaer C. Vasomotion has chloride-dependency in rat mesenteric small arteries. Pflugers Arch. 2008;457(2):389–404. doi: 10.1007/s00424-008-0532-3. [DOI] [PubMed] [Google Scholar]
- 190.Mauban JR, Wier WG. Essential role of EDHF in the initiation and maintenance of adrenergic vasomotion in rat mesenteric arteries. Am J Physiol Heart Circ Physiol. 2004;287(2):H608–16. doi: 10.1152/ajpheart.01084.2003. [DOI] [PubMed] [Google Scholar]
- 191.Sell M, Boldt W, Markwardt F. Desynchronising effect of the endothelium on intracellular Ca2+ concentration dynamics in vascular smooth muscle cells of rat mesenteric arteries. Cell Calcium. 2002;32(3):105–20. doi: 10.1016/s0143-4160(02)00036-2. [DOI] [PubMed] [Google Scholar]
- 192.Keener JP, Sneyd J. Mathematical physiology. 2. New York, NY: Springer; 2009. [Google Scholar]
- 193.LaBelle EF, Polyak F. Phospholipase C beta 2 in vascular smooth muscle. J Cell Physiol. 1996;169(2):358–63. doi: 10.1002/(SICI)1097-4652(199611)169:2<358::AID-JCP15>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 194.Seppey D, Sauser R, Koenigsberger M, Beny JL, Meister JJ. Does the endothelium abolish or promote arterial vasomotion in rat mesenteric arteries? Explanations for the seemingly contradictory effects. J Vasc Res. 2008;45(5):416–26. doi: 10.1159/000124283. [DOI] [PubMed] [Google Scholar]
- 195.Meyer JU, Lindbom L, Intaglietta M. Pacemaker induced diameter oscillations at arteriolar bifurcations in skeletal muscle. Prog Appl Microcirc. 1983;12:264–9. doi: 10.1152/ajpheart.1987.253.3.H568. [DOI] [PubMed] [Google Scholar]
- 196.Wolfle SE, Navarro-Gonzalez MF, Grayson TH, Stricker C, Hill CE. Involvement of nonselective cation channels in the depolarisation initiating vasomotion. Clin Exp Pharmacol Physiol. 2010;37:536–43. doi: 10.1111/j.1440-1681.2009.05350.x. [DOI] [PubMed] [Google Scholar]
- 197.Kapela A, Bezerianos A, Tsoukias NM. A mathematical model of vasoreactivity in rat mesenteric arterioles: I. Myoendothelial communication. Microcirculation. 2009;16(8):694–713. doi: 10.3109/10739680903177539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jacobsen JC, Aalkjaer C, Matchkov VV, Nilsson H, Freiberg JJ, Holstein-Rathlou NH. Heterogeneity and weak coupling may explain the synchronization characteristics of cells in the arterial wall. Philos Transact A Math Phys Eng Sci. 2008;366(1880):3483–502. doi: 10.1098/rsta.2008.0105. [DOI] [PubMed] [Google Scholar]
- 199.Bartlett PJ, Young KW, Nahorski SR, Challiss RA. Single cell analysis and temporal profiling of agonist-mediated inositol 1,4,5-trisphosphate, Ca2+, diacylglycerol, and protein kinase C signaling using fluorescent biosensors. J Biol Chem. 2005;280(23):21837–46. doi: 10.1074/jbc.M411843200. [DOI] [PubMed] [Google Scholar]
- 200.Skupin A, Kettenmann H, Winkler U, Wartenberg M, Sauer H, Tovey SC, et al. How does intracellular Ca2+ oscillate: by chance or by the clock? Biophys J. 2008;94(6):2404–11. doi: 10.1529/biophysj.107.119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Seppey D, Sauser R, Koenigsberger M, Beny JL, Meister JJ. Intercellular calcium waves are associated with the propagation of vasomotion along arterial strips. Am J Physiol Heart Circ Physiol. 2010;298(2):H488–96. doi: 10.1152/ajpheart.00281.2009. [DOI] [PubMed] [Google Scholar]