Abstract
Human pluripotent stem cells (hPSCs) are a powerful tool for modeling brain development and disease. The human cortex is composed of two major neuronal populations, projection neurons and local interneurons. Cortical interneurons comprise a diverse class of cell types expressing the neurotransmitter GABA. Dysfunction of cortical interneurons has been implicated in neuropsychiatric diseases, including schizophrenia, autism and epilepsy.
Here we demonstrate the highly efficient derivation of human cortical interneurons in a NKX2.1::GFP hESC reporter line. Manipulating the timing of SHH activation yields three distinct GFP+ populations with specific transcriptional profiles, neurotransmitter phenotypes and migratory behaviors. Further differentiation in a murine cortical environment yields parvalbumin and somatostatin expressing neurons that exhibit synaptic inputs and electrophysiological properties of cortical interneurons. Our study defines the signals sufficient to model human ventral forebrain development in vitro and lays the foundation for studying cortical interneuron involvement in human disease pathology.
INTRODUCTION
Human pluripotent stem cells (hPSCs) are a powerful tool for studying human development and disease and for applications in regenerative medicine. The use of hPSCs differentiated towards central nervous system lineages has been of particular interest given the lack of effective therapies for many neurodegenerative and neuropsychiatric disorders and the availability of protocols to efficiently direct neuronal specification in vitro (Chambers et al., 2009). Early studies using hPSCs have been primarily geared towards neurodegenerative disorders, which are known to affect specific neuron types such as midbrain dopamine neurons in Parkinson’s disease (PD; (Kriks et al., 2011)) or motor neurons in amyotrophic lateral sclerosis (ALS; (Dimos et al., 2008)) and spinal muscular atrophy (SMA; (Ebert et al., 2009)). More recent studies suggest the possibility of tackling complex neuronal disorders such as Schizophrenia (Brennand et al., 2011) or autism-related syndromes (Marchetto et al., 2010; Pasca et al., 2011). Unlike in PD, ALS or SMA, the neuron types critical for modeling schizophrenia or autism are less well defined, and no attempts have been made to direct neuron subtype identity in those studies. There has been considerable progress in establishing protocols for the derivation of human ESC-derived cortical projection neurons (Espuny-Camacho et al., 2013; Shi et al., 2012). However, inhibitory neurons, such as cortical interneurons may be particularly important in schizophrenia or autism (Insel, 2010; Lewis et al., 2005). We have previously demonstrated the derivation of cortical interneurons using a Lhx6::GFP reporter mouse ESC line (Maroof et al., 2010). However, the efficiency of cortical interneuron generation was low, and it was uncertain whether those conditions would apply for generating human cortical interneurons from PSCs. Modeling human cortical interneuron development in vitro is of particular interest as their developmental origin is controversial with studies suggesting considerable differences across mammalian species (Letinic et al., 2002; Yu and Zecevic, 2011). Furthermore, the protracted in vivo development of several cortical interneuron types (Anderson et al., 1995), represents an additional challenge for modeling their differentiation using human cells in vitro.
Here we present a small molecule-based strategy for the efficient induction of human cortical interneurons. Exposure to the tankyrase inhibitor XAV939 enhances forebrain differentiation. Using an NKX2.1::GFP hESC reporter line, we demonstrate the selective derivation of three distinct ventral forebrain precursor populations by combining XAV939 treatment with the timed activation of SHH signaling. Importantly, results in both hPSCs and in human embryos reveal differences between mouse and human forebrain development such as the human-specific, yet transient, FOXA2 expression within the ventral forebrain. Finally, mature functional properties and expression of late cortical interneuron markers, including parvalbumin and somatostatin, demonstrate the feasibility of studying human cortical interneuron differentiation from hPSCs in vitro despite their protracted development in vivo.
RESULTS
Combined inhibition of Wnt and activation of SHH signaling triggers efficient induction of NKX2.1+ neural precursors
The first step in modeling cortical interneuron development is the robust induction of forebrain precursors. Specification of anterior/forebrain fate is considered a default program during neural differentiation of hPSCs (Chambers et al., 2009; Eiraku et al., 2008; Espuny-Camacho et al., 2013; Gaspard et al., 2008). However, not all cell lines adopt anterior neural fates at equal efficiencies (Kim et al., 2011; Wu et al., 2007). Patterning factors secreted within differentiating cultures such as fibroblast growth factors (FGFs), Wnts or retinoids can suppress forebrain induction and trigger the induction of caudal cell fates. We recently reported that early, high-dose SHH treatment also suppresses forebrain markers such as FOXG1 via inhibition of DKK1 induction (Fasano et al., 2010). This is a concern for deriving cortical interneurons that are thought to require strong SHH activation at the NKX2.1+ progenitor stage (Xu et al., 2010). Here we tested whether pharmacological inhibition of WNT signaling can improve FOXG1 induction and subsequently enable the controlled, SHH-mediated ventralization towards NKX2.1+ forebrain progenitor fates (Figure 1A).
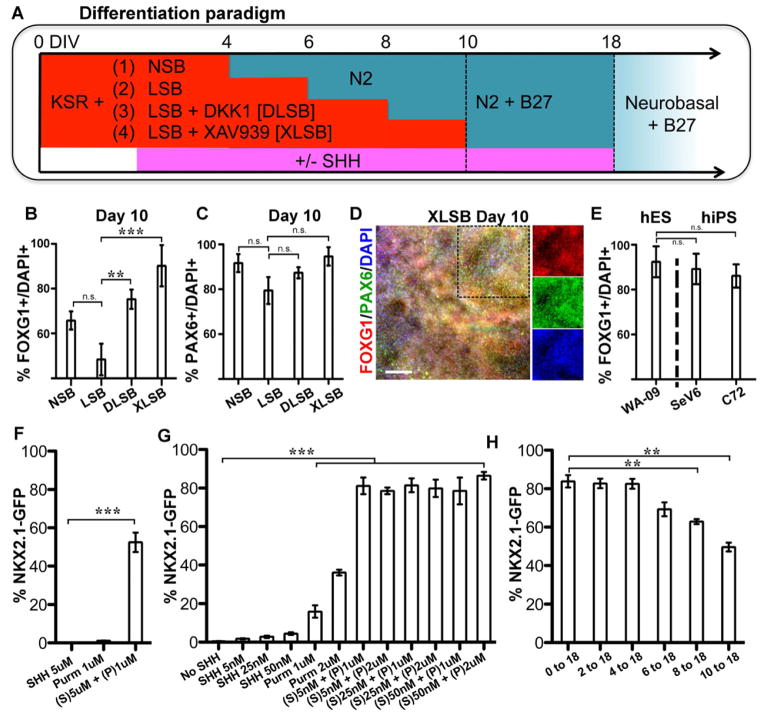
Figure 1. Wnt inhibition and activation of SHH signaling yields highly efficient derivation of forebrain fates and NKX2.1 induction.
(A) Schematic of the differentiation protocol in the dual SMAD inhibition paradigm to generate anterior neural progenitors. NSB: Noggin+ SB431542. LSB: LDN193189+ SB31542.
B–E): When either DKK1 or XAV939, both Wnt signaling antagonists, were added to the dual SMAD inhibition protocol (DLSB or XLSB), there was a significant increase in the percentage of FOXG1+ cells (B) without loss of PAX6 expression (C): ** p < 0.01; *** p < 0.001; using ANOVA followed by Scheffe test. D) Representative immunofluorescent image for FOXG1 (red) and PAX6 (green) expression at day 10 following XLSB treatment. Single channel fluorescent images of marked region are shown in the three right panels E) Robust telencephalic specification using XLSB was also observed at comparable efficiencies in human induced pluripotent stem cells (hiPSC lines SeV6, C72; n = 4). F–H) Addition of SHH signaling to the XLSB protocol significantly enhanced the production of NKX2.1::GFP expressing progenitors. (F) 5nM SHH (Sonic C24II) and 1μm Purmorphamine, added from day 4, showed synergistic effects in inducing NKX2.1::GFP expression at day 10 (*** p < 0.001; compared to SHH). A range of concentrations of SHH and Purmorphamine are compared at day 18 in (G), and again co-treatment was greatly superior to quite high concentrations of either SHH or purmorphamine alone (*** p < 0.001; compared to no SHH using ANOVA followed by Scheffe test). (H) Delaying the timing of SHH exposure between 2 and 10 days of differentiation did not dramatically affect the efficiency of NKX2.1::GFP induction measured at day 18 (*** p < 0.001 compared to 0–18 using ANOVA followed by Scheffe test). P: purmorphamine, S: Sonic hedgehog. Data are from hESC line HES-3 (NKX2.1::GFP) in panels B,C,F,G,H from hESC line WA-09/H9 (panel D) and from hESC line WA-09/H9 and hiPSC lines SeV6 and C72 (panel E). Scale bar in (D) represents 125μm. Data in (B,C,E–H) are represented as mean ± SEM.
Neural differentiation of hESCs via the dual SMAD-inhibition protocol (Chambers et al., 2009) using Noggin + SB431542 (NSB) robustly induced FOXG1+/PAX6+ precursors. Replacement of Noggin with the ALK2/3 inhibitor LDN-193189 (LSB) induced PAX6 expression equally well but showed a trend towards lower percentages of FOXG1+ cells (Figure 1B,C). Adding recombinant DKK1 or the tankyrase inhibitor XAV939 (Huang et al., 2009), inhibitors of canonical Wnt signaling, enhanced FOXG1 expression in LSB-treated cultures (Figure 1B–D). Importantly, the effect of XAV939 on FOXG1 induction was consistent (Figure 1E) across multiple independent hESC (HES-3 and WA-09) and human iPSC lines (C72 line (Papapetrou et al., 2009), SeV6 (Kriks et al., 2011)). Therefore, the use of three small molecules (XAV939, LDN-193189, and SB431542; termed XLSB) enables rapid and robust induction of forebrain fates across human ESCs and iPSC lines.
We next wanted to test our ability to induce ventral fates using XLSB in the presence of SHH activators (Figure 1A). The transcription factor NKX2.1 is a marker of ventral prosencephalic progenitor populations (Sussel et al., 1999; Xu et al., 2004). Using a previously established NKX2.1::GFP knock-in reporter hESC line (Goulburn et al., 2011), we observed GFP induction by day 10 following activation of the SHH pathway by recombinant SHH (R&D Systems, C-25II) and the smoothened activator purmorphamine (Figure 1F). We found maximal induction of NKX2.1::GFP at day 18 upon treatment with purmorphamine at 1 μM and with SHH at 5nM (Figure 1G), a condition referred to as “SHH” for the remainder of the manuscript. Our past studies on the derivation of hESC derived floor plate cells demonstrated that early treatment with SHH (day 1 of differentiation) is critical for the efficient induction of FOXA2 (Fasano et al., 2010). In contrast, we observed here that cells exposed to SHH at late differentiation stages (day 10 of the XLSB protocol) remain competent for inducing NKX2.1::GFP as measured by FACS (Figure 1H). Therefore, XLSB+SHH treatment represents a strategy to generate NKX2.1+ progenitors at high efficiencies.
Timing of SHH exposure induces distinct ventral progenitor populations
The efficient induction of NKX2.1 largely independent of the timing of SHH exposure raised the question of whether timing impacts the subtype of NKX2.1+ neural progenitors generated. Analogous to rodents, NKX2.1 is expressed during early neural human development in both the FOXG1+ telencephalon as well as in the FOXG1-negative ventral diencephalon (Figure 2A, (Kerwin et al., 2010; Rakic and Zecevic, 2003). During embryonic mouse development NKX2.1 expression in the telencephalon (FOXG1+) is restricted to the ventral (subcortical) domain. In contrast, reports in human fetal tissue suggested that NKX2.1 may not be restricted to the ventral forebrain, instead extending into the dorsal forebrain and cortical anlage (Rakic and Zecevic, 2003; Yu and Zecevic, 2011). However, those studies were largely based on the analysis of second trimester human fetuses making it difficult to distinguish whether NKX2.1+ cells were induced in the dorsal forebrain or migrated dorsally after induction in the ventral domain (Fertuzinhos et al., 2009). By performing immunocytochemical analyses in early stage human embryos (Carnegie Stage 15, ~ 38 days post conception), we observed restriction of NKX2.1 expression to the ventral forebrain (Figure 2B–E) comparable to the developing mouse CNS. The NKX2.1 domain in the human embryonic ventral forebrain was further subdivided into an OLIG2+ ganglionic eminence and an OLIG2-negative preoptic area anlage (Figure 2C,D).
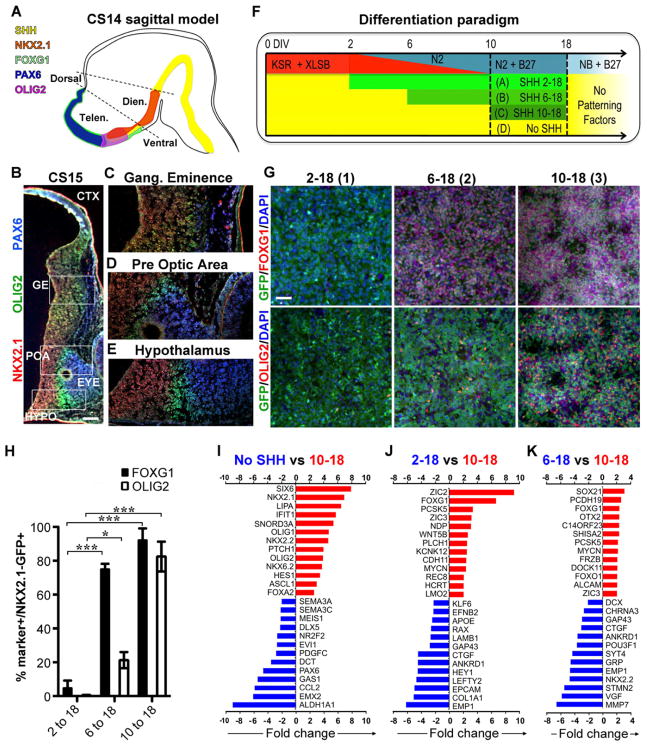
Figure 2. Timing of SHH exposure determines the regional identity of NKX2.1::GFP expressing progenitors.
A) Model of human prosencephalon (sagittal view at Carnegie stage 14 (CS14)) with expression of forebrain patterning markers based on published data (Kerwin et al., 2010). B–E) Coronal (oblique) hemisection of the human prosencephalon at Carnegie stage 15 (CS15) demonstrate expression of NKX2.1, OLIG2, and PAX6. NKX2.1 and OLIG2 are expressed in various regions throughout the ventral prosencephalon, whereas PAX6 is restricted to the dorsal prosencephalon and the eye. The expression of these proteins is non-overlapping, except in the ganglionic eminence (C) where OLIG2 and NKX2.1 are co-expressed. The scale bar in (B) represents 200μm. F) Schematic illustration of the distinct time periods of SHH and purmorphamine treatment used in combination with the XLSB protocol. G,H) Immunofluorescence for OLIG2 and FOXG1 in NKX2.1::GFP line at day 18 of differentiation. Treatment with SHH after day 6 (6-18 and 10-18 group) significantly increases the percentage of NKX2.1::GFP+ cells that co-express FOXG1 as quantified in (H). The scale bar in (G) represents 50μm Treatment with SHH after day 10 (10-18 group) enhanced the derivation of NKX2.1::GFP+ cells co-expressing OLIG2, (data are mean ± SEM; * p < 0.05, *** p < 0.001, compared to 2-18 using ANOVA followed by Scheffe test). Expression of FOXG1, NKX2.1 and OLIG2 indicates a pattern characteristic of ganglionic eminence (Tekki-Kessaris et al.). I–K) Microarray data from cells sorted for NKX2.1::GFP expression at day 18 of differentiation, comparing gene expression levels between the SHH day 10-18 protocol versus no SHH (I), day 10-18 versus day 2-18 protocol (J), and day 10-18 versus 6-18 protocol (K). Red bars indicate genes expressed at higher levels in the SHH 10-18 protocol, blue bars indicate genes expressed at lower levels in day 10-18 protocol. All changes are significant at p < 0.001. Figure 2, see also Figure S1 & Table S1.
To address whether in vitro timing of SHH exposure affects the regional identity of the resulting hPSC-derived NKX2.1::GFP+ cells we compared the identity of sorted cells at day 18 of differentiation (Figure 2F). In agreement with our previous results on floor plate induction (Fasano et al., 2010), we observed that early SHH exposure (2-18) suppressed FOXG1 induction (Figure 2G, left panel) despite robust induction of NKX2.1::GFP (see Figure 1H). In contrast both 6-18 and 10-18 treatment groups showed expression of FOXG1, but differed in OLIG2 expression (Figure 2G, middle and right panels). We hypothesize that differential expression of FOXG1 and OLIG2 reflect the distinct NKX2.1+ precursor domains observed during early human development (Figure 2A–E) and mimic the Olig2+ domains during mouse forebrain development (Flames et al., 2007). More than 90% of the NKX2.1::GFP+ cells in the 6-18 and 10-18 protocol co-expressed FOXG1 and nearly 80% of the GFP+ cells in the 10-18 protocol co-expressed OLIG2 (Figure 2H).
The impact of timing of SHH exposure on the derivation of specific ventral precursor populations was further examined by global transcriptome analysis (Figure 2I–K, Table S1, all raw data are available on GEO: http://www.ncbi.nlm.nih.gov/geo/: Accession # pending). A direct comparison of day 10-18 versus untreated cultures (No SHH) confirmed the robust induction of ventral specification markers such as NKX2.1, NKX2.2, ASCL1, SIX6, OLIG2, and NKX6.2, which were induced at the expense of dorsal forebrain markers such as EMX2 and PAX6 (Figure 2I). There were no significant differences in the expression of general anterior markers such as FOXG1 and OTX2 (Figure 2I), supporting the notion that both populations are of forebrain identity. Comparison of day 10-18 to day 2-18 treated cultures (Figure 2J) illustrated differences in the expression of anterior markers such as FOXG1 versus diencephalic markers such as RAX. In contrast, day 10-18 versus day 6-18 treated cultures showed less pronounced differences in forebrain marker expression (Figure 2K). Overall, our data demonstrate that the window of SHH treatment significantly impacts identity of hPSC-derived NKX2.1 precursors.
One surprising finding from the gene expression data was the induction of FOXA2 in all SHH treated cultures (Figure 2I). Expression of FOXA2, a marker thought to be largely restricted to the floor plate within the developing CNS, was confirmed at day 18 of differentiation in NKX2.1::GFP+ cells of all three SHH treatment paradigms (Figure S1A,B). To address whether FOXA2 expression represents an in vitro artifact following strong extrinsic activation of SHH signaling, or whether FOXA2 expression occurs in the telencephalon during in vivo development, we performed histological analyses in the developing mouse and human forebrain. In mouse embryos (E11.5) FOXA2 expression within the mouse prosencephalon was segregated from the FOXG1 and NKX2.1 domains (Figure S1C). However, immunohistochemical analysis for FOXA2 in primary human forebrain tissue (CS15 embryo) confirmed expression in the telencephalic NKX2.1+ ganglionic eminence (Figure S1D–F). These in vivo data are in agreement with the in vitro hPSC differentiation data demonstrating (transient) FOXA2 expression during human ventral forebrain development.
Neuronal differentiation and migratory properties of hESC derived NKX2.1+ precursors
Cortical interneurons are important for balancing excitation and inhibition within cortical circuitry and play critical roles in controlling developmental plasticity. Cortical interneuron dysfunction has been implicated in various pathological conditions such as epilepsy, autism or schizophrenia (Baraban et al., 2009; Insel, 2010; Lewis et al., 2005; Penagarikano et al., 2011). Our data demonstrate that day 10-18 treated cells express cortical interneuron progenitor markers such as OLIG2, MASH1, and NKX6.2. At day 18 of differentiation NKX2.1+ cells exhibit progenitor cell properties based on Nestin and Ki67 expression. Prolonged culture in the absence of extrinsic SHH activation led to a gradual decrease in Ki67 expression (Figure 3A–C, upper panels) and increased percentages of cells expressing the neuronal precursor markers DCX and TUJ1 (Figure 3A–C, lower panels). In addition, many GFP+ cells in the 10-18 and 2-18 conditions express GABA and subsequently calbindin (Figure 3A–C, lower panels), a marker of tangentially-migrating cortical interneuron precursors (Anderson et al., 1997). Importantly, western blot analysis at day 32 showed that LHX6 protein, a marker of post-mitotic, migratory cortical interneuron precursors in the mouse, was selectively enriched in the 10-18 condition. In contrast, the hypothalamic marker RAX was selectively enriched in the 2-18 condition (Figure 3D). Additional immunocytochemical data showed expression of DLX2 and ASCL1 in most neuronal progeny derived from the day 10-18 NKX2.1::GFP+ progenitors (Figure 3E–G). These data indicate that following withdrawal of extrinsic SHH activators, NKX2.1::GFP+ progenitors give rise to region-specific postmitotic neurons including immature cortical interneurons.
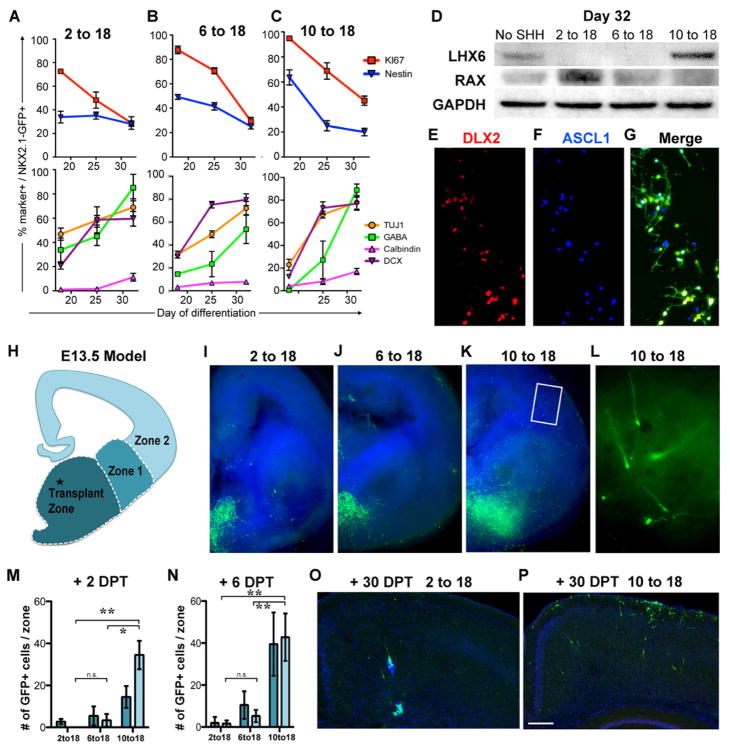
Figure 3. Conversion from cycling neural progenitors to neuronal precursors and the assessment of their migratory potential.
A–C) At day 18 cells from the three indicated protocols were subjected to FACS for NKX2.1::GFP expression then replated and evaluated for co-labeling with markers indicated. For all three protocols there was a decline in co-labeling with markers of progenitors (upper panels: red line, nestin; blue line Ki67), and an increase in markers of neuronal differentiation (lower panels: green, GABA; yellow, TUJ1; purple, doublecortin (DCX); pink, calbindin). Data are mean ± SEM. D) Western blotting showed an increase in the hypothalamic-enriched protein RAX in the 2 to 18 condition, and an increase in the medial ganglionic eminence (MGE)-enriched protein LHX6 in the 10 to 18 condition. Cells were sorted for NKX2.1::GFP prior to analysis. E–G) At day 32, many of the NKX2.1::GFP+ cells from the 10 to 18 condition also expressed DLX2 and ASCL1. H–N) Grafting of day 32-sorted NKX2.1::GFP+ cells into the MGE of E13.5 coronal mouse slice cultures. H) Schematic of coronal hemisection demonstrating the site of transplantation and the zones for quantification of migration. I,J) In both the 2 to 18 and the 6 to 18 conditions, very few cells migrated into zone 1 and fewer into zone 2. K,L) Only the 10 to 18 condition demonstrated significant and robust migration into the cortical and striatal regions, with many GFP+ cells exhibiting bipolar morphologies consistent with a migratory cell (L). The regions where GFP+ cells were detected were quantified two (M) and six (N) days post transplantation DPT (data are mean ± SEM; * p < 0.05; ** p < 0.01 using ANOVA followed by Scheffe test). O,P) Transplantation of day 32 sorted NKX2.1::GFP+ cells (day 10 to 18 protocol) into the neocortex of neonatal mice followed by their evaluation in fixed sections at postnatal day 30. In marked contrast to the MGE-like cells from the SHH 10-18 protocol (P), neither the SHH 2-18 protocol (O) nor the SHH 6-18 protocol (not shown) resulted in extensive migration from the graft site. The scale bar in (P) represents 200μm. See also Figure S2 & Figure S3
We next asked whether human ESC-derived putative cortical interneuron precursors (day 10-18 group) exhibit the characteristic migratory potential observed for primary cortical interneurons in the mouse (Anderson et al., 1997) and human (Letinic et al., 2002) brain. In both species, major subclasses of cortical interneurons undergo tangential migration on their way from the ventral telencephalon into the cortex (Anderson et al., 1997; Fertuzinhos et al., 2009; Letinic et al., 2002). NKX2.1::GFP+ cells at day 32 of differentiation were collected by FACS and injected into forebrain slices isolated from embryonic day 13.5 mouse embryos. The cell injection was carefully targeted to the medial ganglionic eminence under microscopic visual guidance (Figure 3H). The migratory potential of NKX2.1::GFP+ cells and their ability to reach the cortex (see Zone 2 in Figure 3H) was assessed for each treatment group (2-18, 6-18 and 10-18 of SHH treatment; Figure 3I–L) At day 2 (Figure 3M) and, more pronounced at day 6 (Figure 3N) after injection, GFP+ cells were observed migrating from the injection site towards the cortex (zone 2). Remarkably, human cells from the 10-18 treatment, but not the two other treatment groups, showed a robust propensity for migration into the neocortical portions of the murine slice (Figure 3I–N). These data suggest that day 10-18 treated cells exhibit migratory properties previously described for primary mouse MGE-derived interneuron precursors (Sussel et al., 1999; Wichterle et al., 1999).
To further probe the migratory capacity of the day 2-18 versus 10-18 treated cells, we transplanted FACS-isolated NKX2.1::GFP+ cells into the neonatal neocortex of, genetically immuno-compromised mice. Four weeks after transplantation we observed extensive migration, in both the radial and tangential planes within the mouse cortex but only for the day 10-18 group (Figure 3O,P). Similar results were obtained upon transplantation into the adult cortex (Figure S2) though overall migration of NKX2.1::GFP+ cells by day 30 in vivo was less extensive compared to the neonatal grafts.
To determine the extent of interneuron precursor maturation in vivo, we assessed morphological appearance and the expression of interneuron-related markers in the grafted cells from the day 10-18 group (Figure S3). At 1–2 months after grafting into the neonatal cortex most human cells retained undifferentiated bipolar or unipolar morphologies and NKX2.1 expression. Furthermore, essentially all grafted cells expressed the neuronal precursor marker DCX, including those with multipolar morphologies (Figure S3). As it occurs in rodents, the large majority of human interneuron precursors expressed GABA (46/51 GFP+ cells located at the section surface; Figure S3). In contrast, there was no co-labeling for parvalbumin (PV) or somatostatin (SST), two proteins marking the major subclasses of Nkx2.1-lineage interneurons. These data suggest that even by six or 8 weeks post-transplant, the Nkx2.1-GFP+ cells had not yet differentiated into mature interneurons (Figure S3).
One explanation for the apparent lack of differentiation of the grafted GFP+ cells in the mouse host cortex could be that NKX2.1 is downregulated following maturation, as occurs in mouse cortical interneurons (Marin et al., 2000), resulting in the loss of GFP expression. To test this possibility sections were examined for expression of SC121, a human-specific pan-neuronal marker (Kelly et al., 2004). As expected, all GFP+ cells also expressed SC121 (Figure S3). At 6 weeks after transplantation, less than 10% of human cells were single positive for SC121 and lacked GFP expression (25 out of 372 SC121+ cells). In sum, perhaps due to the protracted rate of cortical interneuron maturation in vivo, transplantation studies did not result in mature cortical interneurons within the first two months of transplant.
Phenotypic and synaptic maturation of NKX2.1+ neurons in vitro
We have previously studied cortical interneuron development using a co-culture system in which mouse embryonic MGE-derived progenitors are plated over a “feeder culture” composed mainly of mouse cortical pyramidal neurons and glia (Xu et al., 2004). To determine whether aspects of human interneuron maturation would be accelerated in this system relative to the xenografts, NKX2.1::GFP+ cells were collected by FACS at day 32 and replated onto cultures of dissociated embryonic mouse cortex (Figure 4A). The cortical cells were isolated from E13.5 mouse embryos, a stage prior to the immigration of the ventrally-derived interneurons into the dorsal neocortex (Anderson et al., 1997). Thus, this co-culture system mimics aspects of normal development with human NKX2.1+ cells developing in the presence of the glutamatergic cortical pyramidal neurons. Studies were performed in parallel using GFP+ cells derived from each of the three SHH treatment regimens (Figure 4B–E). Roughly 80% of the GFP+ cells from the 10-18 treated “MGE-like” cultures, versus 40% from the 2-18 “hypothalamic-like” and 15% from the 6-18 culture, expressed GABA. Likewise, the 6-18 culture that is enriched for telencephalic (FOXG1+) cells but lacks expression of the interneuron precursor marker LHX6 (Figure 3D), was enriched for choline acetyltransferase (ChAT) neurons.
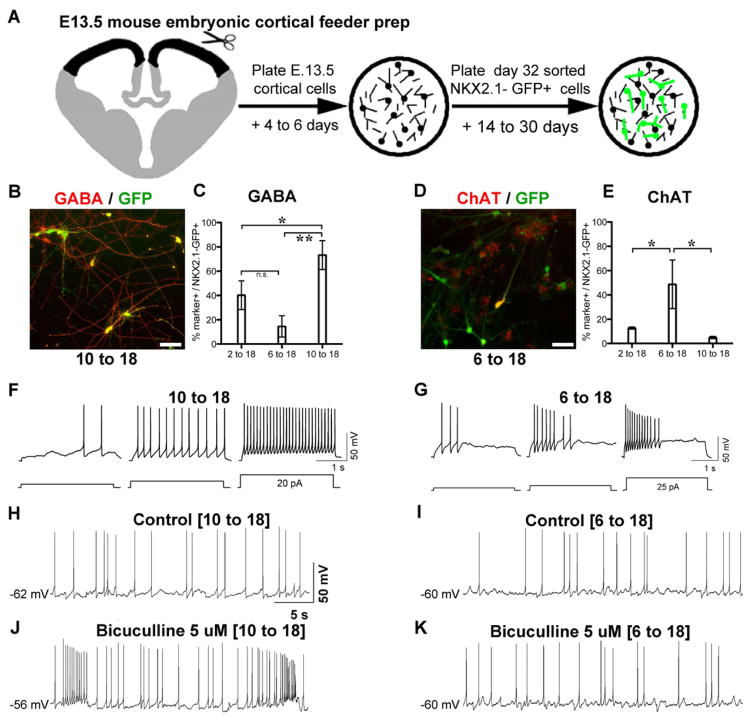
Figure 4. Maturation of NKX2.1+ cells into physiologically active neurons.
A) Preparation of cortical excitatory neuron cultures from embryonic day 13.5 (E13.5) mice, onto which human NKX2.1::GFP+ cells (after FACS at day 32) are plated. The co-culture system was critical for promoting neuronal maturation given the protracted in vivo maturation rates of NKX2.1+ cells. B–E) After 30 days in vitro (DIV), cultures from the SHH 10-18 conditions are enriched for NKX2.1::GFP+ cells that co-express GABA (B, quantified in C: mean ± SEM; * p < 0.05; ** p < 0.01 using ANOVA followed by Scheffe test). In contrast only the SHH 6-18 condition was enriched for NKX2.1::GFP co-labeling with choline acetyl transferase (ChAT) (D, quantified in E: mean ± SEM; * p < 0.05 using ANOVA followed by Scheffe test). F, G) Spiking patterns of SHH day 10-18 (F) and SHH day 6-18 (G) neurons recorded at 28 DIV. Action potentials were initiated by protocols shown at bottom. H–K) Spontaneous spiking was recorded from cultures enriched for GABAergic (SHH day 10 to 18) and cholinergic (6 to 18) neurons in the absence (H, I) and the presence (J,K) of the GABAA receptor antagonist bicuculline. Bicuculline had little effect on the spontaneous firing activity in the 6 to 18 condition, consistent with the lack of GABAergic cells from either the mouse feeder or the human NKX2.1::GFP+ cells generated by this protocol. The scale bars (BD) represent 50μm.
We next determined whether GFP+ cells from day 10-18 treated cultures (enriched for GABA neurons), undergo GABAergic synaptic transmission. Whole-cell patch-clamp electrophysiological studies (Figure 4F–K) of identified GFP+ cells showed spontaneous firing in both day 10-18 and day 6-18 treated cultures (Figure 4H,I). However, only the day 10-18 group showed modulation of the firing rate of NKX2.1::GFP+ cells in response to the GABA-A receptor antagonist bicuculline (Figure 4J). Since the cortical feeders contained very few murine interneurons (in both day 10-18 and day 6-18 co-culture conditions), these results indicate that hPSC-derived GABAergic neurons mediate the inhibitory synaptic output. Accordingly, in the day 6-18 cultures in which GABAergic neurons are more rare (Figure 4C), the firing rates following bicuculline exposure remained unchanged (Figure 4K).
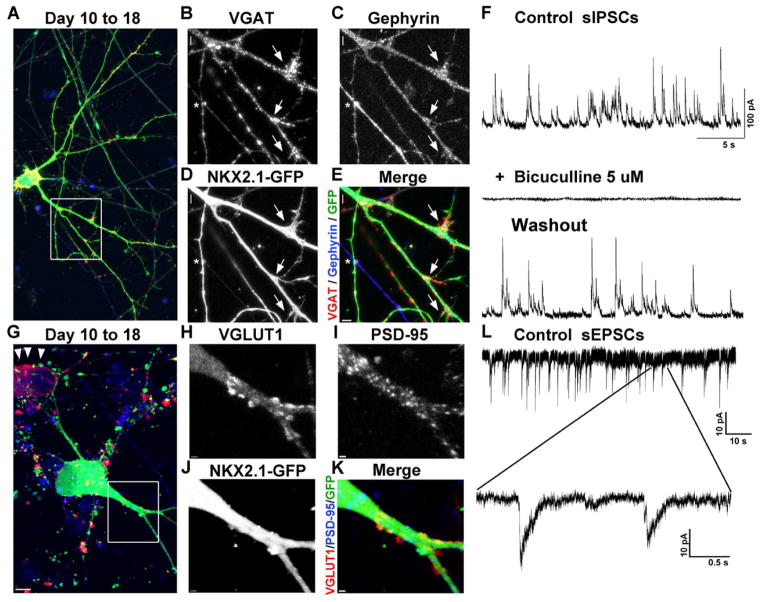
To further analyze synaptic inputs onto the NKX2.1::GFP+ cells from the day 10-18 protocol, we examined spontaneous postsynaptic currents and localization of inhibitory or excitatory synaptic markers. GFP+ cells cultured on the mouse cortical feeder for 30 days expressed high levels of vesicular GABA transporter (VGAT), present within the presynaptic terminal of GABAergic synapses. The subcellular localization of VGAT within GFP+ cells closely matched expression of the GABAergic postsynaptic marker gephyrin (Figure 5A–E). Whole-cell patch clamp analyses demonstrated that NKX2.1::GFP+ cells receive spontaneous inhibitory postsynaptic currents (sIPSCs; Figure 5F) which are reversibly blocked by the addition of the GABA-A receptor antagonist bicuculline. In addition, NKX2.1::GFP+ cells also receive excitatory inputs, as demonstrated by the presence of vesicular glutamate transporter 1 (VGLUT1) expression adjacent to GFP+ putative dendrites, and co-labeling with the post-synaptic excitatory synapse marker PSD-95 (Figure 5G–5K). Consistent with the presence of glutamatergic synaptic inputs, spontaneous excitatory postsynaptic currents (sEPSCs; Figure 5L) were also readily detected in the NKX2.1::GFP+ neurons. Additional analysis of spontaneous synaptic activity in NKX2.1::GFP+ cells is presented in Figure S4 and Table S2.
Figure 5. NKX2.1::GFP+ GABAergic interneurons receive both excitatory and inhibitory synaptic inputs.
A–E) Collapsed z-stack confocal image showing NKX2.1::GFP+, vesicular GABA transporter (VGAT; red in A), and the post-synaptic GABAergic marker gephyrin (blue in A). The dendrites of this GFP+ cell that co-label with gephyrin are receiving VGAT-expressing pre-synaptic terminals (arrows). In addition, a GFP+ axonal process formed a VGAT+ pre-synaptic terminal adjacent to a GFP negative, gephyrin-expressing post-synaptic process (asterisk). F) Whole-cell patch clamp reveals spontaneous inhibitory postsynaptic currents (sIPSCs) recorded from an NKX2.1::GFP+ neuron (SHH 10 to 18 protocol), which are reversibly blocked by the addition of the GABA-A receptor antagonist bicuculline. G-K) Collapsed z-stack confocal image showing NKX2.1::GFP, vesicular glutamate transporter 1 (VGLUT1; red in G), and the post-synaptic marker PSD-95 (blue; C). This GFP+ cell has dendrites that co-label with PSD-95 that are adjacent to VGLUT1-expressing pre-synaptic terminals. Note the presence of a GFP negative cell expressing VGLUT1 (red; G arrowheads), confirming the presence of excitatory glutamatergic neurons in the culture. L) Consistent with the apparent presence of glutamatergic synaptic inputs, spontaneous excitatory postsynaptic currents (sEPSCs) were detected in the NKX2.1::GFP+ neurons (10 to 18). All cells were plated on mouse cortical feeder following FACS for NKX2.1::GFP at day 32. See also Figure S4.
Cortical interneurons include a diverse set of neuron types with distinct roles in cortical development and function (Batista-Brito and Fishell, 2009). The relatively rapid pace of maturation in our mouse cortical feeder system allowed us to assess co-expression of more mature interneuron markers and to define neurotransmitter phenotypes beyond GABA and ChAT. In the day 2-18 group we observed a large percentage of GFP+ cells expressing TH (Figure 6A), consistent with the dopaminergic nature of NKX2.1-lineage hypothalamic neurons (Yee et al., 2009). Immunohistochemistry showed high percentages of nNOS cells in GFP+ cells from the day 2-18 and Calbindin in those from the day 10-18 group (Figure 6B,C). We also observed expression of markers characteristic of differentiated cortical interneurons, including SST and PV (Figures 6D,E), again mainly in the day 10-18 group. Representative images for each subgroup marker, co-expressed together with NKX2.1::GFP, are presented in Figure 6F–J. No VIP or calretinin expressing neurons were observed suggesting the lack of cells exhibiting features of the caudal ganglionic eminence (Xu et al., 2004). The derivation of NKX2.1+/PV+ was particularly remarkable given the late developmental expression of this marker in cortical interneurons in vivo (Zecevic et al., 2011). Only a subset of the cells counted as GFP+/PV+ by the automated image analyses (Operetta high content scanner – see Material & Methods) showed high levels of PV expression and exhibited interneuron-like morphologies (about 5% of total GFP+ cells (J)). Our results point to the need to further optimize the derivation of selective cortical interneuron subtypes. However, the fact that PV+ hPSC-derived neurons could be generated at all suggests that the mouse cortical co-culture strategy facilitates expression of cortical interneuron subtype markers.
Figure 6. Neurochemical profiling of NKX2.1::GFP+ cells grown on mouse cortical feeders for 30 DIV.
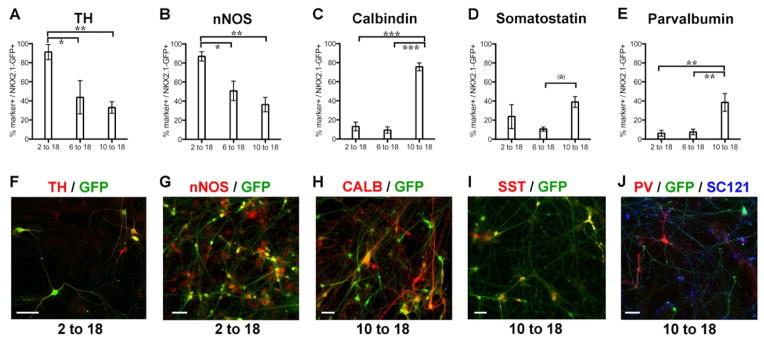
Cells were labeled by immunofluorescence for the markers indicated, and results quantified in graphs (A–E: mean ± SEM; * p < 0.05, ** p < 0.01, *** p < 0.001 using ANOVA followed by Scheffe test: (*) p < 0.05 when compared directly to 6-18 group. p-value did not reach significance in standard Scheffe test: p = 0.08). Panels (F–J) show representative images of cellular labeling. In the SHH 2 to 18 condition, most of the cells co-labeled with tyrosine hydroxylase (TH; A, F) and nNOS (B, G). In the 10 to 18 condition, many of the GFP+ cells co-labeled with calbindin (Calb; C, H), somatostatin (SST; D, I), and parvalbumin (E, J) each of which is present in subpopulations of mature cortical interneurons in humans. All cells were plated on mouse cortical feeder following FACS for NKX2.1::GFP at day 32. The scale bars (F–J) represent 50μm. See also Figure S5, Figure S6 & Table S2.
Given the protracted maturation of Nkx2.1-GFP+, putative interneuron precursors in vivo, we were surprised to obtain morphological, marker-based and physiological evidence of interneuron-like differentiation in the co-culture system. We next tested whether similar results can be obtained with growing the same Nkx2.1::GFP+ cells on cultures enriched for human cortical projection neurons. The human cortical feeders were obtained upon differentiation of hESCs (modified XLSB conditions) followed by FACS for CD44−/CD184+/CD24+ neurons (Yuan et al., 2011) (Figure S5A–D). The cortical identity of the hESC-derived neurons was confirmed by the expression of VGLUT1, MAP2, CTIP2, and SATB2. Only very few GFAP+ or Nestin+ cells were present under those conditions (Figure S5E–T).
Interestingly, we observed a consistent difference in electrophysiological maturation of NKX2.1+ cells plated on mouse versus human cortical feeders as shown in Table S2. Recordings were obtained at either 14–16 DIV or 20–30 DIV for the mouse feeder condition, and 20–30 DIV for the human cortical feeder condition. Analysis of the basic electrophysiological properties of both the 10 to 18 and 6 to18 groups plated on mouse feeders showed more mature characteristics at later time-points of differentiation. There was significant hyperpolarization of the resting membrane potential (RMP) with time, as well as a decrease in the input resistance (Ri). In addition, the action potentials became faster and narrower as evidenced by the decrease in the rise time and half width. In contrast, GFP+ neurons from the day 10-18 condition that were recorded 20–30 days after plating on the human feeder layer retained relatively immature characteristics, comparable to the measurements seen in the younger 14–16 DIV neurons plated onto a mouse feeder layer. The RMP, action potential rise time, action potential half width, and input resistance were significantly different in the cortical feeder condition when compared to the 10-18 neurons on mouse feeders recorded at the same DIV range. Sample traces depicting qualitative firing properties under the various conditions are shown in Figure S6.
These preliminary findings suggest that the pace of functional maturation is influenced by the local environment, though the corresponding mechanisms remain to be determined. While it is possible that species-specific maturation rates trigger the timing of cortical interneuron maturation (e.g. by regulating activity), it is also possible that there are simply more astrocytes in mouse versus human feeders (Johnson et al., 2007). Future studies should include co-culture with primary cortical neurons of human embryonic origin to further corroborate our findings. Independent of the mechanism, our data demonstrate that the mouse co-culture conditions enhance maturation of hESC-derived cortical interneurons and enable functional in vitro studies.
DISCUSSION
The use of WNT-inhibitory molecules such as DKK1 has been previously proposed for the derivation of telencephalic and optic progenitors during mouse and human ESC differentiation (Lamba et al., 2006; Watanabe et al., 2005). We demonstrate that the tankyrase-inhibitor XAV939 (Huang et al., 2009) can replace recombinant DKK1 for enhancing forebrain induction using the dual-SMAD inhibition protocol. The use of only three small molecules (XLSB protocol) renders our induction conditions well-defined, cost-effective and robust across multiple cell lines. XLSB thus represents a general platform for generating forebrain lineages including cortical interneurons.
A recent paper demonstrated the importance of SHH dosage during in vitro ventral forebrain specification towards GSX2+, NKX2.1-negative progenitors capable of generating medium spiny striatal neurons (Ma et al., 2012). In addition to SHH-dose, timing can also affect the efficiency of ventral cell fate specification (Fasano et al., 2010). A remarkable feature of the current study is that the difference in timing of SHH activation is sufficient to trigger the generation of distinct ventral progenitors of divergent anterior-posterior identity. We have previously reported the derivation of hESC-derived progenitors expressing markers of the hypothalamic anlage (Kriks et al., 2011) following early activation of SHH signaling in the presence of FGF8. Our day 2-18 data suggest that addition of FGF8 is not critical for directing hypothalamic progenitor fate. Cholinergic neuron derivation from NKX2.1::GFP+ progenitors of the 6-18 group, should be of great value for modeling and treating disorders associated with loss of cognitive function. For example in Alzheimer’s disease basal forebrain cholinergic neurons are amongst the neurons most vulnerable during early stages of the disease (Whitehouse et al., 1982). However, the main focus of the current study is the derivation of human cortical interneuron lineages triggered in the day 10-18 protocol
Our study points to several surprising differences in human versus mouse forebrain development such as human-specific expression of FOXA2 in hESC derived ventral forebrain progenitors and in CS15 human forebrain tissue. We speculate that transient FOXA2 expression reflects a prolonged requirement for SHH signaling during human development given the obvious differences in brain size and extended proliferation of SHH dependent progenitors. To further address this hypothesis, it will be important to establish a time course of FOXA2 expression during human ventral forebrain development and to determine whether FOXA2 is linked to SHH expression as commonly observed in other FOXA2+ CNS domains such as the floor plate (Placzek and Briscoe, 2005).
Past studies have reported species differences in cortical interneuron development, based on the presence of ASCL1+ cells within the second trimester human fetal cortex (Letinic et al., 2002). Those data led to the hypothesis that many, and perhaps most human cortical interneurons are born within the cortex itself (Letinic et al., 2002). In contrast, studies in the mouse have demonstrated a subcortical origin of most if not all cortical interneurons (Fogarty et al., 2007; Xu et al., 2004). While our study was not specifically geared towards addressing this issue, we found restricted NKX2.1 expression within the ventral forebrain in CS15 human embryo and a complete lack of expression in the dorsal forebrain (see also Allen Brain Atlas, http://human.brain-map.org/). Furthermore, the ability of hESC-derived cortical interneurons to migrate in our slice culture assay and the developing murine cortex in vivo suggests that human cortical interneuron precursors have a similar capacity for tangential migration as their mouse counterparts. However, a distinctive feature in hESC-derived cortical interneurons was the persistent expression of NKX2.1. Nkx2.1 is extinguished in mouse cortical interneuron precursors by the time they leave the MGE prior to entering the cortex (Marin et al., 2000). Our in vitro data are consistent with findings in the human neocortex in vivo showing the presence of postmitotic NKX2.1+ neurons (Fertuzinhos et al., 2009). However, our data do not rule out the possibility that a subset of our human ESC-derived cells correspond to striatal interneurons, as those share markers of cortical interneurons in the mouse while retaining NKX2.1 expression (Marin et al., 2000).
Current paradigms of modeling human psychiatric disease such as schizophrenia are featuring the use of patient-specific iPSC-derived neurons (Brennand et al., 2011; Marchetto et al., 2010; Pasca et al., 2011). However, those published studies were performed in mixed neural cultures of unclear neuronal subtype identity and with limited characterization of subtype specific synaptic and functional properties. There is a convergence of post-mortem findings that attempt to link genetic defects to psychiatric disorders, such as the interneuron-associated Erbb4 receptor in schizophrenia (Fazzari et al., 2010). Therefore it is essential to have access to purified populations of mature cortical interneurons for modeling human disease. Our results demonstrate that the highly efficient derivation of cortical interneurons is possible following timed exposure to developmental cues.
We report that putative hESC-derived GABAergic interneurons receive synaptic inputs from both other human interneurons and from excitatory mouse projection neurons. In addition, cells with neurochemical properties of cortical interneurons adopt fairly mature physiological properties within 30 days of co-culture. Future studies will be required to address the mechanisms of accelerated in vitro maturation of the NKX2.1::GFP+ neurons on mouse cortical cultures and to determine whether species-specific timing factors are involved as suggested from our preliminary studies using hESC-derived cortical feeders. However, our data demonstrate that the proposed culture system can yield synaptically active cortical interneurons in vitro, a key prerequisite for modeling cortical interneuron pathologies in psychiatric disorders such as schizophrenia or autism. The generation of hESC-derived PV expressing neurons and the presence of relatively rapid spiking, non-accommodating neurons in these cultures is of particular interest given the implications of PV interneuron dysfunction in schizophrenia (Lewis et al., 2005). Fast-spiking PV+ cortical interneurons are observed late during primate prenatal development and continue their maturation into early adulthood (Anderson et al., 1995; Insel, 2010). Given the important role of PV+ neurons under various pathological conditions our data suggest that disease modeling of such states may be feasible using our differentiation strategies. Another key for the future will be the development of protocols that allow the enriched generation of specific cortical interneuron subgroups such as somatostatin+ versus PV+ cells. Our previous results in MGE progenitors indicate that this decision may be yet again under control of SHH signaling, with higher levels promoting somatostatin+ and lower levels promoting PV+ neurons (Xu et al., 2010).
Finally, future studies will be required to address the full in vivo potential of human in vitro derived cortical interneurons for applications in regenerative medicine. Our current study illustrates the remarkable migratory potential of the hESC-derived cortical interneurons upon transplantation into the neonatal mouse cortex. Transplantation studies into several adult CNS models of disease will be of particular interest given the potential use of cortical interneuron grafts in modulating pathological seizure activity (Baraban et al., 2009), treating aspects of Parkinson’s disease (Martinez-Cerdeno et al., 2010) and inducing learning and plasticity within the postnatal brain (Southwell et al., 2010). One specific challenge for such transplantation studies is the protracted maturation of grafted hPSC-derived cortical interneuron precursors in vivo. Preliminary longer-term transplantation studies into the adult mouse cortex confirm their continued slow maturation rate with NKX2.1+ putative interneuron precursors retaining immature growth cones and showing limited integration at 3 months post grafting (data not shown). Those data are in contrast to our in vitro co-culture work where rapid functional integration and phenotypic maturation was readily achieved. In sum, this study provides a framework for the generation of distinct ventral prosencephalic neuron types that can be used to study various aspects of development and serves as a powerful in vitro platform to study dysfunction of specific neuron types implicated in a myriad of neuropsychiatric diseases.
MATERIAL & METHODS
hESC culture and neural differentiation
hESCs (WA-09, HES-3 (NKX2.1::GFP) and iPSCs (C72, SeV6) were maintained on mouse embryonic fibroblasts (MEFs) and dissociated with Accutase (Innovative Cell Technologies) for differentiation or dispase for passaging (Chambers et al., 2009). Differentiation media were described previously (Kriks et al., 2011): KSR- and N2 medium for neural induction, and Neurobasal medium + B27 (Gibco) and N2 supplements (Invitrogen) for neuronal differentiation. Small molecule compounds: XAV939 (2 μM; Stemgent;), LDN193189 (100 nM; Stemgent), SB431542 (10 μM; Tocris), and Purmorphamine (1–2 μM; Calbiochem), ascorbic acid (200 μM) and dibutyryl-cyclic AMP (dbcAMP, 200 μM; both from Sigma). Recombinant growth factors: SHH (C25II; 50–500 ng/ml), Noggin (125 ng/ml), DKK1 (250 ng/ml), BDNF (10 ng/ml; all R&D Systems) and FGF2 (5–10 ng/ml; Promega).
E13.5 slice migration analysis
Coronal telencephalic slices (250 μm) were prepared as described previously (Anderson et al., 2001) and maintained in Neurobasal/B27 with 5 ng/ml FGF2 (Promega). hESC-derived NKX2.1::GFP+ cells were isolated by FACS and carefully injected (~ 5,000 cells) into periventricular region of the MGE using an oocyte microinjector (Nanoinject II; Drummond). After 2–6 days cultures were fixed and immunostained. For cell counting, Zone 1 was defined as the region from the ventricular zone of the lateral ganglionic eminence extending to the pallial-subpallial border. Zone 2 was defined as the region from the lateral cortex through the neocortex to the cortical hem.
Mouse and human cortical culture preparation
The cortical feeder cultures were prepared by dissociating dorsal cortices from 250μm coronal slices of E13.5 mouse brain, and cultured as described previously (Xu et al., 2010). For human cortical feeder preparation, cells were subjected to XLSB-mediated neural induction in the presence of cylcopamine and purified for CD24+/CD44−/CD184+ at day 32 (see Supplemental Experimental Procedures for details).
Gene expression profiling
Total RNA was isolated at day 18 of differentiation from FACS sorted NKX2.1::GFP+ cells from three varying differentiation conditions and a “No SHH” condition that did not contain any GFP-expressing cells (Trizol, Sigma). Samples for each group in triplicate were processed for Illumina bead arrays (Illumina HT-12) by the MSKCC genomics core facility according to the specifications of the manufacturer.
Immunocytochemical analyses
Cultures were fixed in 4% paraformaldehyde and processed for immunocytochemistry. Secondary antibodies were species-specific Alexa-dye conjugates (Invitrogen). Use of primary antibodies is summarized in Table S3. For automated quantification (Operetta, PE) see Supplemental Experimental Procedures
Transplantation studies
Transplantation into the neonatal cortex of NOD-SCID IL2RG−/− mice was conducted as described previously (Wonders et al., 2008). In brief, ~ 50 × 103 cells were injected at following coordinates from bregma (2.0mm A, 2.5mm L, 1.0mm D), targeting cortical layers 3–6. All animal experiments were carried out in accordance with institutional and NIH guidelines.
Human Tissue Analysis
Tissue derived from aborted human fetuses was obtained from the Department of Gynecology, University Bern, Switzerland. The study was approved by the Ethics Committee of the Medical Faculty of the University of Bern, Switzerland and the Ethics Committee of the State Bern, Switzerland (No. 52/91, 71/94, 188/95). Written consent was given by the women seeking abortion.
Statistical analysis
For all experiments analysis was derived from at least three independent experiments. Statistical analysis was performed using ANOVA followed by a Scheffe test to for significance among individual groups (Systat v13).
FACS
All cells were dissociated using accutase (Stem cell technologies) for thirty minutes, then resuspended in Neurobasal/B27 with Y27632 (10μM; Tocris) and sorted on a BD FACS Aria II (SSC gating/FSC gating/DAPI negative exclusion gating for live cells). For post-sort analysis, data was processed using FlowJo (Treestar) software.
Electrophysiology
Whole-cell voltage and current clamp recordings were performed as described previously (Ying et al., 2007). Details are provided in Supplementary Methods).
Supplementary Material
HIGHLIGHTS.
Inhibition of WNT signaling enhances forebrain induction using LSB protocol
Differences in timing of SHH exposure induce distinct ventral forebrain fates
hESC-derived cortical interneurons exhibit characteristic migratory properties.
In vitro co-culture yields SOM+ and PV+ interneurons with mature physiology.
Acknowledgments
We would like to thank J. Hendrikx (SKI Flow Cytometry Core), A. Viale (SKI Genomics Core lab), M. Leversha (Molecular Cytogenetics core) and E. Tu & M. Tomishima (SKI stem cell facility) for excellent technical support. We further thank the Department of Gynecology (Directors: M. Mueller & D. Surbek), University of Bern, Switzerland for providing us with the human fetal tissue. Studies using the NKX2.1::GFP hESC line was supported by grants from NYSTEM (SA) and from the C.V. Starr Foundation (SA and LS). Experiments using hESC line WA-09 or iPSC lines were supported by NIMH grant 1RC1MH089690 (SA and LS) and the Swiss National Science Foundation No. 31003A_135565 (HRW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson SA, Classey JD, Conde F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67:7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Southwell DG, Estrada RC, Jones DL, Sebe JY, Alfaro-Cervello C, Garcia-Verdugo JM, Rubenstein JL, Alvarez-Buylla A. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15472–15477. doi: 10.1073/pnas.0900141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Fishell G. The developmental integration of cortical interneurons into a functional network. Curr Top Dev Biol. 2009;87:81–118. doi: 10.1016/S0070-2153(09)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Ebert AD, Yu J, Rose FF, Jr, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Espuny-Camacho I, Michelsen Kimmo A, Gall D, Linaro D, Hasche A, Bonnefont J, Bali C, Orduz D, Bilheu A, Herpoel A, et al. Pyramidal Neurons Derived from Human Pluripotent Stem Cells Integrate Efficiently into Mouse Brain Circuits In Vivo. Neuron. 2013;77:440–456. doi: 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Fasano CA, Chambers SM, Lee G, Tomishima MJ, Studer L. Efficient Derivation of Functional Floor Plate Tissue from Human Embryonic Stem Cells. Cell Stem Cell. 2010;6:336–347. doi: 10.1016/j.stem.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, Lerma J, Marin O, Rico B. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- Fertuzinhos S, Krsnik Z, Kawasawa YI, Rasin MR, Kwan KY, Chen JG, Judas M, Hayashi M, Sestan N. Selective Depletion of Molecularly Defined Cortical Interneurons in Human Holoprosencephaly with Severe Striatal Hypoplasia. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty M, Grist M, Gelman D, Marin O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. Journal of Neuroscience. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, Espuny-Camacho I, Herpoel A, Passante L, Schiffmann SN, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- Goulburn AL, Alden D, Davis RP, Micallef SJ, Ng ES, Yu QC, Lim SM, Soh CL, Elliott DA, Hatzistavrou T, et al. A targeted NKX2.1 human embryonic stem cell reporter line enables identification of human basal forebrain derivatives. Stem Cells. 2011;29:462–473. doi: 10.1002/stem.587. [DOI] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Weick JP, Pearce RA, Zhang SC. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci. 2007;27:3069–3077. doi: 10.1523/JNEUROSCI.4562-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin J, Yang Y, Merchan P, Sarma S, Thompson J, Wang X, Sandoval J, Puelles L, Baldock R, Lindsay S. The HUDSEN Atlas: a three-dimensional (3D) spatial framework for studying gene expression in the developing human brain. J Anat. 2010;217:289–299. doi: 10.1111/j.1469-7580.2010.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lee G, Ganat Y, Papapetrou EP, Lipchina I, Socci ND, Sadelain M, Studer L. miR-371-3 Expression Predicts Neural Differentiation Propensity in Human Pluripotent Stem Cells. Cell Stem Cell. 2011;8:695–706. doi: 10.1016/j.stem.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Ma L, Hu B, Liu Y, Vermilyea SC, Liu H, Gao L, Sun Y, Zhang X, Zhang SC. Human Embryonic Stem Cell-Derived GABA Neurons Correct Locomotion Deficits in Quinolinic Acid-Lesioned Mice. Cell Stem Cell. 2012;10:455–464. doi: 10.1016/j.stem.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Anderson SA, Rubenstein JL. Origin and molecular specification of striatal interneurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:6063–6076. doi: 10.1523/JNEUROSCI.20-16-06063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroof AM, Brown K, Shi SH, Studer L, Anderson SA. Prospective Isolation of Cortical Interneuron Precursors from Mouse Embryonic Stem Cells. Journal of Neuroscience. 2010;30:4667–4675. doi: 10.1523/JNEUROSCI.4255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Noctor SC, Espinosa A, Ariza J, Parker P, Orasji S, Daadi MM, Bankiewicz K, Alvarez-Buylla A, Kriegstein AR. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell. 2010;6:238–250. doi: 10.1016/j.stem.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetrou EP, Tomishima MJ, Chambers SM, Mica Y, Reed E, Menon J, Tabar V, Mo Q, Studer L, Sadelain M. Stoichiometric and temporal requirements of Oct4, Sox2, Klf4, and c-Myc expression for efficient human iPSC induction and differentiation. Proc Natl Acad Sci U S A. 2009;106:12759–12764. doi: 10.1073/pnas.0904825106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca SP, Portmann T, Voineagu I, Yazawa M, Shcheglovitov A, Pasca AM, Cord B, Palmer TD, Chikahisa S, Nishino S, et al. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat Med. 2011;17:1657–1662. doi: 10.1038/nm.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek M, Briscoe J. The floor plate: multiple cells, multiple signals. Nat Rev Neurosci. 2005;6:230–240. doi: 10.1038/nrn1628. [DOI] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Emerging complexity of layer I in human cerebral cortex. Cereb Cortex. 2003;13:1072–1083. doi: 10.1093/cercor/13.10.1072. [DOI] [PubMed] [Google Scholar]
- Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15:477–486. S471. doi: 10.1038/nn.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP. Cortical plasticity induced by inhibitory neuron transplantation. Science. 2010;327:1145–1148. doi: 10.1126/science.1183962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Tekki-Kessaris N, Woodruff R, Hall AC, Gaffield W, Kimura S, Stiles CD, Rowitch DH, Richardson WD. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development. 2001;128:2545–2554. doi: 10.1242/dev.128.13.2545. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, Watanabe Y, Mizuseki K, Sasai Y. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8:288–296. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci. 1999;2:461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Taylor L, Welagen J, Mbata IC, Xiang JZ, Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol. 2008;314:127–136. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Xu J, Pang ZP, Ge W, Kim KJ, Blanchi B, Chen C, Sudhof TC, Sun YE. Integrative genomic and functional analyses reveal neuronal subtype differentiation bias in human embryonic stem cell lines. Proc Natl Acad Sci U S A. 2007;104:13821–13826. doi: 10.1073/pnas.0706199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. Journal of Neuroscience. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Guo L, Moore H, Waclaw RR, Campbell K, Anderson SA. Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates. Neuron. 2010;65:328–340. doi: 10.1016/j.neuron.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee CL, Wang Y, Anderson S, Ekker M, Rubenstein JL. Arcuate nucleus expression of NKX2.1 and DLX and lineages expressing these transcription factors in neuropeptide Y(+), proopiomelanocortin(+), and tyrosine hydroxylase(+) neurons in neonatal and adult mice. J Comp Neurol. 2009;517:37–50. doi: 10.1002/cne.22132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SW, Jia F, Abbas SY, Hofmann F, Ludwig A, Goldstein PA. Dendritic HCN2 channels constrain glutamate-driven excitability in reticular thalamic neurons. Journal of Neuroscience. 2007;27:8719–8732. doi: 10.1523/JNEUROSCI.1630-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Zecevic N. Dorsal radial glial cells have the potential to generate cortical interneurons in human but not in mouse brain. Journal of Neuroscience. 2011;31:2413–2420. doi: 10.1523/JNEUROSCI.5249-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan SH, Martin J, Elia J, Flippin J, Paramban RI, Hefferan MP, Vidal JG, Mu Y, Killian RL, Israel MA, et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One. 2011;6:e17540. doi: 10.1371/journal.pone.0017540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Hu F, Jakovcevski I. Interneurons in the developing human neocortex. Dev Neurobiol. 2011;71:18–33. doi: 10.1002/dneu.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.