Abstract
Objective
To measure and compare the perioral stiffness among three groups of pediatric subjects: a group of patients with a repaired cleft lip (and palate) who had a secondary lip revision surgery (revision), another group of patients with repaired cleft lip (and palate) who did not have secondary surgery (nonrevision), and a group of noncleft “normal” patients (noncleft).
Design
A parallel, three-group, nonrandomized clinical trial.
Participants
A total of 16 patients with repaired cleft lip/palate who did not have lip revision, 13 patients with repaired cleft lip/palate who had lip revision surgery and were tested at 18 to 24 months postsurgery, and 27 noncleft patients.
Analysis
Nonparticipatory perioral stiffness was sampled using a recently developed face-referenced measurement technology known as OroSTIFF. Perioral stiffness, derived as a quotient from resultant force and interangle lip span, was modeled with multilevel regression techniques. Real-time calculation of the perioral stiffness function demonstrated a significant quadratic relation between imposed interangle stretch and resultant force for each of the three groups.
Results
This nonlinear stiffness growth function was significantly elevated in the nonrevision patients compared with the noncleft controls and is likely due to the presence of scar tissue in the upper lip; it was significantly lower among patients with cleft lip/palate who completed lip revision surgery.
Conclusion
This study demonstrates the efficacy of applying an objective measurement to map differences in perioral tissue biomechanics among patients born with orofacial clefts.
Keywords: biomechanics, cheiloplasty, cleft lip, displacement, elastic recoil, force, lower lip, upper lip
Abnormalities in lip function may be attributed to impairments in the regulation of muscle forces of the circumoral/facial region and/or to the presence of scar tissue prevalent among patients with a repaired cleft lip, both of which alter the biomechanical properties of the muscle fibers (Trotman et al., 2007a). Such abnormalities contribute to disorders of facial movement, oral continence, eating, speech production, and oral access (Stranc and Fogel, 1984; Trotman et al., 2000; Trotman et al., 2005). Trotman and coworkers (1998, 2000, 2005) found isolated and quantifiable areas of impaired circumoral soft tissue movements in patients with repaired cleft lip and palate that were related to the effects of the original cleft defect as well as the subsequent scarring that resulted from the primary or initial surgical repair of the lip. Studies on facial kinematics (Trotman et al., 2000; Trotman et al., 2005; Trotman et al., 2007a; Trotman et al., 2007b) support the possibility that in a child with a repaired cleft lip, abnormalities in the upper lip may influence the function of the lower lip. Moreover, subsequent lip revision surgery improves the soft tissue movements of the lip on average but with considerable individual variation—some patients have greater impairment in their movements (get worse); whereas, others have less impairment (show an improvement). Speech also is affected. Pilot studies on lip coordination during speech movements in a small sample of patients with repaired cleft lip conducted by Rutjens et al. (2001) and van Lieshout et al. (2002) demonstrated that young subjects who had recent lip surgery were most likely to show asynchronies in lip movements. Thus, quantifiable measures of lip movements have a demonstrated value for the assessment of lip impairment in patients with cleft lip. To date, however, our understanding of the effects of lip scarring on the underlying tissue biomechanics in this patient population, with particular reference to the stiffness of the lip tissues, remains largely unknown.
A common approach for quantifying muscle stiffness involves subjecting a specific muscle system (e.g., whole body, limb, jaw, lip) to a set amount of displacement (ΔX) and then measuring the force (ΔF) that the system produces. The ratio of the resultant force to the displacement yields a stiffness quotient (ΔF/ΔX) that can be used to compare muscle stiffness properties among patients with different disorders. For example, the stiffness profiles and/or force dynamics for the perioral system show differences when compared among healthy young adults (Barlow and Rath, 1985; Barlow and Müller, 1991), adults with traumatic brain injury (Barlow and Burton, 1990), adults with Parkinson disease (Hunker et al., 1982; Caligiuri, 1989; Barlow et al., 1998; Chu et al., 2009; Chu et al., 2010), and individuals with upper motor neuron syndrome (Barlow and Abbs, 1986). Likewise, for patients who have a repaired cleft lip, it can be expected that there is a disordered stiffness mechanism associated with the scarred upper lip/circumoral muscle system that affects facial animation and reinforces the important role of muscle and tissue biomechanics in facial animation for speech, gesture, and eating (Müller et al., 1985; Chu et al., 2010).
The present study was designed to measure and compare the perioral stiffness among three groups of pediatric subjects: a group of patients with a repaired cleft lip (and palate) who had a secondary lip revision surgery (revision), another group of patients with repaired cleft lip (and palate) who did not have a lip revision (nonrevision), and a group of noncleft “normal” patients (noncleft). The perioral stiffness was measured using a recently developed face-referenced technology known as OroSTIFF (Chu et al., 2010) that does not require head restraint. The following hypotheses were considered: (1) Patients with repaired cleft lip who did not have secondary lip revision surgery (nonrevision group) would manifest scarring of the upper lip and a higher perioral stiffness than the noncleft normal patients; and (2) patients with a repaired cleft lip who received lip revision surgery (revision group) would manifest even greater perioral stiffness than the nonrevision cleft and noncleft patients due to the increased scarring that would be expected from the additional lip revision surgery.
Materials and Methods
Subjects
Subjects were recruited from those attending the University of North Carolina School of Dentistry Orthodontic and Craniofacial Clinics and were part of a larger clinical trial funded by the National Institutes for Dental and Craniofacial Research (Trotman et al., 2007b). Inclusion criteria were as follows: subject interest and parent willingness to participate in the study, an ability to comprehend verbal instructions, and for the subjects, a previously repaired complete unilateral or bilateral cleft lip with or without a cleft of the palate. Exclusion criteria were the following: previous orthognathic or facial soft tissue surgery, diabetes, collagen vascular disease and/or systemic neurologic impairment, inability to comprehend or perform tests due to cognitive or hearing impairment, and a lip revision surgery within the past 2 years. Subjects who met the selection criteria were recruited and screened in the clinic. No subject was excluded from participation on the basis of sex, race, or ethnic background. The purpose and protocol of the study was explained to the subject(s) and parent(s), and informed consent and assent were obtained. Consent and Health Insurance Portability and Accountability Act documents were approved by the School of Dentistry Human Subjects Institutional Review Board.
In the original ongoing clinical trial, three groups of subjects (specifically, revision, nonrevision, and noncleft) were followed longitudinally. Patients in the revision group were asked to perform a battery of tests at six separate sessions as follows: 3 months before and just prior to the revision surgery; and then at 3, 12, 18, and 24 months after the surgery. The nonrevision and noncleft subjects underwent testing at similar time points. OroSTIFF testing began much later than the other tests in the trial. For the present study, only OroSTIFF cross-sectional data for the revision subjects for the sessions 18 and 24 months after surgery were collected and analyzed as well as follow-up data at similar time points for the nonrevision and noncleft groups. Thus, no presurgery data were included in the present analysis. The final cross-sectional sample for this study comprised 56 subjects that included 13 patients in the revision group (RR; age = 14.51 ± 4.93 years), 16 patients in the nonrevision group (NR; age = 16.56 ± 3.00 years), and 27 patients in the noncleft group (NC; age = 17.37 ± 3.17 years). Subject demographics are given in Table 1.
TABLE 1.
Demographic and Clinical Variables by Group*
| Variable | NC (N = 27) | NR (N = 16) | RR (N = 13) |
|---|---|---|---|
| N (%)/Mean (SD)
|
N (%)/Mean (SD)
|
N (%)/Mean (SD)
|
|
| Age (y) | 17.38 (3.17) | 16.56 (3.00) | 14.51 (4.93) |
| Age–lip revision (y) | 11.7 (4.4) | ||
| Gender | |||
| Female | 11 (40.7%) | 8 (50.0%) | 7 (53.8%) |
| Male | 16 (59.3%) | 8 (50.0%) | 6 (46.2%) |
| Cleft orientation | |||
| Right | 5 (31.3%) | 4 (30.8%) | |
| Left | 9 (56.3%) | 8 (61.5%) | |
| Both | 2 (12.5%) | 1 (7.7%) | |
| Lip/palate | |||
| Lip only | 4 (25.0%) | 4 (30.8%) | |
| Lip and palate | 12 (75.0%) | 9 (69.2%) | |
| Orthodontic maxillary expansion | |||
| Yes | 1 (8.3%) | ||
| No | 11 (91.7%) | ||
| Alveolar bone graft | |||
| Yes | 3 (25.0%) | ||
| No | 9 (75.0%) | ||
NC = noncleft group; NR = nonrevision cleft group; RR = surgically revised cleft lip group.
Protocol
Subjects were seated in a comfortable chair and instructed to look straight ahead with the facial muscles relaxed and to remain speechless and motionless. A 1-cm interincisal bite block was molded (KERR Xtrude-XP™, Romulus, Michigan) for each subject to immobilize the mandible during the perioral stiffness sampling protocol, which lasted 2 minutes. Bipolar Ag/AgCl surface electrodes were placed over the orbicularis oris superior and orbicularis oris inferior muscles to monitor background electromyographic activity. A thin-wall, tubular, stainless steel face-referenced OroSTIFF device (mass = 40.7 g) was coupled bilaterally to the oral angles via lip saddles and was supported on the mental symphysis with a double-adhesive tape collar for vertical stabilization (Fig. 1). The details of the OroSTIFF device and calibration are described in Chu et al. (2010), but a brief description is provided here. A pneumatic actuator was pressurized manually with a 10-cc syringe, which in turn imposed an interangle stretch of approximately 20 mm. A 30-gauge blunt-tip cannula, vented to atmosphere, was coupled in parallel with the OroSTIFF pneumatic system. This cannula provided a fixed resistive load (essentially, a controlled air leak) upon which the perioral recoil force would act to allow the equal-arm scissor cantilevers to return to their initial lip aperture resting position (L0 + 15 mm).
FIGURE 1.
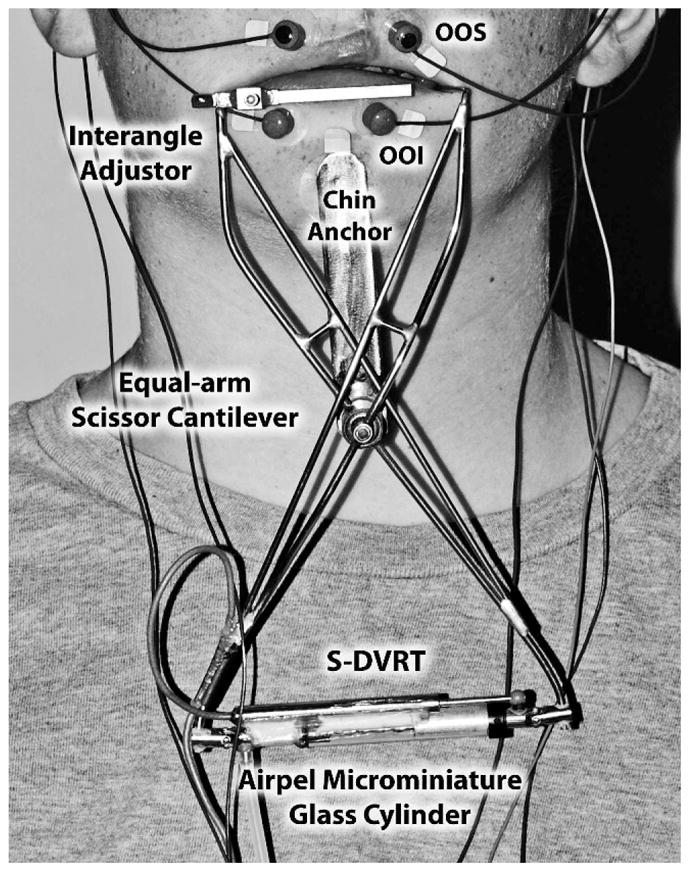
Placement of the OroSTIFF device.
The interangle oral aperture at rest provides an estimate of resting muscle length (L0) and was measured with a digital caliper for each subject. The OroSTIFF interangle span was initialized to L0 + 15 mm for all subjects. A series of five interangle stretch trials were completed while simultaneously sampling force and displacement in real time with custom software (OroSTIFF v.2.1, Communication Neuroscience Laboratories, Lawrence, KS) written in LabVIEW™8.0 (National Instruments, Austin, TX). Individual interangle stretch trials were completed within 10 seconds, and the entire stiffness protocol was completed within 2 minutes for each subject.
Data Acquisition and Analysis
Identifying Nonlinear Segment of Force-Displacement Curve
Air pressure within the microminiature pneumatic glass-cylinder actuator and the displacement signal from the differential variable reluctance transducer (DVRT) were digitized at 2,000 samples/s at 16-bit resolution. Stiffness coefficients (N/mm) were calculated automatically during the phase of elastic recoil for each of five trials as the low-mass interangle yokes of the OroSTIFF device returned to the subject’s interangle rest position. The stiffness coefficient was calculated as the change in force over a 1-mm change in interangle span and was evaluated sequentially at 1-mm intervals. The absolute number of stiffness points along the recoil trajectory depends on the maximum interangle span achieved. To determine stiffness for a specific span, a 100-point running cubic spline was evaluated at 0.5 mm above and below the desired span (for example, force was evaluated at 19.5 and 18.5 mm to calculate stiffness for a nominal span of 19 mm). The cubic spline allows force to be determined at regular displacement intervals.
Statistics
Data and Statistical Analysis of Force Versus Displacement (ΔF/ΔX)
A multilevel regression analysis was conducted in which the perioral stiffness was measured through a series of five interangle stretch trials (level 1) for each subject (level 2), using SAS version 9.1 (SAS Institute, Cary, NC). An unconditional means model (i.e., null model) was initially fitted to examine the intraclass correlation (ICC) and determine significance of the random variance components. This unconditional means model is defined as Stiffnessij = γ00 + uoj + rij, where uoj ~N(O,τ00) and rij ~N(O,σ2) for trial i and participant j. This model can be viewed as a one-way random effects analysis of variance model. Then, trial-level and subject-level predictors and cross-level interaction terms were introduced into the null model along with their random effects. The final model included two trial-level predictors that represent the linear and quadratic regression slopes of the interangle span on the perioral stiffness. This model also included the sex (male versus female) and group (RR, NR, NC) variables and their cross-products as the subject-level predictors. Correspondingly, the cross-level interaction terms included in the model represented the sex by group differences in the linear and quadratic regression slopes. Male subjects and the NC group were used as reference groups for comparisons. Multilevel regression analysis does not allow group-wise variance estimates. However, for example, the squared standard error (SE) for the dummy-coded group variable provides an estimate of the group difference in variance separately between NR and NC and between RR and NC. The variance component covariance structure was chosen for these models because it minimized the Akaike Information Criterion and Bayesian Information Criterion. The final model can be written by
where and rij ~N(O,σ2) for trial i and participant j. The F refers to female; whereas, D refers to interangle displacement. The random effect for the D2 was not included in the final model because its inclusion made the Newton-Raphson algorithm for optimizing residual likelihood function yield a nonpositive definite covariance matrix.
Results
The Regression Result of Force Versus Displacement (ΔF/ΔX)
The multilevel analysis indicated that the mean stiffness scores across all subjects and all trials was .058 (SE = .002, t55 = 31.83, p < .01). The estimated among-subject and among-trial variances were .00017 and .00136, respectively. Both variances were significantly different from zero (p <.01), and 11.1% of the total variance occurred at the subject level, indicating that the stiffness scores vary within subject with less variation among subjects. The parameter estimates from the final model are given in Table 2. The subjects in the NC group showed a quadratic increase of their perioral stiffness scores along the displacement continuum, γ̂20 = .00035, t5208 = 13.81, p < .01. More important, the subjects in the NR group showed a significantly greater quadratic increase than those in the NC group, γ̂21 = .00009, t5208 = 2.19, p < .05. The quadratic increase did not differ between NC and RR groups, γ̂22 = .00008, t5208 = 1.74, p = .08. No sex and sex by group differences were found in the regression slopes of the perioral stiffness score. For the NR group, the stiffness score increased by (γ̂20+γ̂21 = .00035 + .00009) .00044 points with each 1-unit increase in the displacement. For the RR group, the stiffness score increased by (γ̂20+γ̂22 = .00035 + .00008) .00042 points with each 1-unit increase in the displacement.
TABLE 2.
Parameter Estimates From the Final Model*
| Fixed Effect | Estimate | SE | t | p | |
|---|---|---|---|---|---|
| Trial level | |||||
| Intercept | .03792 | .00257 | 14.74 | <.01 | |
| Displacement (D) | −.00162 | .00066 | −2.46 | <.05 | |
| D2 | .00035 | .00003 | 13.81 | <.01 | |
| Subject level | |||||
| NR | .00577 | .00445 | 1.30 | .20 | |
| RR | .00349 | .00489 | 0.71 | .48 | |
| Female (F) | −.00168 | .00401 | −0.42 | .68 | |
| NR × F | −.00387 | .00650 | −0.59 | .55 | |
| RR × F | −.00407 | .00693 | −0.59 | .56 | |
| Cross-level | |||||
| NR × D | −.00188 | .00114 | −1.65 | .10 | |
| RR × D | −.00237 | .00124 | −1.91 | .06 | |
| F × D | −.00037 | .00102 | −0.37 | .71 | |
| NR × F × D | .00001 | .00165 | 0.00 | 1.00 | |
| RR × F × D | .00203 | .00176 | 1.16 | .25 | |
| NR × D2 | .00009 | .00004 | 2.19 | <.05 | |
| RR × D2 | .00008 | .00005 | 1.74 | .08 | |
| F × D2 | −.00007 | .00004 | −1.74 | .08 | |
| NR × F × D2 | .00005 | .00006 | 0.83 | .41 | |
| RR × F × D2 | −.00008 | .00006 | −1.21 | .23 | |
|
| |||||
| Random Effect | Estimate | SE | z | p | |
|
| |||||
| σ2 | .00063 | .00001 | 50.77 | <.01 | |
| τ00 | .00003 | .00001 | 2.63 | <.01 | |
| τ11 | .00000 | .00000 | – | – | |
| Residual ICC | .047 | ||||
|
|
.536 | ||||
|
|
.817 | ||||
| −2 × log likelihood | −23,396.3 | ||||
| AIC | −23,390.3 | ||||
| BIC | −23,384.2 | ||||
D = interangle displacement; NR = nonrevision cleft group; RR = surgically revised cleft lip group; ICC = intraclass correlation coefficient; SE = standard error; AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion.
The residual ICC showed that 4.7% of total residual variance occurred at the subject level. The squared multiple correlations indicated that approximately 53.6% and 71.7% of the variances were explained at the trial level and the subject level, respectively. The likelihood-ratio (LR) tests suggested that both the random effects for the intercept (LR χ2 = 18.00, p < .01) and linear slope (LR χ2 = 505.30, p < .01) were tenable. The difference in group variance between NR and NC was .000020 and between RR and NC was .000024, indicating greater variance in the RR group.
The equations for the estimated stiffness scores were as follows.
These equations were used to derive the average estimated stiffness scores for males and females in each group (see Fig. 2).
FIGURE 2.
The average estimated stiffness scores for males and females in each group: normal noncleft controls (NC), nonrevision cleft (NR), and surgically revised cleft lip (RR) groups.
Discussion
The OroSTIFF measure described in this study is obtained by sampling force and displacement at the corners of the mouth, which represents a composite measure of stiffness for the upper and lower lips. Therefore, any defect in either the upper or lower lip will manifest in the composite stiffness score. This study found that patients with a repaired cleft lip who did not have lip revision surgery (nonrevision subjects) manifest steeper quadratic slopes and greater mean interangle stiffness scores when compared with noncleft patients. This finding confirmed the first hypothesis of the study. It appears that the scar tissue in the upper lip as a result of the initial lip repair limits tissue displacement and constricts the oral opening. Previous studies (Trotman et al., 2007a) have shown that this scarring also results in a decreased ability of these patients to consistently maintain midline target forces with the upper lip; whereas, the lower lip shows midline compensations for the upper lip inconsistency in force regulation. In some instances, the compensations occur in facial soft tissues beyond the circumoral region. Thus, it appears that the localized upper lip defect of a cleft broadly impacts the functioning of the circumoral soft tissue-muscle system.
The second hypothesis of this study was related to the effects of additional lip (revision) surgery on the perioral stiffness. Contrary to our a priori prediction of an even greater increase in lip stiffness following revision surgery, the lip revision subjects in the current study did not differ significantly from the noncleft subjects. This preliminary finding supports the notion that at least in the short term (18 to 24 months), upper lip revision surgery may improve the soft tissue biomechanics of the circumoral region. Additional study is needed to consider the timing of lip revision surgery relative to growth patterns of the face and nasolabial region on orofacial biomechanics. For example, depending on sex and the specific dimension being measured, maxillary and mandibular growth reaches 95% to 98% of the adult dimension between 12 and 15 years of age (Farkas et al., 1992a); whereas, growth of the upper lip cutaneous height, upper lip vermilion height, and total upper lip height attain 94% to 98% of the adult dimension earlier, between 5 and 11 years (Farkas et al., 1992b). When considered in the context of the present study, it appears that most of the lip soft tissue growth was well advanced for the subjects in the revision group before they received the upper lip revision surgery. The mean age of revision surgery in the present study was 11.7 years. The increased circumoral stiffness observed in the nonrevision group likely reflects the combined effects of tissue scarring from the initial surgery to close the cleft lip/palate and subsequent growth of the facial skeleton and nasolabial tissues. A presurgery versus postsurgery longitudinal study and biomechanical analysis would provide more definitive results to test this hypothesis. These studies currently are under way in our laboratory.
Acknowledgments
This study was supported in part by NIH R01 DE13814 (Trotman), NIH P30 DC005803 (Barlow), and the Sutherland Foundation KUAE 38457X (Barlow). The authors express gratitude to Douglas Kieweg for engineering support and Ms. Ada Rey and Ms. Erin Duffy for assistance with participant recruitment and data collection.
Contributor Information
Dr. Steven M. Barlow, Department of Speech-Language-Hearing, Neuroscience, Human Biology, and Bioengineering, and Director, Communication Neuroscience Laboratories, University of Kansas, Lawrence, Kansas.
Dr. Carroll-Ann Trotman, Department of Orthodontics, and Associate Dean, Office of Academic Affairs, University of Maryland, Baltimore, Maryland.
Dr. Shin-Ying Chu, Department of Speech-Language-Hearing: Sciences and Disorders, Communication Neuroscience Laboratories, University of Kansas, Lawrence, Kansas.
Dr. Jaehoon Lee, Center for Research Methods and Data Analysis, University of Kansas, Lawrence, Kansas.
References
- Barlow SM, Abbs JH. Fine force and position control of select orofacial structures in the upper motor neuron syndrome. Exp Neurol. 1986;94:699–713. doi: 10.1016/0014-4886(86)90248-7. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Burton M. Ramp-and-hold force control in the upper and lower lips: developing new neuromotor assessment applications in traumatically brain injured adults. J Speech Lang Hear Res. 1990;33:660–675. doi: 10.1044/jshr.3304.660. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Iacono RP, Paseman LA, Biswas A, D’Antonio LD. The effects of experimental posteroventral pallidotomy on force and speech aerodynamics in Parkinson’s disease. In: Cannito MP, Yorkston KM, Beukelman DR, editors. Speech Motor Control. Baltimore: Paul H. Brookes; 1998. pp. 117–156. [Google Scholar]
- Barlow SM, Müller EM. The relation between interangle span and in vivo resultant force in the perioral musculature. J Speech Lang Hear Res. 1991;34:252–259. doi: 10.1044/jshr.3402.252. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Rath EM. Maximum voluntary closing forces in the upper and lower lips of humans. J Speech Lang Hear Res. 1985;28:373–376. doi: 10.1044/jshr.2803.373. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP. The influence of speaking rate on articulatory hypokinesia in parkinsonian dysarthria. Brain Lang. 1989;36:493–502. doi: 10.1016/0093-934x(89)90080-1. [DOI] [PubMed] [Google Scholar]
- Chu SY, Barlow SM, Kieweg D, Lee J. OroSTIFF: face-referenced measurement of perioral stiffness in health and disease. J Biomech. 2010;43:1476–1482. doi: 10.1016/j.jbiomech.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu SY, Barlow SM, Lee J. Nonparticipatory stiffness in the male perioral complex. J Speech Lang Hear Res. 2009;52:1353–1359. doi: 10.1044/1092-4388(2009/08-0101). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas LG, Posnick JC, Hreczko TM. Growth patterns of the face: a morphometric study. Cleft Palate Craniofac J. 1992a;29:308–315. doi: 10.1597/1545-1569_1992_029_0308_gpotfa_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Farkas LG, Posnick JC, Hreczko TM, Pron GE. Growth patterns of the nasolabial region: a morphometric study. Cleft Palate Craniofac J. 1992b;29:318–324. doi: 10.1597/1545-1569_1992_029_0318_gpotnr_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Hunker CJ, Abbs JH, Barlow SM. The relationship between parkinsonian rigidity and hypokinesia in the orofacial system: a quantitative analysis. Neurology. 1982;32:749–754. doi: 10.1212/wnl.32.7.749. [DOI] [PubMed] [Google Scholar]
- Müller EM, Milenkovic PH, MacLeod GE. Perioral tissue mechanics during speech production. In: Eisenfeld J, DeLisi C, editors. Mathematics and Computers in Biomedical Application. Amsterdam: Elsevier; 1985. pp. 363–371. [Google Scholar]
- Rutjens CA, Spauwen PH, van Lieshout PHHM. Lip movement in patients with a history of unilateral cleft lip. Cleft Palate Craniofac J. 2001;38:468–475. doi: 10.1597/1545-1569_2001_038_0468_lmipwa_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Stranc MF, Fogel ML. Lip function: a study of oral continence. Br J Plast Surg. 1984;37:550–557. doi: 10.1016/0007-1226(84)90148-6. [DOI] [PubMed] [Google Scholar]
- Trotman CA, Barlow SM, Faraway JJ. Functional outcomes of cleft lip surgery. Part III: measurement of lip forces. Cleft Palate Craniofac J. 2007a;44:617–623. doi: 10.1597/06-138.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman CA, Faraway JJ, Essick GK. Three-dimensional nasolabial displacement during movement in repaired cleft lip and palate patients. Plast Reconstr Surg. 2000;105:1273–1283. doi: 10.1097/00006534-200004040-00003. [DOI] [PubMed] [Google Scholar]
- Trotman CA, Faraway JJ, Phillips C. Visual and statistical modeling of facial movement in patients with cleft lip and palate. Cleft Palate Craniofac J. 2005;42:245–254. doi: 10.1597/04-010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman CA, Phillips C, Essick GK, Faraway JJ, Barlow SM, Losken HW, van Aalst J, Rogers L. Functional outcomes of cleft lip surgery. Part I: study design and surgeon ratings of lip disability and need for lip revision. Cleft Palate Craniofac J. 2007b;44:598–606. doi: 10.1597/06-124.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman CA, Stohler CS, Johnston LE., Jr Measurement of facial soft tissue mobility in man. Cleft Palate Craniofac J. 1998;35:16–25. doi: 10.1597/1545-1569_1998_035_0016_mofstm_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- van Lieshout PHHM, Rutjens CAW, Spauwen PHM. The dynamics of interlip coupling in speakers with a repaired unilateral cleft-lip history. J Speech Lang Hear Res. 2002;45:5–19. doi: 10.1044/1092-4388(2002/001). [DOI] [PubMed] [Google Scholar]


