Abstract
To determine whether steroid avoidance in pediatric kidney transplantation is safe and efficacious, a randomized, multicenter trial was performed in 12 pediatric kidney transplant centers. One hundred thirty children receiving primary kidney transplants were randomized to steroid-free (SF) or steroid-based (SB) immunosuppression, with concomitant tacrolimus, mycophenolate, and standard dose daclizumab (SB group) or extended dose daclizumab (SF group). Follow-up was 3 years post-transplant. Standardized height Z score change after 3 years follow-up was −0.99±2.20 in SF vs. −0.93±1.11 in SB; p=0.825. In subgroup analysis, recipients under 5 years of age showed improved linear growth with SF compared to SB treatment (change in standardized height Z score at 3 years −0.43±1.15 vs. −1.07±1.14; p=0.019). There were no differences in the rates of biopsy-proven acute rejection at 3 years after transplantation (16.7% in SF vs. 17.1% in SB; p=0.94). Patient survival was 100% in both arms; graft survival was 95% in the SF and 90% in the SB arms (p=0.30) at 3 years follow-up. Over the three year follow-up period, the SF group showed lower systolic BP (p=0.017) and lower cholesterol levels (p=0.034). In conclusion, complete steroid avoidance is safe and effective in unsensitized children receiving primary kidney transplants.
Keywords: Pediatric, kidney transplantation, growth, corticosteroids, graft function, side effects
INTRODUCTION
Corticosteroids have been the cornerstone of maintenance immunosuppression therapy in pediatric renal transplantation over the last half century) (1). Unfortunately, steroid use is a two-edged sword, with side effects that are particularly serious for children, including growth retardation with reduced final adult height (2;3), body disfigurement (often leading to medication nonadherence), hypertension, hyperlipidemia, diabetes mellitus, acne, osteopenia and increased infection risk (4;5).
Early attempts at steroid withdrawal in renal transplant recipients were associated with high rates of subsequent acute rejection (6;7), leading to the belief that chronic steroid usage resulted in immune dependence (5;8–11). More recent trials of steroid withdrawal or avoidance in adult recipients, who received induction therapy and more powerful maintenance therapy, have shown variable results. Some analyses have shown an increased acute rejection risk (12–14), while others have not (15;16). Meta-analyses have demonstrated that steroid avoidance and steroid withdrawal strategies in kidney transplantation are not associated with increased mortality or graft loss, despite an increase in acute rejection (17–20).
In pediatric transplantation, it is possible to withdraw (21) or avoid steroids (22) if other immunosuppressive agents are given in large doses (18). However, such a strategy could induce a state of over-immunosuppression with an increased risk of such complications as post transplant lymphoproliferative disorder (23). A steroid-free protocol for pediatric kidney transplant recipients was developed at Stanford University. This protocol replaced steroids with extended daclizumab induction for the first 6 months after transplantation, followed by a two-drug tacrolimus and mycophenolate mofetil maintenance immunosuppression protocol (24;25).
At the single center level, in comparison to a historical matched cohort, this protocol was associated with improved linear growth, reduced hypertension and hyperlipidemia, without an associated increase in acute rejection (24;25). Other single-center studies confirmed these findings (26). However, the studies were not prospective and included few African-American recipients, a traditionally high risk group. We therefore conducted a three year prospective, randomized, multi-center study of steroid-avoidance versus standard steroid-based immunosuppression in children receiving primary kidney transplants between 2004 and 2006.
MATERIALS AND METHODS
Study Design and Patients
Pediatric subjects, age 0 to 21 years, who received a primary kidney transplant from a deceased or living donor, were enrolled following IRB approval, informed consent, and, where appropriate, patient assent. This multi-center study used a randomized, open-label, parallel group design. Treatment with steroids within the 6 months prior to transplantation, en-bloc kidney transplantation, high panel reactive antibody (PRA) levels (>20%), pregnancy, transplantation of a solid organ or bone marrow or hematopoietic stem cell transplant in addition to a kidney, HIV positivity/AIDS, hypersensitivity to tacrolimus, mycophenolate mofetil (MMF), prednisone, Cremophor, HCO-60, or murine products, and the inability to measure height accurately were exclusion criteria. Donor kidney exclusion criteria included donor age >55 years, non-heart beating donor kidneys, cold ischemia time >20 hours for simple cold storage, and expected maximum cold ischemia time >30 hours for perfusion preservation.
Subjects were randomized (1:1) to a traditional low dose steroid-based (SB) immunosuppression regimen (steroids, standard daclizumab induction until the second month post-transplant, and maintenance immunosuppression with tacrolimus (Prograf®, Astellas Pharma) and MMF (CellCept®, Hoffman-La Roche) or a steroid-free (SF) immunosuppression regimen (prolonged daclizumab induction until the sixth month post-transplant, tacrolimus and MMF). For both the SF arm and the SB arm, oral tacrolimus was administered from immediately pre-operatively to recipients >5 years of age at a starting dose of 0.1 mg/kg/dose BID for living donor recipients and 0.1 mg/kg/dose QD for deceased donor recipients. Recipients <5 years of age received tacrolimus from immediately preoperatively at 0.15 mg/kg/dose BID (two preoperative doses) for living donor recipients and 0.15 mg/kg/dose QD (one preoperative dose) for deceased donor recipients. Postoperatively, the oral tacrolimus dose was 0.07 mg/kg/dose BID adjusted subsequently to achieve target levels of 12–14 ng/ml from day 0–7, 10–12 ng/ml from week 2–8, 7–10 ng/ml from week 9–12 and 5–7 ng/ml after 12 weeks. Evidence of tacrolimus toxicity on any protocol biopsy resulted in a further lowering of the tacrolimus target level to 4–6 ng/ml before the first year and 3–5 ng/ml after the first year post-transplantation. Intravenous MMF was dosed at 1200 mg/m2/day in 2 divided doses pre-operatively and for the first 48 hours postoperatively. Oral MMF was dosed at 600–900 mg/m2/day in 2 divided doses, the dose ranging because of tolerability and side effects of MMF. This regimen was used in both the SF and the SB arm.
Extended daclizumab (Zenapax®, Hoffman-La Roche) dosing was the investigational product for evaluation (BB-IND-10127 held by MS from 1999–2004, and NIAID from 2004-current). The dosing for daclizumab for the SF arm was 2 mg/kg pre-transplant followed by 1 mg/kg at weeks 2, 4, 6, 8, 11 and months 4, 5, and 6. For the SB arm, daclizumab was given at a dose of 1mg/kg peri-operatively and then at week 2, 4, 6 and 8. In the SB arm, MMF and tacrolimus were dosed in a manner similar to the SF protocol. In the SB arm, prednisone 10 mg/kg was given peri-operatively followed by 2 mg/kg/day in subjects weighing <40 kg and 1.5 mg/kg/day in subjects weighing >40 kg. The prednisone dosing was tapered as follows: by the end of weeks 1, 2, 4, 6, 12, and 16, dosages were 0.5, 0.4, 0.3, 0.2, 0.15, and 0.1 mg/kg/day respectively. The prednisone dose of 0.1 mg/kg was achieved by no later than 6 months post transplant.
Concomitant medications included intravenous gancyclovir or oral valgancyclovir for anti-viral prophylaxis for minimum the first 100 days post-transplantation and trimethoprim/sulfamethoxazole (Septra®) for pneumocystis prophylaxis for a minimum first 6 months post-transplantation.
All patients had protocol renal biopsies planned at 0, 6, 12, and 24 months after transplantation. Biopsies for indication were performed at times of graft dysfunction. Clinical acute rejection was defined as an acute rejection episode, associated with graft dysfunction, based on a greater than 10% rise in serum creatinine from baseline values, and confirmed through central pathological reading of the biopsies according to the updated Banff classification (27;28). Clinical T-cell mediated rejection was treated with 3 pulses of intravenous corticosteroid (10mg/kg) with the immediate return to SF maintenance therapy in the SF arm. Vascular rejection was treated with thymoglobulin with steroid premedication (1mg/kg/dose). Subclinical borderline Banff grade rejection was treated with immunosuppression intensification without steroid pulsing. There was no fixed protocol for treatment of steroid-resistant rejection, which was treated per center protocol.
Guidelines for removing individual subjects from study therapy were biopsy proven acute cell mediated rejection episode not responsive to treatment, biopsy proven acute rejection episode recurring within 3 months, delayed graft function defined as requirement for dialysis in the first week post-transplant or <25% decline in serum creatinine in the first 72 hours after transplant.
Statistical Analysis
All analyses were conducted on the intent to treat (ITT) population. The primary efficacy endpoint of this study was the difference linear growth between the two treatment groups at 1 year. Standardized Z-scores (SDS) were computed following a formula ([measured height–average height in the reference population]/standard deviation of height in the reference population), using an age- and gender-specific calculation provided by the NHANES III 2000 Growth Data set. From an analysis of 1 year results from nearly 2000 NAPRTCS post 1995 transplants, the standard deviation of 1 year change in height SDS is 0.7. Sample sizes of 65 in each study arm achieve 81.3% power, at a 5% significance level using a two-sided test, to detect a 0.5 standard deviation shift. A sensitivity analysis of the primary efficacy endpoint was conducted, with comparisons between the last observation carried forward (LOCF) method versus other approaches including differing distribution assumptions and multiple imputation.
The primary safety endpoint was the rate at 12 months of biopsy proven acute rejection according to the updated Banff classification (27;28). Sample sizes of 65 from the steroid group and 65 from the no steroid group achieve 75% power at a 5% significance level using a one-sided equivalence test of proportions when the proportion in the steroid group is 0.15 and the proportion in the no steroid group being tested for equivalence is 0.15 and the maximum allowable difference between these proportions that still results in equivalence (the range of equivalence) is 0.15. The study was not powered to definitively evaluate small differences in rejection rate.
Secondary endpoints included patient and graft survival, incidence of delayed graft function, acute rejection, renal function as determined by the Schwartz method (29;30), incidence of hyperlipidemia (fasting serum triglyceride levels >140 mg/dl and/or fasting serum cholesterol levels >190 mg/dl), hypertension (defined as present based on actual values of systolic or diastolic blood pressure norms for age), anemia, leucopenia, infectious complications, surgical complications, incidence and duration of re-hospitalizations, incidence of biopsy proven PTLD, incidence of post-transplant diabetes mellitus (fasting plasma glucose >126 mg/dl, based on the definition used by the American Diabetes Association, requiring treatment with either insulin or hypoglycemia agents), body disfigurement, and incidence of return to steroid therapy for subjects assigned to the SF arm.
Student’s t-test was used for continuous, and Chi-square-test for categorical variables when comparing the two treatment groups. When sample sizes were small, nonparametric tests such as the Wilcoxon test for continuous variables or Fisher’s exact test for two-way classification tables, respectively, were used for hypothesis testing. Mixed model repeated measures were used for analyses of continuous outcomes, such as change in growth from baseline and blood pressure levels. The Restricted Maximum Likelihood (REML) method, as implemented in SAS PROC MIXED, was used for variance estimation to account for repeated observations. Kaplan-Meier method was used for time-to-event analyses and log-rank test was used to test differences in the median time-to-event. Data values were reported as mean±standard deviation for patient demographic information, and were reported as mean±standard error of the mean for the clinical outcomes. P values ≤0.05 were considered statistically significant. All statistical analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Patient characteristics
One hundred thirty subjects were enrolled for randomization into either study arm from 12 different US pediatric transplant programs. More SB patients were enrolled in two centers, resulting in unequal final numbers in the 2 arms, with 60 in the SF and 70 in the SB groups. Demographic characteristics (Table 1) were similar between the two treatment groups. A quarter of the patients were African-Americans in both groups. Approximately 60% of recipients received deceased donor grafts (Table 1). Of the total patients enrolled, 10 patients in the SF arm and 18 patients in the SB arm were dropped from the study. The causes of termination were graft failure in 7 cases, investigator’s decision in 3 cases, loss to follow-up in 4 cases, relocation in 8 cases, protocol deviation in 2 cases, consent withdrawal in 3 cases and another reason in 1 case. None of the grafts was lost due to delayed graft function (DGF), defined as requirement of dialysis in the first post-transplant week. DGF occurred in 11.7% (7/60) of patients in SF group and in 10.0% (7/70) of patients in the SB group (p=0.784), but was not a reason for protocol break in either arm. Five patients from the SF study group (8.3%) switched to steroids. They remained in the study but were transitioned to “reduced follow-up”. In total, 134 indication biopsies of adequate quality were performed, 69 in the steroid-base study arm and 65 in the steroid-free arm.
Table 1.
Baseline Patient Characteristics
| Baseline Patient Characteristics | |||
|---|---|---|---|
| Steroid-free | Steroid-based | P value | |
| N | 60 | 70 | |
| Recipient | |||
| Mean age (yrs)(median; range) | 11.8 ± 5.4(12.7; 1.5–19.9) | 11.9 ± 6.1 (11.9; 1.0–20.3) | 0.93 |
| Infant: ≤ 5 yrs old (%) | 18.3% | 21.4% | 0.66 |
| 5–12 years old (%) | 30.0% | 22.9% | 0.36 |
| >12 years old (%) | 51.7% | 55.7% | 0.64 |
| Mean age (yrs) males | 11.3 ± 5.5 | 12.1 ± 6.6 | 0.55 |
| Mean age (yrs) females | 12.9 ± 5.2 | 11.6 ± 5.4 | 0.42 |
| Gender: females (%) | 33% | 40% | 0.43 |
| Mean height (cm)(median; range) | 137.6 ± 26.5 (143; 75.8–183) | 136.0 ± 33.3 (143; 71.0–183) | 0.77 |
| Height Z-score | −1.2 ± 1.4 | −1.3 ±1.3 | 0.70 |
| Mean weight (kg)(median; range) | 40.2 ± 18.9 (40.6; 10–85) | 40.7 ± 22.9 (41.3; 8.8–113) | 0.86 |
| Mean BMI (kg/m2) | 19.9 ± 4.1 | 20.0 ± 4.1 | 0.90 |
| Mean BSA (m2) | 1.2 ± 0.4 | 1.2 ± 0.5 | 1.00 |
| Race (1,2,3,4) † | 55%, 25%, 8%, 12% | 50%, 27%, 4%, 19% | 0.56 |
| CMV D+R− | 46.7% | 42.9% | 0.66 |
| EBV D+R− | 13.3% | 11.4% | 0.74 |
| Transfusions 0, 1–5, >5, unknown (%) | 48.3, 40.0%, 6.7%, 5.0% | 64.3%, 24.3%, 8.6%, 2.9% | 0.08 |
| Dialysis mode pre-transplant (none, hemodialysis, peritoneal dialysis, both) | 20%, 36.7%, 20.0%, 23.3% | 14.3%, 41.4%, 21.4%, 22.9% | 0.39 |
| Cold ischaemia time (mins) | 705.1 ± 479.1 | 716.6 ± 416.5 | 0.90 |
| Cause of ESRD (1,2,3,4,5,6,7,8)* | (8%,3%,18%,2%,12%,15%,3%,38%) | (10%,1%,16%,11%,11%,9%,9%,33%) | 0.33 |
| Mean eGFR (ml/min 1.73 m2) | 14.2 ± 9.6 | 13.8 ± 8.1 | 0.81 |
| Mean Hematocrit (%) | 33.9 ± 6.4 | 34.4 ± 6.8 | 0.71 |
| Mean white cell count (ul) | 8.7 ± 3.9 | 7.8 ±2.8 | 0.12 |
| Mean serum cholesterol (mg/dl) | 160.8 ± 40.2 | 148.5 ± 40.9 | 0.12 |
| Mean serum triglyceride (mg/dl) | 177.1 ± 89.7 | 164.8 ± 109.9 | 0.53 |
| Mean Systolic blood pressure (mmHg) | 126.4 ± 19.4 | 122.7 ± 20.3 | 0.30 |
| Mean Diastolic blood pressure (mmHg) | 74.7 ± 15.9 | 72.1 ± 15.4 | 0.35 |
| Donor | |||
| Mean donor age (median; range) | 29.1 ± 10.1 (28.5; 2–47) | 27.4 ± 10.0 (26.5; 5–54) | 0.34 |
| Gender: females (%) | 47% | 40% | 0.44 |
| Donor Type (LRD) | 40% | 34% | 0.59 |
| Mean HLA match LRD | 2.5 ± 1.9 | 1.9 ±1.4 | 0.06 |
| Mean HLA match DD | 0.6 ± 0.9 | 0.7 ± 0.8 | 0.85 |
1=White; 2=Black/African American; 3=Asian; 4=Other
1=glomerulonephritis; 2=polycystic kidney disease; 3=dysplasia; 4=reflux nephropathy; 5=obstructive uropathy; 6=FSGS; 7=congenital nephrotic syndrome; 8=others
Primary Efficacy Endpoint: Growth
For the overall study ITT population there was no significant difference between SF and SB groups with respect to linear growth at three years following transplant between the two treatment groups (change in standardized height Z score −0.99±2.20 in SF vs. −0.93±1.11 in SB; p=0.786). There was also no significant difference for linear growth at three years follow-up overall for a combined cohort of all children with growth potential defined as females <16 years and males <18 years (change in standardized height Z score −0.92±2.29 in SF vs. −0.96±1.16 in SB; p=0.732). Within the African-American subgroup, the increase in Z-score was 0.38 (SE 0.148) in the SF group and 0.25 (SE 0.124) in the SB group (p=0.518).
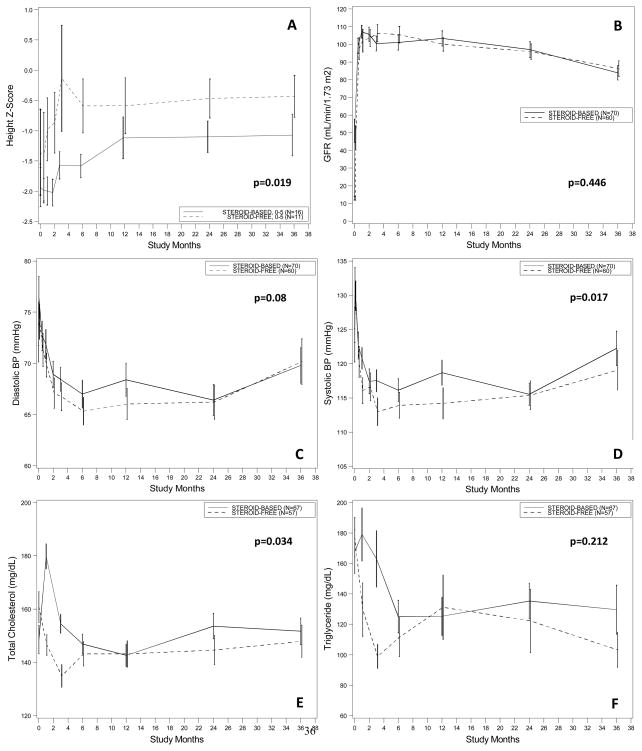
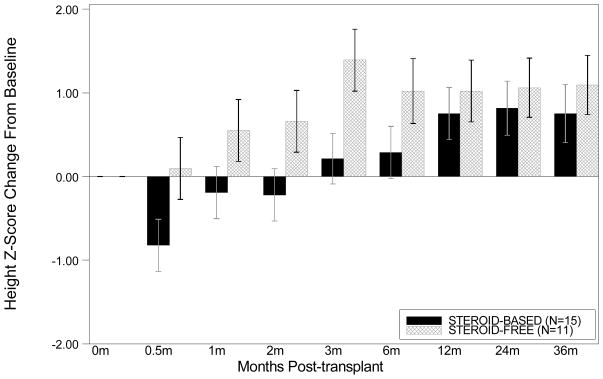
There were 27 children <5 years of age (11 in the SF and 16 in the SB arms). This group showed a numerically greater growth rate at 3 years post-transplantation (change in standardized height Z −0.43±1.15 vs. −1.07±1.14; p=0.844 with LOCF analysis), and when this was adjusted for baseline height, the treatment effect was significant (mixed model group effect p=0.019; Figure 1A). Change in height Z-score from baseline tended to be different between the SF and the SB arm in the first months after transplantation, but this effect was lost by 1 year after transplantation (Wilcoxon p=0.79 at 1 year and p=0.84 at 3 years after transplantation)(Figure 2).
Figure 1. Estimated group mean standardized change in growth (Z-score) among infants and young children (A), mean eGFR level (by Schwartz method) (B), mean diastolic (C) and systolic (D) blood pressure levels and serum cholesterol (E) and triglyceride (F) levels from transplantation up to three year.
Values are estimated group means ± Standard Error (SE) from a repeated measure mixed model with treatment and time (in study month) as main effects, and treatment by time interaction. The P value for overall treatment effect in each analysis is given.
Figure 2. Change in height Z-score from baseline amongst infants and young children < 5 years of age.
Change in height Z-score from baseline tended to be different between the SF and the SB arm in the first months after transplantation, but this effect was lost by 1 year after transplantation (Wilcoxon p=0.79 at 1 year and p=0.84 at 3 years after transplantation).
Primary Safety Endpoint: Acute Rejection
Acute rejection in for-cause biopsies
By 1 year after transplantation, biopsy-proven acute clinical rejection occurred in 13.3% of SF vs. 11.4% of SB patients (p=0.74) (Table 2). Also when borderline changes were included, the incidence of rejection was comparable between both study groups (20.0% in SF vs. 18.6% in SB; p=0.84) by 1 year after transplantation. Over the 3 years of follow-up, there was no significant difference in the rate of biopsy-proven acute clinical T-cell mediated rejection (16.7% in SF vs. 17.1% in SB; p=0.94). Also when borderline changes were included, no difference was noted at 3 years after transplantation (26.7% in SF vs. 28.6% in SB; p=0.81). There was also no difference in the incidence of steroid-resistant acute rejection (19.0% of acute rejections in SF vs. 25.8% in SB; p=0.74). Antibody-mediated rejection was seen in only 4 indication biopsies from 3 different patients (2 in the SF arm and 1 in the SB arm). There was no difference in the number of subjects who had recurrent acute rejection within 3 months (0% of acute rejections in SF vs. 25.0% [N=2] in SB; p=0.1). There was no difference in the time to first acute rejection between groups (detailed histological analysis in accompanying submission by Naesens et al (31))
Table 2.
Secondary End-Points
| Secondary end-points | |||
|---|---|---|---|
| Steroid-free treatment group | Steroid-based treatment group | P value | |
| Number of patients | 60 | 70 | |
| Patient survival by 3 years | 60/60 (100%) | 70/70 (100%) | NA |
| Graft survival by 3 years | 57/60 (95.0%) | 63/70 (90.0%) | 0.30 |
| Delayed graft function | 4/60 (6.7%) | 1/70 (1.4%) | 0.18 |
| Graft function (Schwartz eGFR, mL/min/1.73m2) | |||
| At 1 year (mean ± SD; median) | 100 ± 28.8; 102 (N=56) | 103 ± 33.9; 101 (N=63) | 0.90 |
| At 3 years (mean ± SD; median) | 86.3 ± 30.5; 89 (N=49) | 83.9 ± 28.5; 85 (N=48) | 0.72 |
| Acute T-cell mediated rejection in indication biopsies (graft dysfunction) | |||
| Cumulative prevalence at 1 year | 8/60 (13.3%) | 8/70 (11.4%) | 0.74 |
| Cumulative prevalence at 3 years | 10/60 (16.7%) | 12/70 (17.1%) | 0.94 |
| Borderline changes or T-cell mediated rejection in indication biopsies (graft dysfunction) | |||
| Cumulative prevalence at 1 year | 12/60 (20.0%) | 13/70 (18.6%) | 0.84 |
| Cumulative prevalence at 3 years | 16/60 (26.7%) | 20/70 (28.6%) | 0.81 |
| Acute T-cell mediated rejection in protocol biopsies (stable graft function) | |||
| At 6 months | 5/47 (10.6%) | 6/53 (11.3%) | 0.91 |
| At 12 months | 0/35 (0.0%) | 2/46 (4.3%) | 0.21 |
| At 24 months | 0/33 (0.0%) | 2/42 (4.8%) | 0.20 |
| Borderline changes in protocol biopsies (stable graft function) | |||
| At 6 months | 4/47 (8.5%) | 5/53 (9.4%) | 0.87 |
| At 12 months | 6/35 (17.1%) | 2/46 (4.3%) | 0.06 |
| At 24 months | 4/33 (12.1%) | 1/42 (2.4%) | 0.09 |
Acute rejection in protocol biopsies
Central pathological reading of the 256 post-transplant protocol biopsies, performed in patients with stable graft function (47, 35 and 33 at respectively 6, 12 and 24 months after transplantation in the SF treatment arm, and 53, 46 and 42 in the SB treatment arm), showed that the risk for subclinical T-cell mediated rejection was similar between both treatment arms at each protocol biopsy time point (Table 2). There was also no significantly increased risk for borderline changes in protocol biopsies. The incidence of subclinical antibody-mediated rejection was too low to allow for statistical analysis.
This study was not powered to examine small differences in rejection rates in patient subgroups. Nevertheless, since the African-American group has traditionally been considered as high risk for steroid avoidance protocols, we analyzed the results in this subgroup separately. At 12 months, the biopsy proven AR rates were 13.3% (SF group; 2 episodes in 15 subjects) versus 31.1 % (SB group, 6 episodes in 19 subjects, p=0.26). At 36 months, the rates were almost identical (BPAR rate 33.3% in SB, 5 episodes in 15 subjects vs.36.8%in SF, 7 episodes in 19 subjects, p=1.0).
Secondary End-points
Patient and graft survival
Patient survival was 100% in both arms at 3 years; graft survival was 95% in the SF and 90% in the SB arms (p=0.30) at 3 years follow-up (Table 2). The causes of the 7 graft losses in SB group were acute rejection (n=2), chronic rejection (n=3), medication non-adherence (n=1), and recurrence of original kidney disease (n=1). The three graft losses in the SF arm were due to medication non-adherence (n=2) and recurrence of original kidney disease (n=1).
Graft function
Between the two groups, there were no significant differences in graft function based on eGFR (ml/min/1.73m2)by the Schwartz formula 29, 30 for ITT SF and SB population (100.5±4.8 for SF vs. 102.3±4.5 for SB at 1 year; 93.7±4.9 for SF vs. 95.9±4.6 for SB at 2 years; 83.0±5.0 for SF vs. 80.8±4.9 for SB at 3 years; mixed model group effect p=0.446)(Figure 1B). In addition, there was no significant difference regardless of whether or not the patients had an AR episode (101.5±4.8 for SF vs. 102.3±4.5 for SB at 1 year; 93.7±4.9 for SF vs. 95.9±4.6 for SB at 2 years; 83.0±5.0 for SF vs. 80.8±4.9 for SB at 3 years; mixed model group effect p=0.64). Delayed graft function occurred in 6.7% of patients in the SF group vs. 1.4% of patients in the SB group (p=0.18).
Diabetes mellitus
There was no statistical difference in post-transplant diabetes between the groups at the 3 years post-transplantation (1.7% in SF vs. 5.7% in SB; p=0.373).
Body mass index and body disfigurement
There was no significant difference in body mass index between two groups at the 3 years post-transplantation (26.6±5.1 vs. 24.1±6.6; p=0.17). Cushingoid facies was observed significantly more frequently at 3 years in the SB arm compared to the SF arm (respectively in 34% in SB vs. 2.1% in SF; P<0.001), although it is important to note that this is a subjective parameter and the current study was not conducted in a double-blind fashion.
Hypertension and hyperlipidemia
Standardized systolic blood pressure was significantly lower in SF vs. SB over the first 3 years after transplantation (mixed model group effect p=0.017) and there was a non-significant trend towards lower diastolic BP in SF vs, SB (mixed model group effect p=0.08) (Figures 1C and 1D). The SF group had a significantly lower total cholesterol level than SB group (mixed model group effect p=0.034). The overall treatment effect for triglyceride level was not significant (p=0.212) (Figures 1E and 1F).
Infections, malignancies and hospitalizations
There was no increase in the number of hospitalizations in the SF group vs. the SB group. There was no difference in the time to first re-hospitalization (Figure 3, p=0.346 log-rank test), and 80% of SF and 86% of SB patients had one or more hospitalizations post-transplantation. There was no PTLD in both groups. Five SF subjects (8.3%) and 6 SB subjects (8.6%) had BK viremia (p=0.96). Of these, BK nephritis occurred in 3 patients in the SB group vs. 1 in the SF group (P=0.62). Eleven SF subjects and 12 SB subjects reported having Cytomegalovirus infection or viremia (p=0.26). Other relevant adverse events are listed in Table 3.
Table 3. Overall incidence of serious and non-serious adverse events regardless of relationship to study medication.
Presented are adverse events with an occurrence of ≥10% of patients in either treatment group or with a significant difference between groups or of clinical importance. Excluded are adverse events related to the transplanted organ, abnormal laboratory values and complications of surgery.
| Steroid-free treatment group | Steroid-based treatment group | |
|---|---|---|
| Non-serious adverse events | ||
| Number of subjects with at least one non-serious adverse event | 56/60 (93,3%) | 64 (91,4%) |
| Blood and lymphatic system disorders | 37 (66,1%) | 38 (59,4%) |
| Anemia | 25 (44,6%) | 21 (32,8%) |
| Neutropenia | 24 (42,9%) | 21 (32,8%) |
| Leukopenia | 14 (25,0%) | 12 (18,8%) |
| Gastrointestinal disorders | 29 (51,8%) | 27 (42,2%) |
| Diarrhoea | 18 (32,1%) | 19 (29,7%) |
| Vomiting | 11 (19,6%) | 8 (12,5%) |
| Nausea | 6 (10,7%) | 5 (7,8%) |
| Stomatitis | 2 (3,6%) | 0 (0,0%) |
| Infections and infestations | 44 (78,6%) | 47 (73,4%) |
| Urinary tract infection | 14 (25,0%) | 20 (31,3%) |
| Upper respiratory tract infection* | 20 (35,7%) | 10 (15,6%) |
| Otitis media | 9 (16,1%) | 8 (12,5%) |
| CMV infection | 8 (14,3%) | 6 (9,4%) |
| EBV infection | 6 (10,7%) | 4 (6,3%) |
| Metabolism and nutrition disorders | 29 (51,8%) | 36 (56,3%) |
| Hyperkalemia | 12 (21,4%) | 10 (15,6%) |
| Hypophosphatemia | 10 (17,9%) | 12 (18,8%) |
| Hypomagnesemia | 6 (10,7%) | 6 (9,4%) |
| Metabolic acidosis | 5 (8,9%) | 3 (4,7%) |
| Hyperglycemia | 1 (1,8%) | 5 (7,8%) |
| Hyperlipidemia* | 0 (0,0%) | 5 (7,8%) |
| Vascular disorders | 19 (33,9%) | 26 (40,6%) |
| Hypertension | 14 (25,0%) | 19 (29,7%) |
| Musculoskeletal and connective tissue disorders | 7 (12,5%) | 9 (14,1%) |
| Skin and subcutaneous tissue disorders | 5 (8,9%) | 14 (21,9%) |
| Nervous system disorders | 6 (10,7%) | 9 (14,1%) |
| Serious adverse events | ||
| Number of subjects with at least one serious adverse event | 52 (86,7%) | 60 (85,7%) |
| Blood and lymphatic system disorders | 2 (3,8%) | 3 (5,0%) |
| Anemia | 0 (0,0%) | 1 (1,7%) |
| Neutropenia | 0 (0,0%) | 2 (3,3%) |
| Gastrointestinal disorders | 9 (17,3%) | 9 (15,0%) |
| Diarrhoea | 3 (5,8%) | 3 (5,0%) |
| Peritonitis | 0 (0,0%) | 2 (3,3%) |
| Infections and infestations | 25 (48,1%) | 28 (46,7%) |
| Pyelonephritis | 7 (13,5%) | 8 (13,3%) |
| Urinary tract infection | 7 (13,5%) | 7 (11,7%) |
| Gastroenteritis | 3 (5,8%) | 8 (13,3%) |
| CMV infection | 1 (1,9%) | 3 (5,0%) |
| Pneumonia | 3 (5,8%) | 4 (6,7%) |
| Herpes zoster | 1 (1,9%) | 2 (3,3%) |
| Metabolism and nutrition disorders | 7 (13,5%) | 13 (21,7%) |
| Dehydration | 3 (5,8%) | 7 (11,7%) |
| Hyperkalemia | 3 (5,8%) | 2 (3,3%) |
| Post-transplant diabetes mellitus | 1 (1,7%) | 4 (5,7%) |
| Vascular disorders | 4 (7,7%) | 7 (11,7%) |
| Hypertension | 1 (1,9%) | 3 (5,0%) |
| Renal and urinary disorders | 12 (23,1%) | 13 (21,7%) |
| Musculoskeletal and connective tissue disorders | 1 (1,9%) | 0 (0,0%) |
| Skin and subcutaneous tissue disorders | 1 (1,9%) | 1 (1,7%) |
| Nervous system disorders | 3 (5,8%) | 2 (3,3%) |
| Post-transplant lymphoproliferative disorder | 0 (0,0%) | 0 (0.0%) |
P value <0.05
DISCUSSION
This study reports the first 3-year randomized, prospective, multicenter trial in pediatric kidney transplantation that tests the safety and efficacy of a complete steroid avoidance protocol. The study cohorts represent a selected group of unsensitized recipients of first transplants, without prolonged cold ischemia times. This is also the first pediatric clinical trial that compares two different treatment protocols with detailed reporting on the subclinical occurrence of acute rejection and progression of chronic injury, assessed by serial protocol biopsies at pre-specified intervals. The histological substudy, presented separately from the current manuscript (31), showed that subclinical progression of chronic histological damage and subclinical inflammation are not different between both treatment arms.
The excellent patient and graft survival, the absence of any increase in acute rejection, viral and bacterial infections, and the absence of PTLD support earlier single center study results (4;24;25) on the safety of complete steroid avoidance. The low infection and malignancy rates are particularly important as there was a double total dose of daclizumab administered for 4 months longer in the SF than the steroid-based protocol, with equivalent dosing for tacrolimus and MMF in the two treatment arms. Furthermore, given some recent concerns of hypersensitivity with chimeric monoclonal interleukin 2 receptor antibody (basiliximab) infusions (32;33), it is important to note that there were no infusion-related adverse events with the fully humanized daclizumab in either treatment arm. The results of the current study can however not be generalized, and as daclizumab has been withdrawn from the market, it will be necessary to separately evaluate the safety and efficacy of basiliximab for induction in steroid-free immunosuppressive regimens in pediatric kidney transplantation.
The incidence of clinical and subclinical T-cell mediated rejection was similar in the two treatment arms. This is in contradistinction to studies in adults, where there was a higher incidence of acute rejection in steroid avoidance and withdrawal (19). None of the clinical acute rejections in the SF arm recurred within 3 months of the initial rejection episode, while in the SB arm 25% of the acute rejections recurred within 3 months of the initial rejection episode. Although this finding reached only borderline statistical significance, it possibly suggests that the mechanisms of immunological escape during rejection may be different depending on whether or not the patient is being treated with steroids. Though this clinical study cannot dissect the specific impact of extended dose daclizumab from the impact of complete steroid avoidance, it is possible that the prolonged use of daclizumab was a key immunomodulatory agent that diminished the risk of acute rejection recurrence. On the other hand, evaluating literature on the effect of chronic steroids in animal and human models of organ transplantation (5;8–11;34;35), we can hypothesize that the higher rejection recurrence rate in the SB arm may reflect an element of steroid dependence. The clinical significance of the numerically higher rate of borderline changes in protocol biopsies at 12 and 24 months post-transplantation is yet unclear (36), and was counterbalanced by numerically higher number of patients that developed acute T-cell mediated rejection after one year in the SB group.
Another safety parameter is the absence of any negative impact of steroid avoidance on the incidence of DGF. There was some concern whether ischemia reperfusion injury at the time of engraftment would require peri-operative steroid exposure to facilitate immediate graft function (37). Our study suggests that in a low-risk immunologic recipient of a first graft with cold ischemia time of <20 hours, peri-operative steroids may not be required (38). There are currently no data suggesting the best course for steroid avoidance or withdrawal in the high risk pediatric setting. Importantly, our study found that graft function was equivalent in the 2 arms at the end of three post-transplant years.
In terms of efficacy, the primary end point, i.e. linear growth in the overall group, was not different between both treatment arms. However, when linear growth was further stratified for age, there was a significantly greater growth rate for infants and small children under 5 years in the steroid-free group compared to the steroid-based treatment arm. This confirms previous single-center observations (24;25). The similarity in growth between the SF and SB groups for the study population as a whole can be explained by the wide recipient age range in this study. Specifically, our study had a high percentage of adolescent transplant recipients (n=63), as would be expected in a cross section of kidney transplants in the US population as a whole (39). In addition, the prednisone dose in the SB group was low (0.1 mg/kg or less by 6 months) and perhaps blunted the differences in growth usually seen with higher doses of steroids (see below). Finally, it is possible that the effects of steroid avoidance in the smallest recipients persist many years later, as a previous observation noted unprecedented catch-up growth rates, greater than the growth velocity in normal healthy age and gender-matched controls, after 4 years post transplantation (25).
Our 36 month follow up results substantiate our early 12 month results (40) and the 6 month results of the European TWIST study (41). In the TWIST study, the mean treatment group difference in linear height Z score was 0.13 (p<0.005) in children under 5 years age, 0.21 in pre-pubertal (p=0.009) and 0.05 in pubertal children (p=ns). It is, however, interesting that the dose of steroids used in the TWIST steroid-based arm at 6 months (<0.3mg/kg) were always higher than they were in our SB patients. When converting mg/m2 to mg/kg, it turns out that patients in the TWIST received doses of steroids that were different by a factor of 0.5–3. This is particularly true in patients above the weight of 20 kg (data not shown).
Our study also addresses an additional important question, whether steroid avoidance immunosuppression is safe in African American recipients. African American recipients generally have worse outcomes after kidney transplantation. In particular, this group exhibits higher rates of acute rejection and earlier graft loss, especially in earlier steroid withdrawal studies (12). The proportion of African Americans enrolled in adult recipient studies of steroid avoidance (14;42;43)has been too small to judge if these patients would be at risk with a steroid avoidance strategy. Single center reported series also have a small percentage of African American recipients (25;44). However, in the current multi-center prospective trial, African American patients did equally well in terms of acute rejection as other groups with steroid avoidance and did not exhibit any untoward effects, although it should be noted that the number of African American patients in the current study was still relatively low (N=34, 26.1%)to draw firm conclusions.
In conclusion, complete steroid avoidance, combined with effective induction, tacrolimus and mycophenolate mofetil, provides a new therapeutic standard for safe and effective immunosuppression for renal transplantation of low-risk children with end stage renal disease.
Acknowledgments
We are grateful of the support with patient recruitment and sample collections from transplant patients in the SNSO1 multicenter study centers and for invaluable study support from Nikki Williams, Senior Project Manager, Transplantation Immunobiology Branch, NIAID. This study was supported by a grant AI-055795 to Dr. Salvatierra and Dr. Sarwal from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, made as a component of the Cooperative Clinical Trials in Pediatric Transplantation Consortium. We are also grateful to Astellas and Roche Pharmaceuticals for their generous financial support for this trial. Data confidentiality was maintained between the NIH and the SNSO1 centers by Pharmaceutical Product Development, Inc (PPD). We are also grateful of the help from our nurses, clinical coordinators, patients and patient families.
Footnotes
ClinicalTrials.gov Number: NCT00141037
DISCLOSURE
None of the authors have any conflict of interest to declare in relation to this study.
MMS and OS designed the study. MMS, RE, VD, MB, RM, AP, RMD, WH, DK, VMV, EK, HJB and BW were involved in patient inclusion and patient care. LT, JL, LL, TS, MN and JW were involved in data collection and data management. LT, JL, MMS, MN, OS, LL, and TS were responsible for data analysis and data interpretation. MMS, OS, MN, RE, MB, WH, RM and VD wrote the manuscript. MMS, OS, RE, VD, MB, RM, AP, RMD, WH, DK, VMV, EK, HJB and BW critically revised the manuscript. MMS and Pharmaceutical Product Development (PPD) had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Goodwin WE, Mims MM, Kaufman JJ. Human renal transplantation. III. Technical problems encountered in six cases of kidney homotransplantation. J Urol. 1963 Mar;89:349–56. doi: 10.1016/S0022-5347(17)64556-7. [DOI] [PubMed] [Google Scholar]
- 2.Fine RN, Martz K, Stablein D. What have 20 years of data from the North American Pediatric Renal Transplant Cooperative Study taught us about growth following renal transplantation in infants, children, and adolescents with end-stage renal disease? Pediatr Nephrol. 2010 Apr;25(4):739–46. doi: 10.1007/s00467-009-1387-3. [DOI] [PubMed] [Google Scholar]
- 3.Travis LB, Chesney R, McEnery P, Moel D, Pennisi A, Potter D, et al. Growth and glucocorticoids in children with kidney disease. Kidney Int. 1978 Oct;14(4):365–8. doi: 10.1038/ki.1978.138. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Chaudhuri A, Weintraub LA, Hsieh F, Shah S, Alexander S, et al. Subclinical cytomegalovirus and Epstein-Barr virus viremia are associated with adverse outcomes in pediatric renal transplantation. Pediatr Transplant. 2007 Mar;11(2):187–95. doi: 10.1111/j.1399-3046.2006.00641.x. [DOI] [PubMed] [Google Scholar]
- 5.Knight SR, Morris PJ. Azathioprine and Steroids. In: Morris Peter, Knechtle Stuart., editors. Kidney Transplantation: Principles and Practice. 6. Philadelphia: W.B. Saunders; 2008. pp. 220–33. [Google Scholar]
- 6.Stratta RJ, Armbrust MJ, Oh CS, Pirsch JD, Kalayoglu M, Sollinger HW, et al. Withdrawal of steroid immunosuppression in renal transplant recipients. Transplantation. 1988 Feb;45(2):323–8. doi: 10.1097/00007890-198802000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Srinivas TR, Meier-Kriesche HU. Minimizing immunosuppression, an alternative approach to reducing side effects: objectives and interim result. Clin J Am Soc Nephrol. 2008 Mar;3(Suppl 2):S101–16. S101–S116. doi: 10.2215/CJN.03510807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berki T, Tavakoli A, Nagy KK, Nagy G, Nemeth P. Alterations of glucocorticoid receptor expression during glucocorticoid hormone therapy in renal transplant patients. Transpl Int. 2002 Mar;15(2–3):132–8. doi: 10.1007/s00147-002-0397-x. [DOI] [PubMed] [Google Scholar]
- 9.Wiegers GJ, Labeur MS, Stec IE, Klinkert WE, Holsboer F, Reul JM. Glucocorticoids accelerate anti-T cell receptor-induced T cell growth. J Immunol. 1995 Aug 15;155( 4):1893–902. [PubMed] [Google Scholar]
- 10.Sarwal MM, Rianthavorn P, Ettenger R. Kidney Transplantation in Children. In: Morris Peter, Knechtle Stuart., editors. Kidney Transplantation: Principles and Practice. 6. Philadelphia: W.B. Saunders; 2008. pp. 599–629. [Google Scholar]
- 11.Almawi WY, Hess DA, Assi JW, Chudzik DM, Rieder MJ. Pretreatment with glucocorticoids enhances T-cell effector function: possible implication for immune rebound accompanying glucocorticoid withdrawal. Cell Transplant. 1999 Nov;8(6):637–47. doi: 10.1177/096368979900800610. [DOI] [PubMed] [Google Scholar]
- 12.Ahsan N, Hricik D, Matas A, Rose S, Tomlanovich S, Wilkinson A, et al. Prednisone withdrawal in kidney transplant recipients on cyclosporine and mycophenolate mofetil--a prospective randomized study. Steroid Withdrawal Study Group. Transplantation. 1999 Dec 27;68(12):1865–74. doi: 10.1097/00007890-199912270-00009. [DOI] [PubMed] [Google Scholar]
- 13.Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, Van VP. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early(7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg. 2008 Oct;248(4):564–77. doi: 10.1097/SLA.0b013e318187d1da. [DOI] [PubMed] [Google Scholar]
- 14.Vincenti F, Schena FP, Paraskevas S, Hauser IA, Walker RG, Grinyo J. A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. American Journal of Transplantation. 2008 Feb;8( 2):307–16. doi: 10.1111/j.1600-6143.2007.02057.x. [DOI] [PubMed] [Google Scholar]
- 15.Opelz G, Dohler B, Laux G. Long-term prospective study of steroid withdrawal in kidney and heart transplant recipients. American Journal of Transplantation. 2005 Apr;5( 4 Pt 1):720–8. doi: 10.1111/j.1600-6143.2004.00765.x. [DOI] [PubMed] [Google Scholar]
- 16.Hocker B, John U, Plank C, Wuhl E, Weber LT, Misselwitz J, et al. Successful withdrawal of steroids in pediatric renal transplant recipients receiving cyclosporine A and mycophenolate mofetil treatment: results after four years. Transplantation. 2004 Jul 27;78(2):228–34. doi: 10.1097/01.tp.0000133536.83756.1f. [DOI] [PubMed] [Google Scholar]
- 17.Pascual J, Zamora J, Galeano C, Royuela A, Quereda C. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev. 2009;(1):Art. No.: CD005632. doi: 10.1002/14651858.CD005632.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Knight SR, Morris PJ. Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation. 2010 Jan 15;89(1):1–14. doi: 10.1097/TP.0b013e3181c518cc. [DOI] [PubMed] [Google Scholar]
- 19.Sprangers B, Vanrenterghem Y. Steroid avoidance or withdrawal after kidney transplantation: a balancing act. Transplantation. 2010 Aug 27;90(4):350–2. doi: 10.1097/TP.0b013e3181e5927a. [DOI] [PubMed] [Google Scholar]
- 20.KDIGO clinical practice guideline for the care of kidney transplant recipients. American Journal of Transplantation. 2009 Nov;9( Suppl 3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 21.Hocker B, Weber LT, Feneberg R, Drube J, John U, Fehrenbach H, et al. Prospective, randomized trial on late steroid withdrawal in pediatric renal transplant recipients under cyclosporine microemulsion and mycophenolate mofetil. Transplantation. 2009 Mar 27;87(6):934–41. doi: 10.1097/TP.0b013e31819b6d4a. [DOI] [PubMed] [Google Scholar]
- 22.Grenda R, Webb NJ. Steroid minimization in pediatric renal transplantation: Early with-drawal or avoidance? Pediatr Transplant. 2010 Dec;14(8):961–7. doi: 10.1111/j.1399-3046.2010.01403.x. [DOI] [PubMed] [Google Scholar]
- 23.Benfield MR, Bartosh S, Ikle D, Warshaw B, Bridges N, Morrison Y, et al. A randomized double-blind, placebo controlled trial of steroid withdrawal after pediatric renal transplantation. American Journal of Transplantation. 2010 Jan;10(1):81–8. doi: 10.1111/j.1600-6143.2009.02767.x. [DOI] [PubMed] [Google Scholar]
- 24.Sarwal MM, Yorgin PD, Alexander S, Millan MT, Belson A, Belanger N, et al. Promising early outcomes with a novel, complete steroid avoidance immunosuppression protocol in pediatric renal transplantation. Transplantation. 2001 Jul 15;72(1):13–21. doi: 10.1097/00007890-200107150-00006. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Chang A, Naesens M, Kambham N, Waskerwitz J, Martin J, et al. Steroid-Free Immunosuppression Since 1999: 129 Pediatric Renal Transplants with Sustained Graft and Patient Benefits. American Journal of Transplantation. 2009 May 6;9(6):1362–72. doi: 10.1111/j.1600-6143.2009.02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhakta N, Marik J, Malekzadeh M, Gjertson D, Ettenger R. Can pediatric steroid-free renal transplantation improve growth and metabolic complications? Pediatr Transplant. 2008 Dec;12(8):854–61. doi: 10.1111/j.1399-3046.2008.00928.x. [DOI] [PubMed] [Google Scholar]
- 27.Racusen L, Solez K, Colvin R, Bonsib S, Castro M, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999 Feb 1;55(2):713–23. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 28.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008 Apr;8( 4):753–60. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976 Aug;58(2):259–63. [PubMed] [Google Scholar]
- 30.Schwartz GJ, Gauthier B. A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr. 1985 Mar;106(3):522–6. doi: 10.1016/s0022-3476(85)80697-1. [DOI] [PubMed] [Google Scholar]
- 31.Naesens M, Kambham N, Salvatierra O, Benfield M, Ettenger R, Dharnidharka V, et al. Steroid avoidance in pediatric kidney recipients does not influence subclinical inflammation or chronic renal allograft injury. 2011 doi: 10.1111/j.1600-6143.2012.04144.x. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wouters KM, Lane MH, Walker I. Acute hypersensitivity reaction on re-exposure to basiliximab in an infant undergoing heart transplantation. Paediatr Anaesth. 2008 Aug;18( 8):806–7. doi: 10.1111/j.1460-9592.2008.02555.x. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki H, Chikaraishi T, Furuhata S, Tsutsumi H, Miyano S, Nakano T, et al. Anaphylactic reaction after initial exposure of Basiliximab: case reports. Transplant Proc. 2007 Dec;39( 10):3457–9. doi: 10.1016/j.transproceed.2007.08.104. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Mercep M, Ware CF, Ashwell JD. Fas and activation-induced Fas ligand mediate apoptosis of T cell hybridomas: inhibition of Fas ligand expression by retinoic acid and glucocorticoids. J Exp Med. 1995 May 1;181(5):1673–82. doi: 10.1084/jem.181.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almawi WY, Hess DA, Rieder MJ. Significance of enhanced cytokine receptor expression by glucocorticoids. Blood. 1998 Nov 15;92(10):3979–80. [PubMed] [Google Scholar]
- 36.Beimler J, Zeier M. Borderline rejection after renal transplantation--to treat or not to treat. Clin Transplant. 2009 Dec;23( Suppl 21):19–25. doi: 10.1111/j.1399-0012.2009.01105.x. [DOI] [PubMed] [Google Scholar]
- 37.Martins PN, Chandraker A, Tullius SG. Modifying graft immunogenicity and immune response prior to transplantation: potential clinical applications of donor and graft treatment. Transpl Int. 2006 May;19(5):351–9. doi: 10.1111/j.1432-2277.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- 38.Kainz A, Wilflingseder J, Mitterbauer C, Haller M, Burghuber C, Perco P, et al. Steroid pretreatment of organ donors to prevent postischemic renal allograft failure: a randomized, controlled trial. Ann Intern Med. 2010 Aug 17;153(4):222–30. doi: 10.7326/0003-4819-153-4-201008170-00003. [DOI] [PubMed] [Google Scholar]
- 39.Horslen S, Barr ML, Christensen LL, Ettenger R, Magee JC. Pediatric transplantation in the United States, 1996–2005. Am J Transplant. 2007 May;7(Suppl 1):1339–58. doi: 10.1111/j.1600-6143.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 40.Sarwal M, Benfield M, Ettenger R, Dharnidharka V, Mathias R, McDonald R, et al. One year results of a prospective, randomized, multicenter trial of steroid avoidance in pediatric renal transplantation. American Journal of Transplantation. 2008;8(Suppl 2):192. [Google Scholar]
- 41.Grenda R, Watson A, Trompeter R, Tonshoff B, Jaray J, Fitzpatrick M, et al. A randomized trial to assess the impact of early steroid withdrawal on growth in pediatric renal transplantation: the TWIST study. American Journal of Transplantation. 2010 Apr;10( 4):828–36. doi: 10.1111/j.1600-6143.2010.03047.x. [DOI] [PubMed] [Google Scholar]
- 42.Vitko S, Klinger M, Salmela K, Wlodarczyk Z, Tyden G, Senatorski G, et al. Two corticosteroid-free regimens-tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil-in comparison with a standard triple regimen in renal transplantation: results of the Atlas study. Transplantation. 2005 Dec 27;80( 12):1734–41. doi: 10.1097/01.tp.0000188300.26762.74. [DOI] [PubMed] [Google Scholar]
- 43.Rostaing L, Cantarovich D, Mourad G, Budde K, Rigotti P, Mariat C, et al. Corticosteroid-free immunosuppression with tacrolimus, mycophenolate mofetil, and daclizumab induction in renal transplantation. Transplantation. 2005 Apr 15;79(7):807–14. doi: 10.1097/01.tp.0000154915.20524.0a. [DOI] [PubMed] [Google Scholar]
- 44.Silverstein DM, Aviles DH, LeBlanc PM, Jung FF, Vehaskari VM. Results of one-year follow-up of steroid-free immunosuppression in pediatric renal transplant patients. Pediatr Transplant. 2005 Oct;9(5):589–97. doi: 10.1111/j.1399-3046.2005.00345.x. [DOI] [PubMed] [Google Scholar]