Abstract
The astrocytic glutamate transporters (GLT-1, GLAST) are critical for removing excess glutamate from synaptic sites, thereby maintaining glutamate homeostasis within the brain. 17 -Estradiol (E2) is one of the most active estrogen hormones possessing neuroprotective effects both in in vivo and in vitro models, and it has been shown to enhance astrocytic glutamate transporter function (Liang et al. 2002; Pawlak et al. 2005). However, E2 is not clinically optimal for neuroprotection given its peripheral feminizing and proliferative effects; therefore, brain selective estrogen receptor modulators (neuroSERMs) (Zhao et al. 2005) that specifically target estrogenic mechanisms, but lack the systemic estrogen side effects offer more promising therapeutic modality for the treatment of conditions associated with excessive synaptic glutamate levels. This review highlights recent studies from our laboratory showing that E2 and SERMs effectively reverse glutamate transport inhibition in a manganese (Mn)-induced model of glutamatergic deregulation. Specifically, we discuss mechanisms by which E2 restores the expression and activity of glutamatergic neurotransmission. We advance the hypothesis that E2 and related compounds, such as tamoxifen (TX) may offer a potential therapeutic modality in neurodegenerative disorders, which are characterized by altered glutamate homeostasis.
Introduction
Although manganese (Mn) is an essential element required for normal development and for the proper function of multiple enzymes, such as Mn superoxide dismutase, arginase, and glutamine synthase (ASTDR 2000), chronic high levels of Mn exposure represent a toxicological concern (Aschner et al. 2009). Human exposures are commonly associated with battery manufacturing, water purification, as well as bactericidal and fungicide agents (de Tollenaer et al. 2006). Moreover, increased serum Mn levels are observed in chronic liver failure, as result of the inability to excrete the metal via the biliary system (McKinney et al. 2004), as well as in patients on total parenteral nutrition (Aschner and Aschner 2005).
While several tissues are affected by excessive Mn exposure, the central nervous system (CNS) is particularly vulnerable to its toxicity (Bowman et al. 2011). Long-term Mn exposures result in a neurologic syndrome referred to as manganism. Although manganism shares multiple clinical features with Parkinson's disease (PD) (Barbeau 1984; Sloot et al. 1994), the primary sites of damage following Mn neurotoxicity include the globus pallidus (GP) and striatum with the subtantia nigra pars compacta (SNc) generally spared (Olanow 2004). Globus pallidus (GP) neurons have been shown to be selectively vulnerable to Mn intoxication (Gwiazda et al. 2002) with marked neuronal loss and astrocytosis, particularly in the medial segment. The GP contains abundant -aminobutyric acid (GABA) projections (Pal et al. 1999), and the degeneration of these GABAergic neurons leads to decreased inhibitory GABA input to the subthalamic nucleus. Thus, Mn dysinhibits glutamate output to the subtantia nigra (SN) resulting in chronic over-stimulation of dopaminergic (DAergic) neurons (Verity 1999). The exacerbation of dopamine neurotransmission is associated with oxidative stress, which may hasten Mn neurotoxicity; a less severe degeneration occurs in the putamen, the caudate nucleus and substantia nigra pars reticulata (SNr) (Yamada et al. 1986).
Mn preferentially accumulates in astrocytes where it is known to impair glutamate transporter function (Erikson and Aschner 2002; Hazell and Norenberg 1997). There is compelling evidence of glutamate-mediated excitotoxicity in Mn neurotoxicity, and the involvement of astrocytic glutamate transporters in this process (Aschner et al. 1992; Danbolt 2001). Mn injection into the rat striatum induces excitotoxic lesions and pre-treatment with the noncompetitive N-methyl D-aspartate (NMDA) antagonist, MK801, blocks these lesions (Brouillet et al. 1993). Furthermore, Mn decreases astrocytic glutamate uptake (Hazell and Norenberg 1997; Normandin and Hazell 2002) and expression of the astrocytic glutamate transporters, GLAST (EAAT1 in human) (Erikson and Aschner 2002) and GLT-1 (EAAT2 in human) (Deng et al. 2012). Although the molecular mechanisms involved with Mn-induced glutamate dyshomeostasis are not fully understood, recent evidence shows that protein kinase C (PKC) activation is one of the key events in mediating decreased glutamate uptake into astrocytes upon Mn exposure (Sidoryk-Wegrzynowicz et al. 2011).
The neuroprotective effect of 17 -Estradiol (E2) and SERMs is partially mediated by astrocytes
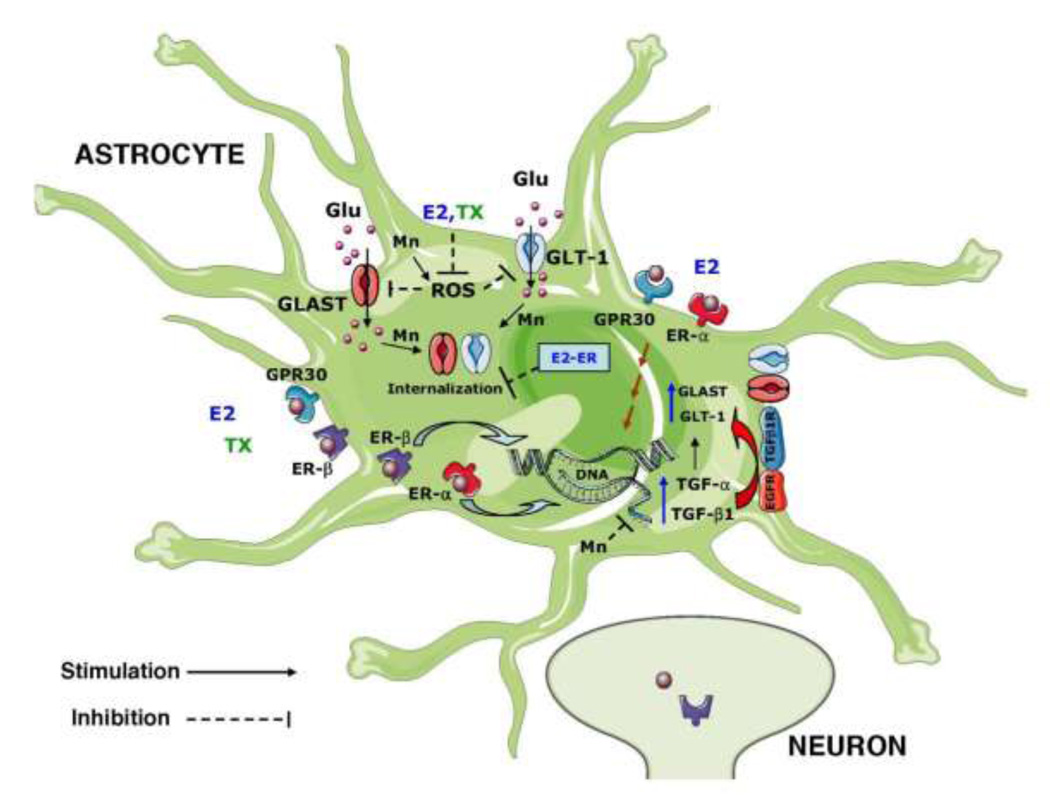
Epidemiological and experimental studies have reported gender effects on the development of specific neuropathologic conditions, including metal-induced neurotoxicity (Beuter et al. 1999; Dorman et al. 2004; Malagutti et al. 2009). In this regard, several studies established that 17 -estradiol (E2), at physiological concentrations, exerts protective effects in a number of neurodegenerative disorders, such as Alzheimer’s disease (AD), PD and acute ischemic stroke (Dhandapani and Brann 2007). Selective estrogen receptor modulators (SERMs) have been in clinical use for the treatment and/or prevention of breast cancer (tamoxifen; TX) and osteoporosis (raloxifene). Both these SERMs are neuroprotective in various in vivo and in vitro models of neurological disorders (Biewenga et al. 2005; Kimelberg et al. 2000). E2 exerts neuroprotective effects both via estrogen receptor (ER)-dependent and -independent mechanisms (Manthey and Behl 2006). Lee and colleagues have demonstrated that E2 protects against Mn-induced oxidative stress and cell death both in neurons and astrocytes (Lee et al. 2009a). While E2 may have a direct protective effect on neurons, emerging evidence points to the astrocytes as key mediators of its effect (Dhandapani et al. 2003; Dhandapani et al. 2005; Sortino et al. 2004; Wilson et al. 2000). Notably, astrocytes express several subtypes of the estrogen receptors (ERs), including ER-α and ER-β, as well as G protein-coupled receptor 30 (GPR30). Although the classical ERs are recognized as the nuclear receptors, E2 also exerts non-classical effects via the plasma membrane-associated ERs. The latter effects are mediated via MAPK/ERK and PI3K/Akt signaling (Dhandapani and Brann 2007). ER- in astrocytes, but not in neurons, appears responsible for E2-induced neuroprotection in experimental autoimmune encephalomyelitis (EAE) (Spence et al. 2011). GPR30 mediated neuroprotection has been invoked in ischemic animal model (Lebesgue et al. 2010). Astrocytes may also contribute to neuroprotection by increasing local estrogen levels via the activation of aromatase, which catalyzes the synthesis of E2 from testosterone (Azcoitia et al. 2001; Gulinello et al. 2006). Astrocytes are also an important source of E2-induced trophic factors, which possess neuroprotective functions. For example, in response to treatment with E2, astrocytes upregulate the expression of growth factors, such as transforming growth factor alpha (TGF-α) and TGF- 1(Fig. 1) (Dhandapani et al. 2003; Duenas et al. 1994; Mahesh et al. 2006). Tamoxifen (TX), acting as an antagonist of the ERs in breast tissue, has been shown to evoke neuroprotective effects by increasing TGF- 1 synthesis in astrocytes (Dhandapani and Brann 2003a). E2 increases the expression of the astroglial glutamate transporters, GLAST and GLT-1, thus reducing extracellular glutamate levels (Fig. 1) (Pawlak et al. 2005). E2 also enhances astroglial glutamate transporter GLAST functions by modulating the trafficking/redistribution of glutamate transporters (Lee et al. 2009b).
Fig. 1.
Putative mechanism associated with the effect of E2/TX on GLT-1 and GLAST expression. GLT-1 expression is increased by treatment with E2/TX in astrocytes. The effect is mediated via the nuclear ER (ER-α and ER-β) as well as GPR30. GPR30 is localized on the plasma membrane and/or endoplasmic reticulum. E2 and TX induce TGF-α as well as TGF- 1. These, in turn, bind to EGFR or TGFR with high affinity and activate TGF- 1 by autocrine/paracrine modes. TGF- 1 mediates the effects of E2/TX on GLT-1 (as well as GLAST) expression by multiple signaling pathways, including EGFR (PI3K, mitogen-activated protein kinase (MAPK), and protein kinase A (PKA) -not shown). Glu: glutamate; GLT-1: glutamate transportert-1; GLAST: glutamate aspartate transporter; ER, estrogen receptor; GPR30, G protein-coupled Receptor 30; TGF 1 R: Receptor for TGF- 1; EGFR: Receptor for TGF- .
Astrocytic glutamate transporters, neurodegeneration and neuroprotection
Glutamate is the major excitatory neurotransmitter in the mammalian CNS. As such, glutamate plays an important role in many CNS functions (Muller et al. 1994) and a number of neurodegenerative diseases have been associated with glutamate transporter impairment, including amyotrophic lateral sclerosis (ALS), AD and PD (Masliah et al. 2000; Sheldon and Robinson 2007). Optimal synaptic glutamate concentrations are regulated mainly by GLAST (Storck et al. 1992) and GLT-1 (Pines et al. 1992), both of which are predominantly localized in astrocytes (Rothstein et al. 1996). In optimal physiological conditions, astrocytes are responsible for neuronal activity regulation and removal of glutamate from the synaptic cleft (80% of Glutamate released from neurons is taken up by astrocytes) (de Vivo et al. 2010). While GLAST is highly expressed in cultured primary astrocytes, GLT-1 is abundantly expressed in vivo, playing a critical role in taking up glutamate from the synaptic cleft. GLT-1 expression in astrocyte cultures is regulated by neuronal factors (Gegelashvili et al. 1997; Schlag et al. 1998; Swanson et al. 1997). Despite the crucial role of astrocytic glutamate transporters in the maintenance of glutamate homeostasis, molecular details regarding the regulation of glial glutamate transporters has yet to be fully understood. In addition to genomic regulation of transporter expression (Figiel et al. 2003), glutamate transporter GLT-1 is also regulated by post-transcriptional mechanisms (Beart and O'Shea 2007; Robinson 2002). Another mechanism invoked for glutamate transporter regulation is trafficking of transporters in response to activation of a variety of signaling molecules, such as PKC and phosphoinositide-3-kinase (PI3K/Akt) (Danbolt 2001; Guillet et al. 2005; Sims et al. 2000).
Since impairment in astrocytic glutamate transporter expression and function represents the key contributing factor to neural pathogenesis, several experimental approaches have been pursued for neuroprotection by enhancing glutamate transporter function. These include transgenic mouse models over-expressing glutamate transporters, pharmacological upregulation of glutamate transporters, as well as transduction of various cell types with exogenous glutamate transporters using viral vectors (Zhou and Sutherland 2004). GLT-1 over-expressing mice exhibit reduced neurological signs of ischemia (Romera et al. 2007). Several approaches have also been advanced in search for novel therapeutic strategies for small molecules that might regulate expression or function of glutamate transporters (Dunlop 2006). -Lactam antibiotics stimulate GLT-1 expression and have been shown to be efficacious both in vitro and in vivo in a mouse model of ALS (Rothstein et al. 2005). Other studies have established that up-regulation of GLT-1 is neuroprotective in models of ALS and cerebral ischemia (Chu et al. 2007; Ganel et al. 2006).
Do growth factors play a role in the E2-enhancing glutamate transporter function in astrocytes?
E2 increases expression of certain growth factors such as transforming growth factor- (TGF- , transforming growth factor- 1 (TGF- 1) and basic fibroblast growth factor (bFGF) in astrocytes (Dhandapani et al. 2005; Ojeda et al. 2006; Siegel and Chauhan 2000) (Fig. 1). E2 has been shown to protect neurons by increasing the expression of astrocytic TGF- 1 in several neurological experimental models, including AD and ischemia (Fig. 1) (Dhandapani et al. 2003; Tesseur et al. 2006; Wyss-Coray 2004). TGF- binds to the EGFR with high affinity (Gomez-Pinilla et al. 1988; Planas et al. 1998) and reduces infarct volume after focal ischemia (Justicia et al. 2001; Justicia and Planas 1999). TGF acts primarily on astrocytes (Junier 2000; Schluter et al. 2002) and the expression of GLAST and GLT-1 is stimulated by EGF/TGF- (Figiel and Engele 2000; Zelenaia et al. 2000) and bFGF (Suzuki et al. 2001) in cultured cortical astrocytes (Fig. 1).
Mn neurotoxicity involves impairment of glutamate transporters, which contributes to pathological neuronal degeneration in the GP and striatum. Although astrocytes are the main cellular target of Mn and E2/SERM-neuroprotection, the mechanisms underlying this effect in Mn-induced glutamate transporter impairment have yet to be fully established. TGF- may play an important role in E2/TX-induced reversal of Mn-impaired GLT-1 expression, given our findings that E2 and TX enhanced GLT-1 function by increasing TGF-α expression, thus, attenuating Mn-induced impairment in astrocytic GLT-1 expression and glutamate uptake (Lee et al. 2012a). Moreover, this E2/TX action was associated with GLT-1 gene expression levels, activating the GLT-1 promoter. Our findings also reveal that TGF- plays an intimate role in E2/TX-induced GLT-1 expression, given that knockdown of TGF-α abolished the E2/TX effect on GLT-1 expression and GLT-1 promoter activity (Lee et al. 2012a). The effect of E2/TX on TGF-α mRNA and protein levels with a concomitant increase in astrocytic glutamate uptake was mediated by the activation of MAPK/extracellular signal-regulated kinase (ERK) and PI3K/Akt signaling pathways (Lee et al. 2012a). We further demonstrated that ERs (ER-α, ER-β, and G protein-coupled receptor 30) were involved in mediating the effects of E2 on the regulation of TGF-α, GLT-1, and glutamate uptake (see Fig. 1 for additional details).
Transcriptional regulation of E2-induced GLT-1 expression via GPR30
Although the classical ERs, ER- and ER- upregulate GLT-1 expression recent studies in our laboratory indicate that GPR30, also known as a G-protein coupled estrogen receptor 1 (GPER), is critically involved in regulating the E2-induced GLT-1 expression (Lee et al. 2012b). This is supported by results in which blocking GPR30 function fully abrogates the effect of G-1, a selective GPR30 agonist, on GLT-1 protein expression. The GPR30-induced increase in GLT-1 expression is mediated at the transcriptional level, via activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF- B) and CREB) pathways. The promoter of the GLT-1 gene contains cis-elements of NF- B and CREB binding sites. The NF- B pathway appears to play a critical role, given that GPR30 not only transactivated EGFR, which is known to regulate GLT-1 expression through NF- B pathway (Ghosh et al. 2011; Sitcheran et al. 2005), but also induced the expression of TGF- , a ligand of EGFR. The NF- B pathway is intimately involved in the G-1-induced enhancement of GLT-1 expression. Both NF- B p50 and NF- B p65, which compose the predominant heterodimer form of NF- B in the brain (Meffert and Baltimore 2005) are involved in G-1-induced GLT-1 regulation, supported by the observation that G-1 induced the binding of these two subunits (p50 and p65) to the cis-element of GLT-1 promoter and activated the NF- B luciferase reporter. The CREB pathway might be another pathway that mediates GPR30-induced GLT-1 expression. G-1/GPR30 activates the G protein-coupled receptor system and the downstream to the cAMP/PKA pathways; furthermore it induces CREB binding to a cAMP response element (CRE) site in the GLT-1 promoter (Lee et al. 2012b). Taken together, these results suggest that E2/TX enhance astrocytic glutamate transporter expression via increased transforming growth factors (both TGF-α and TGF- 1 expression). Furthermore, both E2 and TX effectively reverse Mn-- induced glutamate transport inhibition by restoring its expression and activity, thus offering a potential therapeutic modality in neurodegenerative disorders characterized by altered glutamate homeostasis.
Acknowledgements
This review was supported in part by grants from the National Institutes of Health (NIH) ES R01 10563, ES P30 000267 and GM SC1 089630.
REFERENCES
- Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005;26:353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Herrero Hernandez E, Tjalkens R. Manganese and its role in Parkinson's disease: from transport to neuropathology. Neuromolecular Med. 2009;11:252–266. doi: 10.1007/s12017-009-8083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Gannon M, Kimelberg HK. Manganese uptake and efflux in cultured rat astrocytes. J Neurochem. 1992;58:730–735. doi: 10.1111/j.1471-4159.1992.tb09778.x. [DOI] [PubMed] [Google Scholar]
- ASTDR. Toxicological profile for manganese. Atlanta Georgia: US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry; 2000. pp. 1–466. [Google Scholar]
- Azcoitia I, Garcia-Ovejero D, Chowen JA, Garcia-Segura LM. Astroglia play a key role in the neuroprotective actions of estrogen. Prog Brain Res. 2001;132:469–478. doi: 10.1016/S0079-6123(01)32096-4. [DOI] [PubMed] [Google Scholar]
- Barbeau A. Manganese and extrapyramidal disorders (a critical review and tribute to Dr George C. Cotzias) Neurotoxicology. 1984;5:13–35. [PubMed] [Google Scholar]
- Beart PM, O'Shea RD. Transporters for L-glutamate: an update on their molecular pharmacology and pathological involvement. Br J Pharmacol. 2007;150:5–17. doi: 10.1038/sj.bjp.0706949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuter A, Edwards R, deGeoffroy A, Mergler D, Hundnell K. Quantification of neuromotor function for detection of the effects of manganese. Neurotoxicology. 1999;20:355–366. [PubMed] [Google Scholar]
- Biewenga E, Cabell L, Audesirk T. Estradiol and raloxifene protect cultured SN4741 neurons against oxidative stress. Neurosci Lett. 2005;373:179–183. doi: 10.1016/j.neulet.2004.09.067. [DOI] [PubMed] [Google Scholar]
- Bowman AB, Kwakye GF, Hernandez EH, Aschner M. Role of manganese in neurodegenerative diseases. J Trace Elem Med Biol. 2011;25:191–203. doi: 10.1016/j.jtemb.2011.08.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp Neurol. 1993;120:89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, Kim SJ, Park DK, Jung KH, Song EC, Lee SK, Kim M, Roh JK. Pharmacological Induction of Ischemic Tolerance by Glutamate Transporter-1 (EAAT2) Upregulation. Stroke. 2007;38:177–182. doi: 10.1161/01.STR.0000252091.36912.65. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- de Tollenaer SM, Buysse C, van den Anker JN, Touw DJ, de Hoog M. Life threatening central nervous system manifestations and hypothermia due to maneb intoxication in a child: a case report. Ther Drug Monit. 2006;28:813–815. doi: 10.1097/01.ftd.0000243964.90340.cc. [DOI] [PubMed] [Google Scholar]
- de Vivo L, Melone M, Rothstein JD, Conti F. GLT-1 Promoter Activity in Astrocytes and Neurons of Mouse Hippocampus and Somatic Sensory Cortex. Front Neuroanat. 2010;3:31. doi: 10.3389/neuro.05.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Xu Z, Xu B, Xu D, Tian Y, Feng W. The Protective Effects of Riluzole on Manganese-Induced Disruption of Glutamate Transporters and Glutamine Synthetase in the Cultured Astrocytes. Biol Trace Elem Res. 2012 doi: 10.1007/s12011-012-9365-1. [DOI] [PubMed] [Google Scholar]
- Dhandapani K, Brann D. Neuroprotective effects of estrogen and tamoxifen in vitro: a facilitative role for glia? Endocrine. 2003a;21:59–66. doi: 10.1385/endo:21:1:59. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Brann DW. Role of astrocytes in estrogen-mediated neuroprotection. Exp Gerontol. 2007;42:70–75. doi: 10.1016/j.exger.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Mahesh VB, Brann DW. Astrocytes and brain function: implications for reproduction. Exp Biol Med (Maywood) 2003;228:253–260. doi: 10.1177/153537020322800303. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Wade FM, Mahesh VB, Brann DW. Astrocyte-derived transforming growth factor-{beta} mediates the neuroprotective effects of 17{beta}-estradiol: involvement of nonclassical genomic signaling pathways. Endocrinology. 2005;146:2749–2759. doi: 10.1210/en.2005-0014. [DOI] [PubMed] [Google Scholar]
- Dorman DC, McManus BE, Marshall MW, James RA, Struve MF. Old age and gender influence the pharmacokinetics of inhaled manganese sulfate and manganese phosphate in rats. Toxicol Appl Pharmacol. 2004;197:113–124. doi: 10.1016/j.taap.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Duenas M, Luquin S, Chowen JA, Torres-Aleman I, Naftolin F, Garcia-Segura LM. Gonadal hormone regulation of insulin-like growth factor-I-like immunoreactivity in hypothalamic astroglia of developing and adult rats. Neuroendocrinology. 1994;59:528–538. doi: 10.1159/000126702. [DOI] [PubMed] [Google Scholar]
- Dunlop J. Glutamate-based therapeutic approaches: targeting the glutamate transport system. Curr Opin Pharmacol. 2006;6:103–107. doi: 10.1016/j.coph.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Erikson K, Aschner M. Manganese causes differential regulation of glutamate transporter (GLAST) taurine transporter and metallothionein in cultured rat astrocytes. Neurotoxicology. 2002;23:595–602. doi: 10.1016/s0161-813x(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Figiel M, Engele J. Pituitary adenylate cyclase-activating polypeptide (PACAP), aneuron-derived peptide regulating glial glutamate transport and metabolism. J Neurosci. 2000;20:3596–3605. doi: 10.1523/JNEUROSCI.20-10-03596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figiel M, Maucher T, Rozyczka J, Bayatti N, Engele J. Regulation of glial glutamate transporter expression by growth factors. Exp Neurol. 2003;183:124–135. doi: 10.1016/s0014-4886(03)00134-1. [DOI] [PubMed] [Google Scholar]
- Ganel R, Ho T, Maragakis NJ, Jackson M, Steiner JP, Rothstein JD. Selective up-regulation of the glial Na+-dependent glutamate transporter GLT1 by a neuroimmunophilin ligand results in neuroprotection. Neurobiol Dis. 2006;21:556–567. doi: 10.1016/j.nbd.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Danbolt NC, Schousboe A. Neuronal soluble factors differentially regulate the expression of the GLT1 and GLAST glutamate transporters in cultured astroglia. J Neurochem. 1997;69:2612–2615. doi: 10.1046/j.1471-4159.1997.69062612.x. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Yang Y, Rothstein JD, Robinson MB. Nuclear factor-kappaB contributes to neuron-dependent induction of glutamate transporter-1 expression in astrocytes. J Neurosci. 2011;31:9159–9169. doi: 10.1523/JNEUROSCI.0302-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Knauer DJ, Nieto-Sampedro M. Epidermal growth factor receptor immunoreactivity in rat brain Development and cellular localization. Brain Res. 1988;438:385–390. doi: 10.1016/0006-8993(88)91369-8. [DOI] [PubMed] [Google Scholar]
- Guillet BA, Velly LJ, Canolle B, Masmejean FM, Nieoullon AL, Pisano P. Differential regulation by protein kinases of activity and cell surface expression of glutamate transporters in neuron-enriched cultures. Neurochem Int. 2005;46:337–346. doi: 10.1016/j.neuint.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Lebesgue D, Jover-Mengual T, Zukin RS, Etgen AM. Acute and chronic estradiol treatments reduce memory deficits induced by transient global ischemia in female rats. Horm Behav. 2006;49:246–260. doi: 10.1016/j.yhbeh.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwiazda RH, Lee D, Sheridan J, Smith DR. Low cumulative manganese exposure affects striatal GABA but not dopamine. Neurotoxicology. 2002;23:69–76. doi: 10.1016/s0161-813x(02)00002-5. [DOI] [PubMed] [Google Scholar]
- Hazell AS, Norenberg MD. Manganese decreases glutamate uptake in cultured astrocytes. Neurochem Res. 1997;22:1443–1447. doi: 10.1023/a:1021994126329. [DOI] [PubMed] [Google Scholar]
- Junier MP. What role(s) for TGFalpha in the central nervous system? Prog Neurobiol. 2000;62:443–473. doi: 10.1016/s0301-0082(00)00017-4. [DOI] [PubMed] [Google Scholar]
- Justicia C, Perez-Asensio FJ, Burguete MC, Salom JB, Planas AM. Administration of transforming growth factor-alpha reduces infarct volume after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 2001;21:1097–1104. doi: 10.1097/00004647-200109000-00007. [DOI] [PubMed] [Google Scholar]
- Justicia C, Planas AM. Transforming growth factor-alpha acting at the epidermal growth factor receptor reduces infarct volume after permanent middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 1999;19:128–132. doi: 10.1097/00004647-199902000-00002. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Feustel PJ, Jin Y, Paquette J, Boulos A, Keller RW, Jr, Tranmer BI. Acute treatment with tamoxifen reduces ischemic damage following middle cerebral artery occlusion. Neuroreport. 2000;11:2675–2679. doi: 10.1097/00001756-200008210-00014. [DOI] [PubMed] [Google Scholar]
- Lebesgue D, Traub M, De Butte-Smith M, Chen C, Zukin RS, Kelly MJ, Etgen AM. Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats. PLoS One. 2010;5:e8642. doi: 10.1371/journal.pone.0008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Sidoryk-Wegrzynowicz M, Wang N, Webb A, Son DS, Lee K, Aschner M. GPR30 regulates glutamate transporter GLT-1 expression in rat primary astrocytes. J Biol Chem. 2012b doi: 10.1074/jbc.M112.341867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Sidoryk-Wegrzynowicz M, Yin Z, Webb A, Son DS, Aschner M. Transforming growth factor-alpha mediates estrogen-induced upregulation of glutamate transporter GLT-1 in rat primary astrocytes. Glia. 2012a;60:1024–1036. doi: 10.1002/glia.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Sidoryk M, Jiang H, Yin Z, Aschner M. Estrogen and tamoxifen reverse manganese-induced glutamate transporter impairment in astrocytes. J Neurochem. 2009b;110:530–544. doi: 10.1111/j.1471-4159.2009.06105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Yin Z, Milatovic D, Jiang H, Aschner M. Estrogen and tamoxifen protect against Mn-induced toxicity in rat cortical primary cultures of neurons and astrocytes. Toxicol Sci. 2009a;110:156–167. doi: 10.1093/toxsci/kfp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Valla J, Sefidvash-Hockley S, Rogers J, Li R. Effects of estrogen treatment on glutamate uptake in cultured human astrocytes derived from cortex of Alzheimer's disease patients. J Neurochem. 2002;80:807–814. doi: 10.1046/j.0022-3042.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- Mahesh VB, Dhandapani KM, Brann DW. Role of astrocytes in reproduction and neuroprotection. Mol Cell Endocrinol. 2006;246:1–9. doi: 10.1016/j.mce.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Malagutti KS, da Silva AP, Braga HC, Mitozo PA, Soares Dos Santos AR, Dafre AL, de Bem AF, Farina M. 17beta-estradiol decreases methylmercury-induced neurotoxicity in male mice. Environ Toxicol Pharmacol. 2009;27:293–297. doi: 10.1016/j.etap.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Manthey D, Behl C. From structural biochemistry to expression profiling: neuroprotective activities of estrogen. Neuroscience. 2006;138:845–850. doi: 10.1016/j.neuroscience.2005.10.058. [DOI] [PubMed] [Google Scholar]
- Masliah E, Alford M, Mallory M, Rockenstein E, Moechars D, Van Leuven F. Abnormal glutamate transport function in mutant amyloid precursor protein transgenic mice. Exp Neurol. 2000;163:381–387. doi: 10.1006/exnr.2000.7386. [DOI] [PubMed] [Google Scholar]
- McKinney AM, Filice RW, Teksam M, Casey S, Truwit C, Clark HB, Woon C, Liu HY. Diffusion abnormalities of the globi pallidi in manganese neurotoxicity. Neuroradiology. 2004;46:291–295. doi: 10.1007/s00234-004-1179-1. [DOI] [PubMed] [Google Scholar]
- Meffert MK, Baltimore D. Physiological functions for brain NF-kappaB. Trends Neurosci. 2005;28:37–43. doi: 10.1016/j.tins.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Muller WE, Scheuer K, Stoll S. Glutamatergic treatment strategies for age-related memory disorders. Life Sci. 1994;55:2147–2153. doi: 10.1016/0024-3205(94)00395-5. [DOI] [PubMed] [Google Scholar]
- Normandin L, Hazell AS. Manganese neurotoxicity: an update of pathophysiologic mechanisms. Metab Brain Dis. 2002;17:375–387. doi: 10.1023/a:1021970120965. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Roth C, Mungenast A, Heger S, Mastronardi C, Parent AS, Lomniczi A, Jung H. Neuroendocrine mechanisms controlling female puberty: new approaches, new concepts. Int J Androl. 2006;29:256–263. doi: 10.1111/j.1365-2605.2005.00619.x. discussion 286–290. [DOI] [PubMed] [Google Scholar]
- Olanow CW. Manganese-induced parkinsonism and Parkinson's disease. Ann N Y Acad Sci. 2004;1012:209–223. doi: 10.1196/annals.1306.018. [DOI] [PubMed] [Google Scholar]
- Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–238. [PubMed] [Google Scholar]
- Pawlak J, Brito V, Kuppers E, Beyer C. Regulation of glutamate transporter GLAST and GLT-1 expression in astrocytes by estrogen. Brain Res Mol Brain Res. 2005;138:1–7. doi: 10.1016/j.molbrainres.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- Planas AM, Justicia C, Soriano MA, Ferrer I. Epidermal growth factor receptor in proliferating reactive glia following transient focal ischemia in the rat brain. Glia. 1998;23:120–129. doi: 10.1002/(sici)1098-1136(199806)23:2<120::aid-glia3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Robinson MB. Regulated trafficking of neurotransmitter transporters: common notes but different melodies. J Neurochem. 2002;80:1–11. doi: 10.1046/j.0022-3042.2001.00698.x. [DOI] [PubMed] [Google Scholar]
- Romera C, Hurtado O, Mallolas J, Pereira MP, Morales JR, Romera A, Serena J, Vivancos J, Nombela F, Lorenzo P, Lizasoain I, Moro MA. Ischemic preconditioning reveals that GLT1/EAAT2 glutamate transporter is a novel PPARgamma target gene involved in neuroprotection. J Cereb Blood Flow Metab. 2007;27:1327–1338. doi: 10.1038/sj.jcbfm.9600438. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Schlag BD, Vondrasek JR, Munir M, Kalandadze A, Zelenaia OA, Rothstein JD, Robinson MB. Regulation of the glial Na+-dependent glutamate transporters by cyclic AMP analogs and neurons. Mol Pharmacol. 1998;53:355–369. doi: 10.1124/mol.53.3.355. [DOI] [PubMed] [Google Scholar]
- Schluter K, Figiel M, Rozyczka J, Engele J. CNS region-specific regulation of glial glutamate transporter expression. Eur J Neurosci. 2002;16:836–842. doi: 10.1046/j.1460-9568.2002.02130.x. [DOI] [PubMed] [Google Scholar]
- Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int. 2007;51:333–355. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M, Lee E, Mingwei N, Aschner M. Disruption of astrocytic glutamine turnover by manganese is mediated by the protein kinase C pathway. Glia. 2011;59:1732–1743. doi: 10.1002/glia.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain Res Brain Res Rev. 2000;33:199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- Sims KD, Straff DJ, Robinson MB. Platelet-derived growth factor rapidly increases activity and cell surface expression of the EAAC1 subtype of glutamate transporter through activation of phosphatidylinositol 3-kinase. J Biol Chem. 2000;275:5228–5237. doi: 10.1074/jbc.275.7.5228. [DOI] [PubMed] [Google Scholar]
- Sitcheran R, Gupta P, Fisher PB, Baldwin AS. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. EMBO J. 2005;24:510–520. doi: 10.1038/sj.emboj.7600555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloot WN, van der Sluijs-Gelling AJ, Gramsbergen JB. Selective lesions by manganese and extensive damage by iron after injection into rat striatum or hippocampus. J Neurochem. 1994;62:205–216. doi: 10.1046/j.1471-4159.1994.62010205.x. [DOI] [PubMed] [Google Scholar]
- Sortino MA, Chisari M, Merlo S, Vancheri C, Caruso M, Nicoletti F, Canonico PL, Copani A. Glia mediates the neuroprotective action of estradiol on beta-amyloid-induced neuronal death. Endocrinology. 2004;145:5080–5086. doi: 10.1210/en.2004-0973. [DOI] [PubMed] [Google Scholar]
- Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, Cao Y, Bondar G, Lam J, Ao Y, Sandoval F, Suriany S, Sofroniew MV, Voskuhl RR. Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc Natl Acad Sci U S A. 2011;108:8867–8872. doi: 10.1073/pnas.1103833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci U S A. 1992;89:10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Ikegaya Y, Matsuura S, Kanai Y, Endou H, Matsuki N. Transient upregulation of the glial glutamate transporter GLAST in response to fibroblast growth factor, insulin-like growth factor and epidermal growth factor in cultured astrocytes. J Cell Sci. 2001;114:3717–3725. doi: 10.1242/jcs.114.20.3717. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Liu J, Miller JW, Rothstein JD, Farrell K, Stein BA, Longuemare MC. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J Neurosci. 1997;17:932–940. doi: 10.1523/JNEUROSCI.17-03-00932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseur I, Zou K, Esposito L, Bard F, Berber E, Can JV, Lin AH, Crews L, Tremblay P, Mathews P, Mucke L, Masliah E, Wyss-Coray T. Deficiency in neuronal TGF-beta signaling promotes neurodegeneration and Alzheimer's pathology. J Clin Invest. 2006;116:3060–3069. doi: 10.1172/JCI27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verity MA. Manganese neurotoxicity: a mechanistic hypothesis. Neurotoxicology. 1999;20:489–497. [PubMed] [Google Scholar]
- Wilson JX, Peters CE, Sitar SM, Daoust P, Gelb AW. Glutamate stimulates ascorbate transport by astrocytes. Brain Res. 2000;858:61–66. doi: 10.1016/s0006-8993(99)02433-6. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. Transforming growth factor-beta signaling pathway as a therapeutic target in neurodegeneration. J Mol Neurosci. 2004;24:149–153. doi: 10.1385/JMN:24:1:149. [DOI] [PubMed] [Google Scholar]
- Yamada M, Ohno S, Okayasu I, Okeda R, Hatakeyama S, Watanabe H, Ushio K, Tsukagoshi H. Chronic manganese poisoning: a neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol. 1986;70:273–278. doi: 10.1007/BF00686083. [DOI] [PubMed] [Google Scholar]
- Zelenaia O, Schlag BD, Gochenauer GE, Ganel R, Song W, Beesley JS, Grinspan JB, Rothstein JD, Robinson MB. Epidermal growth factor receptor agonists increase expression of glutamate transporter GLT-1 in astrocytes through pathways dependent on phosphatidylinositol 3-kinase and transcription factor NF-kappaB. Mol Pharmacol. 2000;57:667–678. doi: 10.1124/mol.57.4.667. [DOI] [PubMed] [Google Scholar]
- Zhao L, Chen S, Ming Wang J, Brinton RD. 17beta-estradiol induces Ca2+ influx, dendritic and nuclear Ca2+ rise and subsequent cyclic AMP response element-binding protein activation in hippocampal neurons: a potential initiation mechanism for estrogen neurotrophism. Neuroscience. 2005;132:299–311. doi: 10.1016/j.neuroscience.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Zhou J, Sutherland ML. Glutamate transporter cluster formation in astrocytic processes regulates glutamate uptake activity. J Neurosci. 2004;24:6301–6306. doi: 10.1523/JNEUROSCI.1404-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]