SUMMARY
Melanocytes are pigment-producing cells of neural crest origin responsible for protecting the skin against UV-irradiation. Pluripotent stem cell technology offers a novel approach for studying human melanocyte development and disease.
Here we report that timed exposure to activators of WNT, BMP and EDN3 signaling triggers the sequential induction of neural crest and melanocyte precursor fates under dual-SMAD inhibition conditions. Using a SOX10::GFP hESC reporter line, we demonstrate that the temporal onset of WNT activation is particularly critical for human neural crest induction. Subsequent maturation of hESC-derived melanocytes yields pure populations matching the molecular and functional properties of adult melanocytes. Melanocytes from Hermansky-Pudlak and Chediak-Higashi Syndrome patient-specific iPSCs faithfully reproduce the ultrastructural features of disease-associated pigmentation defects. Our data define a highly specific requirement for WNT signaling during neural crest induction and enable the generation of pure populations of hiPSC-derived melanocytes for faithful modeling of human pigmentation disorders.
INTRODUCTION
Epidermal melanocytes are pigment-producing cells found at the basement membrane of the skin where they establish a photo-protective barrier against UV-irradiation. Melanocytes synthesize melanin within specialized lysosome-related structures known as melanosomes which are subsequently transferred to neighboring keratinocytes, giving the skin its characteristic pigmentation. While the developmental biology of melanocytes has been well studied in avian and murine models, the processes underlying melanocyte development in humans remain poorly understood. The derivation of melanocytes from human embryonic stem cells (hESCs) therefore provides a valuable tool for studying human melanocyte development and for modeling disease biology. Previous work on the derivation of melanocytes from murine (Yamane et al., 1999) and human (Fang et al., 2006; Nissan et al., 2011) ESCs relied on stromal co-culture or embryoid body formation in combination with conditioned media from a WNT3a producing stromal cell line to trigger melanocytic differentiation. The lack of a defined culture system has complicated efforts to gain better mechanistic insights into early melanocyte development and maturation.
During development melanocytes arise from a transient, migratory population of cells unique to vertebrates known as the neural crest (NC). The NC is a multipotent population that exhibits a broad differentiation repertoire with distinct fate potentials along axial levels of origin. Our lab had previously established a stromal co-culture based approach for the differentiation of hESCs into NC with PNS and mesenchymal competence, however this population did not efficiently yield melanocyte lineages (Lee et al., 2007). More recently, a neural induction protocol in which hESCs are differentiated under defined dual SMAD inhibition (DSi) conditions was found to support low levels of spontaneous NC induction (Chambers et al., 2009) and the emergence of a pigmented cell population. However, most pigmented cells under those conditions exhibit properties of CNS-derived retinal pigment epithelium rather than melanocyte lineage (Figure S2). Therefore we sought to establish a novel, defined approach for the derivation of a melanocyte competent NC population that would enable us to dissect the mechanistic and temporal signaling requirements underlying NC induction, specification along the melanocyte lineage, and melanocyte maturation.
We now report that activation of canonical WNT signaling is sufficient to drive efficient NC specification at the expense of CNS lineages under defined, dual-SMAD inhibition-based neural induction conditions. We found a brief pulse of WNT activation is sufficient to induce NC with high efficiency. Remarkably, induction is largely insensitive to pharmacological BMP inhibition and is not dependent on sustained WNT activity. However, derivation of the melanoblast lineage required additional exposure to BMP4 and EDN3 to induce KIT+ melanocyte-competent neural crest precursors. We next describe defined and scalable culture conditions for the subsequent differentiation, maturation and long-term maintenance of hESC-derived melanocytes. Finally we confirm the robustness and utility of our melanocyte differentiation paradigm by modeling pigmentation defects in two independent genetic disorders using patient-specific induced pluripotent stem cells (iPSCs). Differentiation into melanocytes, across all control and disease-specific iPSCs, displayed minimal variability. Furthermore the direct comparison of disease and control lines identifies discrete defects in melanosome loading and transfer by ultrastructural analysis. Our results offer novel insights into human NC and melanocyte specification and present a defined and efficient protocol for generating human melanocytes from pluripotent stem cells that faithfully reproduce patient specific pigmentations defects.
RESULTS
Derivation of Neural Crest (NC) from Human ESCs
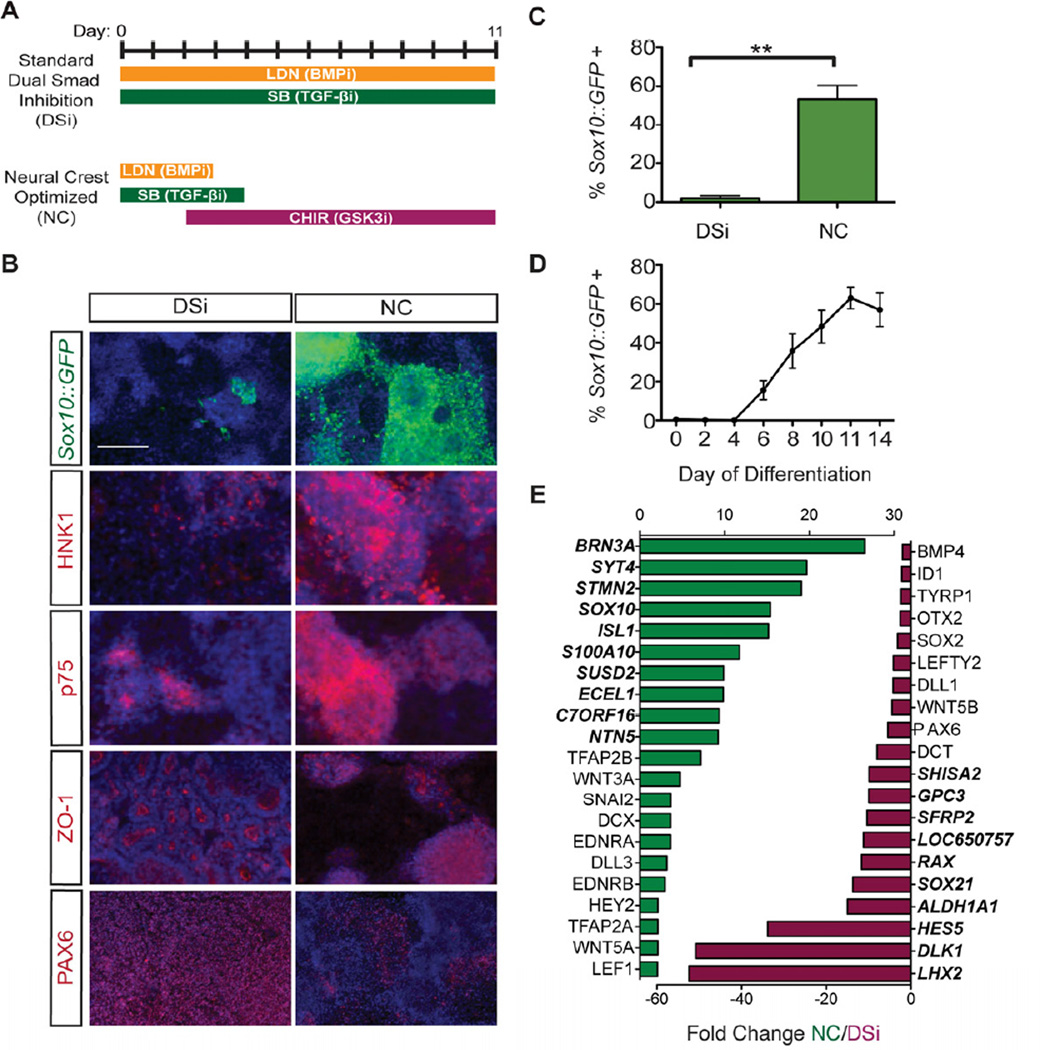
To recapitulate the progressive differentiation that occurs during normal development we established a stepwise differentiation protocol in which human pluripotent stem cells (hPSCs) are first differentiated to the multipotent NC stage before becoming melanoblast precursors capable of maturing into terminally-differentiated melanocytes. In the DSi protocol hESCs are treated for 11 days with two small molecules that inhibit the separate branches of SMAD signaling; LDN-193189 (LDN) which inhibits BMP signaling and SB431542 (SB) which inhibits TGF-β, Activin, and Nodal signaling (Figure 1A). Inhibition of both arms is required to trigger exit from the pluripotent state, prevent trophectoderm formation and block the formation of mesendoderm and non-neural ectoderm (Chambers et al., 2009). However NC specification requires intermediate levels of BMP signaling (Marchant et al., 1998) while activation of TGF-β signaling is thought to be required at a later timepoint to promote epithelial-tomesenchymal transition (Xu et al., 2009). To reconcile these opposing requirements, we hypothesized that withdrawal of BMP and TGF-β inhibitors at an intermediate time points may allow early inhibition of alternate lineages while facilitating NC specification at later stages by restoring endogenous BMP and TGF-β signaling. Studies in avian models have also established a strong requirement for WNT signaling for early NC specification (García-Castro et al., 2002). Wnt signaling was activated in our system using the potent and cost-effective small molecule CHIR99021 (Chir), which functions as an agonist of WNT signaling by selectively inhibiting glycogen synthase kinase 3β (GSK-3β) (Meijer et al., 2004; Ring et al., 2003), although recombinant WNT also efficiently promoted NC induction (Figure S1). Efficiency of NC induction was monitored with a Sox10::GFP hESC reporter line (Chambers et al., 2012), which marks early multipotent NC stem cells and specific NC derivatives including melanocyte progenitors.
Figure 1. Induction and isolation of NC from Sox10::GFP hESCs using a modified dual SMAD inhibition protocol.
(A) A dual SMAD inhibition (DSi) protocol can be modified to support highly efficient induction of a neural crest (NC) population by optimizing BMP, TGF-β, and Wnt signaling. (B) NC conditions support induction of a Sox10::GFP expressing population that co-expresses neural crest markers HNK-1 and p75 while down-regulating ZO-1 and PAX6 expressing CNS. Scale bars represent 200µm. (C) NC optimized conditions increase the yield of Sox10::GFP positive cells greater than 20-fold (53% ± 14%, n=4, p=0.002) over the DSi condition. (D) Using FACS analysis, Sox10::GFP was first detected at day 6 of NC differentiation and peaked by day 11. Sox10::GFP induction efficiency is represented as a percentage of total viable cells. (E) Significantly up- (green) and downregulated (red) genes at day 11 in NC-induced cells compared to DSi cells. Bold genes represent top 10 most differentially regulated genes. All error bars represent the s.e.m of at least three independent experiments. ** p<0.01. See also Figures S1 and S2.
We found that inhibition of BMP, TGF-β and WNT signaling pathways, collectively referred to as the neural crest inductive “NC” condition (Figure 1A), was sufficient to promote robust specification of Sox10::GFP expressing precursors (Figures 1B,C). Compared with the standard DSi protocol, the NC optimized condition increased the yield of Sox10::GFP positive cells greater than 20-fold (53% ± 14%; Figure 1C). Induction of Sox10::GFP occurred rapidly with GFP expression first detectable by flow cytometry at day 6 of differentiation and peaking by day 11 (Figure 1D). As previously suggested, the ratio between CNS and NC differentiation was found to be density dependent under DSi conditions (Chambers et al., 2009) while density-dependent differences in yield were less pronounced under NC conditions (Figure S1). Efficient induction of Sox10::GFP correlated with an increase in HNK-1 and p75 expression two markers previously found to define hESC-derived NC populations (Lee et al., 2007). Sox10::GFP did not however exclusively co-label with HNK-1 and p75 suggesting that these may not represent identical populations of NC (Figure S1). Specifically, the Sox10::GFP positive population contained p75 single positive cells, while the Sox10::GFP negative population also included p75/HNK1 double positive cells. NC conditions did not support the emergence of ZO-1 expressing neural rosettes or CNS-associated PAX6 expression (Elkabetz et al., 2008) (Figure 1B). Comparative gene expression analysis of day 11 DSi and NC cells further confirmed that NC differentiation conditions favored induction of NC associated markers including SOX10, TFAP2A/B, SNAI2, and EDNRA/B as well as genes associated with WNT (WNT3A, LEF1) and Notch signaling (DLL3, HEY2) (Figure 1E, Table S1) while DSi cells expressed high levels of genes associated with CNS (PAX6, HES5) and forebrain state (OTX2, LHX2). Fate specific markers were also induced under both NC and DSi conditions with BRN3A, an early marker of sensory neurogenesis, observed following NC differentiation while genes associated with pigmentation such as tyrosinase-related protein 1 (TYRP1) and dopachrome tautomerase (DCT) were observed under DSi conditions, presumably due to the presence of retinal pigment epithelium cells that can be expanded from DSi conditions (Figure S2), although DCT is also expressed in the mouse forebrain (Steel et al., 1992). Unlike previously reported NC populations derived from hESC using stromal co-culture conditions (Lee et al., 2007), NC-derived cells did not exhibit high levels of HOX gene expression suggesting that these cells correspond to the HOX-negative NC lineage previously shown to be particularly plastic (Creuzet et al., 2005; Le Douarin et al., 2004). AP axial level including HOX gene expression could however be manipulated using caudalizing cues such as FGF2 or retinoic acid (RA) treatment (Figure S1).
NC Induction is driven by a narrow window of GSK-3β inhibition
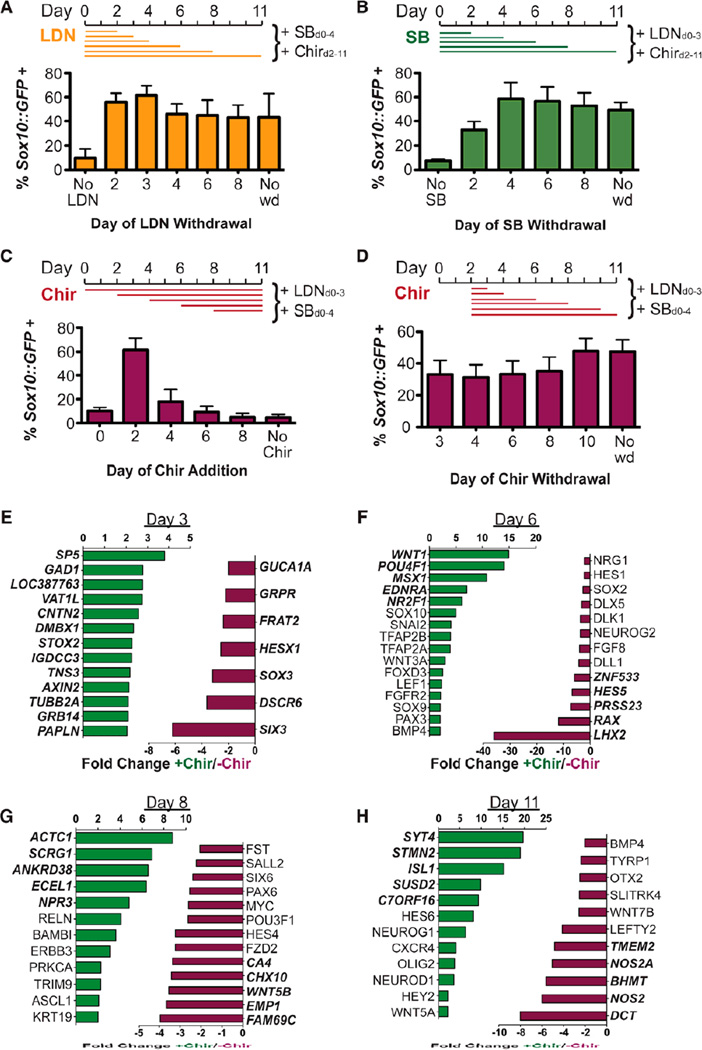
To further investigate the individual contributions of the BMP, TGF-β, and GSK-3β inhibitors to NC specification, we performed timed withdrawal experiments during the 11-day differentiation or omitted factors entirely (Figure 2A–C). While a brief treatment with LDN was required for Sox10::GFP induction, 2–3 days of treatment appeared to be sufficient for optimal NC specification. These findings contrast with a recent report in which treatment with Noggin, another BMP inhibitor used in the original DSi protocol, was found to be dispensable for the induction of a p75+/HNK1+ NC population (Menendez et al., 2011). We observed similar requirements for TGF-β/Activin inhibition. Conditions in which the inhibitor SB was omitted entirely were not permissive for NC differentiation while withdrawal at any point after day 4 allowed for robust Sox10::GFP induction (Figure 2B). Continued treatment with either the BMP or TGF-β inhibitors for the entire 11 days of the differentiation did not negatively impact the Sox10::GFP yield, suggesting that de-repression of endogenous BMP or TGF-β signaling is not essential for triggering NC differentiation (Figure 2A,B).
Figure 2. Neural crest induction is driven by a narrow window of Wnt activation.
BMP inhibitor LDN193189 (LDN, A) and TGF-β inhibitor SB431542 (SB, B) are individually withdrawn at various timepoints within the context of the NC protocol and induction of Sox10::GFP assessed at day 11 by FACS. Treatment with GSK-3β inhibitor CHIR99021 (Chir) is initiated (C) or withdrawn (D) at various timepoints and induction of Sox10::GFP assessed at day 11 by FACS. Sox10::GFP induction efficiency is represented as a percentage of total viable cells. The effects of Chir on gene expression at days 3 (E), 6 (F), 8 (G), and 11 (H) were determined by comparative microarray analysis of NC cells derived in the presence (green) or absence (red) of Chir. Bold genes represent top 5 most differentially regulated genes. All error bars represent the s.e.m of at least three independent experiments. See also Figure S3.
When we investigated the requirements for WNT activation using the small molecule Chir, we observed a narrow window during which Chir treatment is essential for Sox10::GFP induction. While Chir exposure starting at day 2 of the differentiation resulted in optimal Sox10::GFP induction, changing the onset of Chir treatment by as little as 1–2 days resulted in a near complete loss of Sox10::GFP expression (Figure 2C). When we investigated the minimal duration of WNT activity required for Sox10::GFP induction, we found that a single day of Chir treatment was sufficient to achieve 70% of the maximal yield (Figure 2D). To test whether a short pulse of Chir treatment can induce a subsequent wave of endogenous WNT signaling, we performed luciferase-based WNT reporter assays. We observed that β catenin levels remained elevated three days after a single day pulse of Chir treatment, suggesting that endogenous WNT signaling was indeed triggered by brief Chir exposure (Figure S3). However, blocking endogenous WNT signaling after 1–2 days of Chir treatment using the axin stabilizing small molecule XAV-939 (XAV) did not affect the efficiency of NC induction (Figure S3). Our data indicate that WNT signaling is not required beyond a brief inductive pulse to specify NC fate.
We next performed global gene expression analysis to determine the transcripts regulated immediately after Chir exposure (Figures 2E–H). Within 24 hours of Chir addition, the most upregulated gene was SP5, a transcription factor downstream of WNT and beta-catenin signaling implicated in anterior-posterior patterning (Fujimura et al., 2007; Weidinger et al., 2005). Upregulation of AXIN2 and WNT1 at 24 hours and 6 days after Chir addition further confirmed that Chir treatment induces endogenous WNT signaling. In contrast, SIX3, a negative regulator of WNT signaling required for vertebrate forebrain development (Braun et al., 2003; Lagutin et al., 2003; Lavado et al., 2008) and HESX1 and SOX3, two additional forebrain markers, were suppressed following Chir treatment (Figure 2E). By day 6 of the differentiation, the timepoint when Sox10::GFP expression was first observed, induction of various additional NC markers was observed including EDNRA, SOX10, SNAI2, TFAP2A/B, FOXD3, SOX9, and PAX3 (Figure 2F) while various CNS and forebrain markers were suppressed (Figure 2F). By days 8 (Figure 2G) and 11 (Figure 2H) more mature, cell type specific markers were upregulated including ASCL1, EDNRB, and OLIG2. Our global transcriptome profiling data therefore establish a model in which treatment with the GSK-3β inhibitor Chir during the NC protocol first induces a surge of WNT signaling which is followed by markers of NC specification, and a subsequent neurogenic wave.
We next determined whether the surprisingly narrow window during which WNT signaling drives NC specification reflects a unique temporal competence of the cells that respond to NC-inductive WNT signaling. Our gene expression analysis had identified genes induced with 24 hours and 6 days of Chir treatment (Figures 2E, F). We asked whether these genes would also be induced when Chir treatment was delayed until day 4 of differentiation, a timepoint that does not efficiently support NC specification (Figure 2C). We found that many of the genes upregulated within 24 hours of Chir treatment, including those known to be direct targets of WNT signaling, are not induced when cells are treated with Chir at day 4 (Figure S3). In fact, upon delayed Chir treatment, many Chir responsive genes were expressed at levels comparable with DSi conditions. Intriguingly, levels of the negative WNT regulator SIX3, which is usually downregulated upon Chir treatment, remained elevated with late Chir treatment. Induction of late WNT responsive genes was similarly lost (Figure S3). These data indicate that cells lose competency to NC-inductive WNT signals and undergo a default anterior neuroectodermal program.
Lineage Specification and Isolation of Neural Crest-Derived Melanoblasts
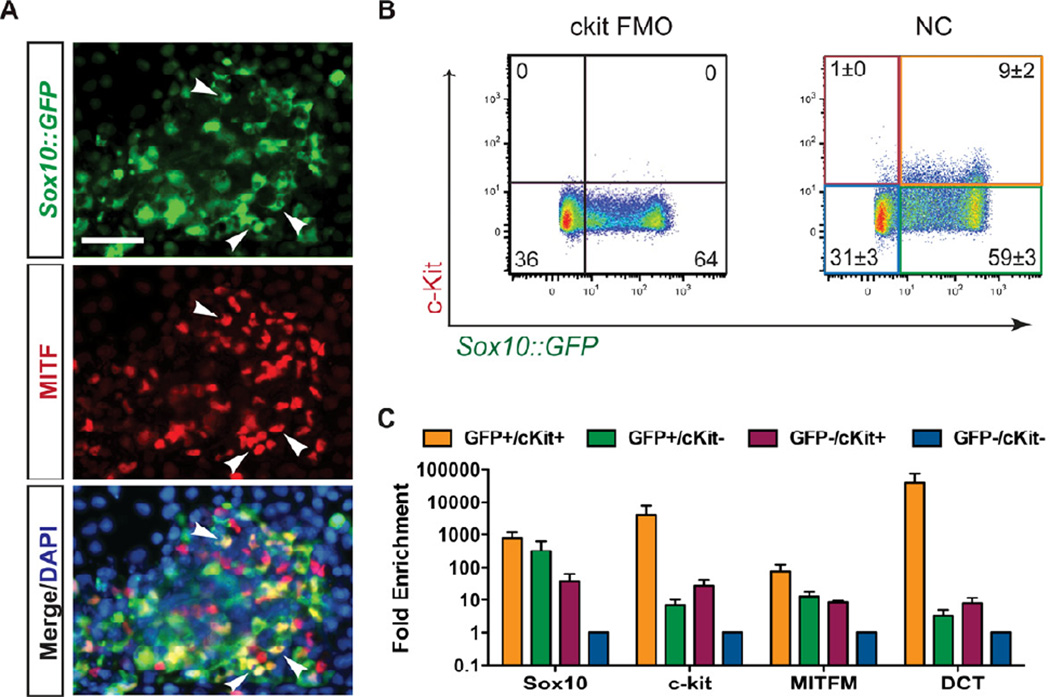
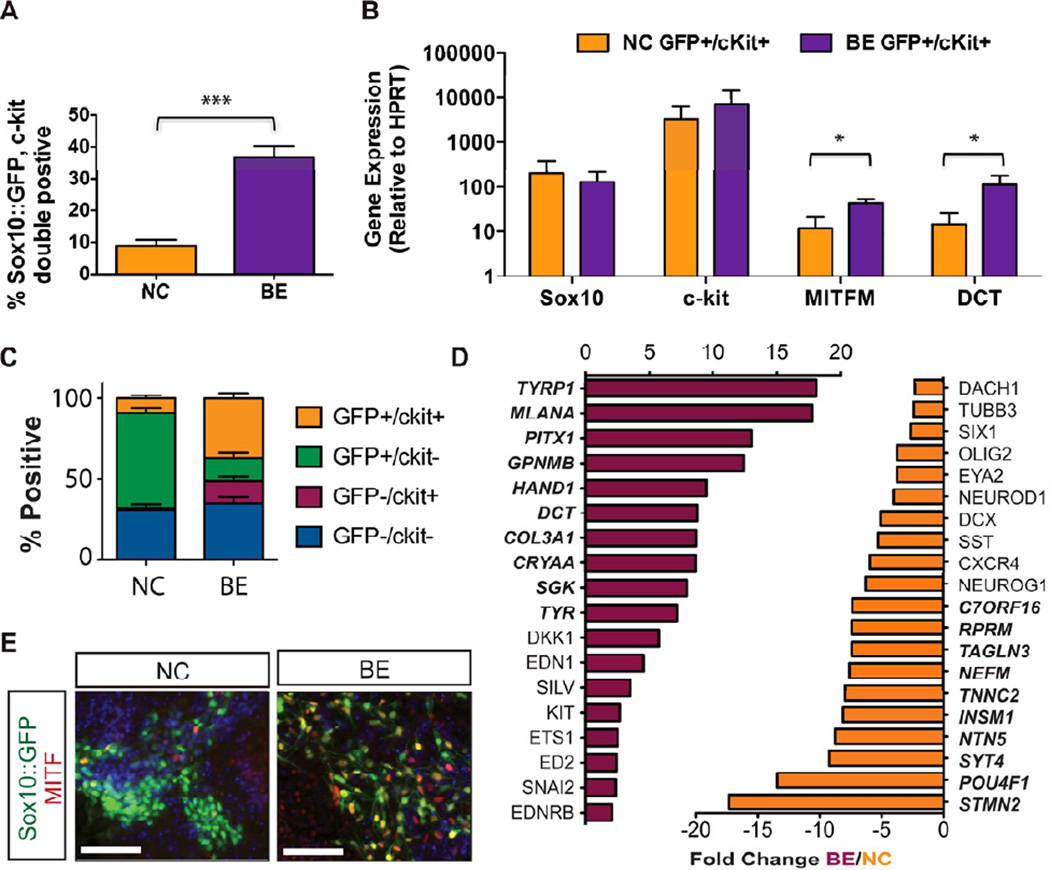
To confirm the melanocyte competence of our NC-derived cells, we identified a subpopulation of presumptive melanoblasts at day 11 of differentiation based on co-expression of Sox10::GFP and microphthalmia-associated transcription factor (MITF) (Figure 3A). MITF positive cells lacking Sox10::GFP expression where also observed, which may be due to downregulation of the BAC reporter, as we observed continued SOX10 protein expression by immunofluorescence (Figure 5E). To prospectively identify and isolate melanoblasts from the heterogeneous population of NC cells, we selected KIT (c-kit) as a putative cell surface marker of early melanocyte progenitors. KIT has previously been identified as uniquely marking the melanocyte-competent sub-fraction of NC cells (Luo et al., 2003; Reid et al., 1995) and plays an important role in melanocyte migration (Kunisada et al., 1998; Yoshida et al., 1996), proliferation (Kunisada et al., 1998; Yoshida et al., 1996), and maturation(Kunisada et al., 1998). Using FACS analysis we confirmed that 9% ± 2% of the population at day 11 of the differentiation co-expressed Sox10::GFP and c-kit, while 59% ± 3% only expressed Sox10::GFP (Figure 3B).
Figure 3. C-kit and Sox10 expression can be used to identify and isolate melanoblasts.
(A) Melanocyte progenitors can be identified in day 11 NC protocol-derived populations by coexpression of Sox10::GFP and the melanocyte transcription factor MITF (arrowheads). Scale bar represents 50µm. (B) Flow cytometry reveals the presence of a Sox10::GFP and c-kit co-expressing population. C-kit “fluorescence minus one” (FMO) was used a negative control for c-kit staining. (C) 4-way FACS sorting for Sox10::GFP and c-kit reveals an enrichment of the melanocyte markers MITFM and Dct in the SOX10/ckit double positive population by qRT-PCR. All error bars represent the s.e.m of at least three independent experiments. See also Figure S4.
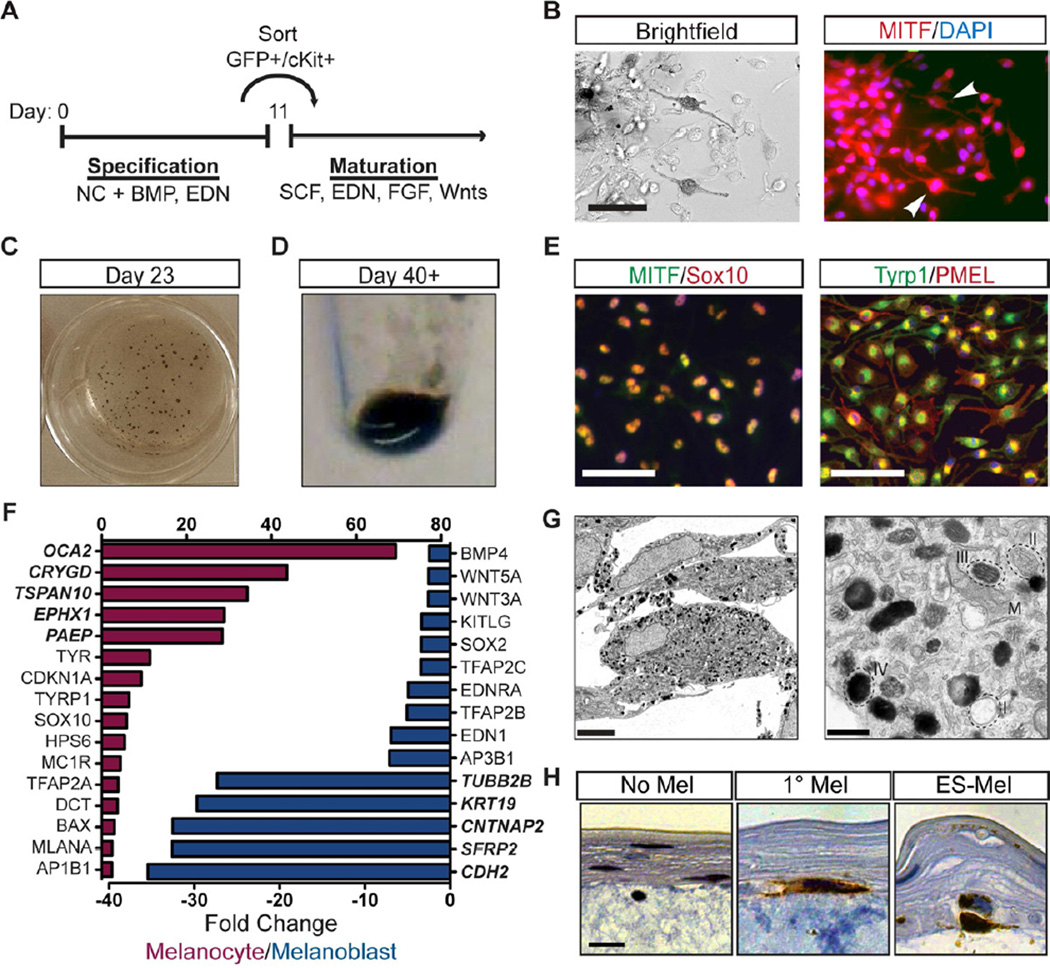
Figure 5. hESC-derived melanoblasts can be matured to a functional, pigmented state.
(A) BE-derived melanocyte progenitors can be matured to a pigmented state following culture in media containing SCF, EDN3, FGF2, BMP, cAMP and WNT signaling factors. (B) Pigmented cells expressing MITF can be observed as early as one week after passaging. Scale bar represents 20µm. (C, D) Cells become progressively more pigmented with macroscopic pigmented clusters discernable in tissue culture wells within 3 weeks (C) and cell pellets of 1×106 cells taking on a darkly pigmented phenotype (D). (E) Extended culture supports the propagation of nearly pure populations of mature melanocytes expressing transcription factors SOX10 and MITF and melanosomal markers TYRP1 and PMEL. Scale bar represents 50µm. (F) Mature melanocytes exhibit elevated levels of mature marker expression (red) and downregulation of neural crest and stem cell-associated markers (blue) compared to melanocyte progenitors in gene expression analyses. Bold genes represent top 5 most differentially regulated genes. (G) Numerous pigmented melanosomes, representing all four stages of melanosome maturation (I–IV), are visible in the cytoplasm of ES-derived melanocytes by electron microscopy. Scale bar represents 5µm (left) and 500nm (right). (H) Tyrosinase positive (IHC brown) ES-derived melanocytes home to the basement membrane in organotypic artificial skin reconstructs. Scale bar represents 2µm. No melanocyte negative control – left, primary melanocytes – middle, ES-melanocytes – right. See also Figure S6.
Purified Sox10::GFP, c-kit co-expressing (GFP+/cKit+) cells expressed higher levels of the melanocyte markers MITF-M and DCT when compared to GFP+/cKit− cells (Fig. 3C), confirming the utility of KIT and Sox10 co-expression as an appropriate strategy for melanoblast isolation. We performed a candidate screen for factors that increase the percentage of GFP+/cKit+ cells. Contrary to predictions based on literature in other model systems, SCF signaling at this timepoint did not enhance melanoblast induction, however we identified BMP and Endothelin 3 (EDN3) as two factors that did enhance melanoblast yield when introduced at day 6 of the differentiation (Figure S4). The role of BMP signaling during melanocyte specification has been well established (Dunn et al., 2000; Nissan et al., 2011; Takeda et al., 2000; Thomas and Erickson, 2008), while endothelins have been implicated in the maintenance and proliferation of melanocyte precursors (Baynash et al., 1994; Dupin and Le Douarin, 2003; Lahav et al., 1996; Reid et al., 1996). Combined treatment with both BMP4 and EDN3 (hereafter referred to as the “BE” condition), significantly increased the percentage of GFP+/cKit+ cells to 37% ± 3% (Figure 4A). We observed robust expression of SOX10, KIT, MITF-M, and DCT within the GFP+/cKit+ population under both NC (Figure 3C) and BE conditions (Figure S5). However, BEderived melanoblasts expressed significantly higher levels of MITF-M and DCT than the same population derived with the NC protocol (Figure 4B), suggesting that treatment with BMP4 and EDN3 not only enhances melanoblast yield but also further drives their maturation. A comparison of the relative proportions of Sox10::GFP and KIT expressing populations following NC and BE treatment revealed that BE conditions increased the yield of double positive melanocyte progenitors, while the percentage of Sox10::GFP single positive NC was reduced (Figure 4C). The percentage of double negative (GFP−/cKit−) however remained unchanged. This change in population makeup could be due to a direct fate switch within the existing NC population. However, we cannot rule out that increased proliferation of the existing melanoblasts, or inhibition of non-melanocyte NC contribute to the BE-mediated increase in melanocyte precursors. Comparative global gene expression profiling of cell populations derived with the NC and BE protocols confirmed the enhanced expression of various melanocyte associated markers, including TYRP1, MLANA, DCT, TYR, and SILV, following treatment with BE (Figures 4D, S5). In contrast, NC-derived populations displayed a gene expression profile associated with sensory and nociceptor neuron fates (Chambers et al., 2012). BE treatment also induced substantial upregulation of COL3A1 and CRYAA (Figure 4D). Our group has previously observed transient expression of COL3A1, and several crystallin transcripts in neural rosette stage cells (Elkabetz et al., 2008). However, future studies are required to address whether COL3A1 and CRYAA are expressed in NC stage melanocyte precursors or represent contamination with another cell type induced under BE conditions, as those gene expression analyses were performed on unpurified BE bulk populations. BE-derived Sox10::GFP cells that co-expressed MITF exhibited an elongated, spindle-like morphology compatible with the appearance of melanocyte progenitors (Figure 4E).
Figure 4. Treatment with BMP4 and EDN3 enhances melanoblast specification.
(A) Additional treatment with BMP4 and EDN3 (BE) beginning at day 6 significantly enhances the yield of Sox10::GFP, c-kit double positive melanocyte progenitors (n=4, p=0.0005). (B) BE-derived melanocyte progenitors express significantly higher levels of MITF and DCT than NC-derived cells by qRT-PCR (n=3, p=0.03(MITF), p=0.05(DCT)). (C) BE treatment increases the yield of Sox10::GFP, c-kit double positive melanocyte progenitors at the expense of Sox10::GFP single positive NC cells. (D) Comparative gene expression analysis identified significantly up- (red) and downregulated (orange) genes at day 11 in BE-induced cells compared to NC cells. Bold genes represent top 10 most differentially regulated genes. (E) BE-derived melanocyte progenitors exhibit spindle-like melanocyte morphology. Scale bars represent 50µm. All error bars represent the s.e.m of at least three independent experiments. * p<0.05. See also Figure S5.
Maturation and Characterization of Melanocytes
Melanoblast progenitors were isolated on day 11 based on Sox10::GFP and KIT expression and replated in maturation media (Figure 5A). We next established defined culture conditions for melanocyte maturation. A previous melanocyte differentiation medium was based on the use of conditioned media from a WNT3A producing murine cell line (Fang et al., 2006). While we found the same media to be sufficient for maturation of BE-derived melanoblasts to a pigmented, terminally differentiated state (Figure S6), we developed defined maturation conditions using a medium containing SCF, EDN3, FGF2, Chir, cyclic AMP, BMP4, and B27 (Figure S6). Under these conditions, pigmented cells were first observed by brightfield microscopy six days after sorting and co-expressed the melanocyte marker MITF (Figure 5B). By day 20 of differentiation, most clusters were pigmented (Figure 5C) and cell pellets became darkly colored by passage five (Figure 5D). Extended culture supported the propagation of an essentially pure population of melanocytes expressing typical melanocyte transcription factors such as MITF and SOX10 and melanosomal markers including tyrosinase-related protein 1 (TYRP1) and premelanosome protein (PMEL) (Figure 5E, S6). A comparison of gene expression profiles of mature melanocytes and day 11 melanoblast progenitors revealed that maturation had supported the induction of several late stage melanocyte markers including oculocutaneous albinism II (OCA2), tyrosinase (TYR), and melan-A (MLANA), while NC markers such as TFAP2C, EDNRA and EDN1 had been downregulated (Figure 5F).
Electron microscopy of human ES-derived melanocytes (ES-melanocytes) revealed the presence of numerous pigmented melanosomes, representing all four stages of melanosome maturation (Figure 5G). In primary skin, melanocytes reside at the basement membrane at the interface of the epidermal and dermal layers. In an organotypic skin reconstruct assay in which melanocytes and keratinocytes were seeded onto a preformed layer of fibroblasts and collagen, ES-melanocytes phenocopied the localization of primary control melanocytes and correctly homed to the basement membrane (Figure 5H). Gene expression profiles of ES-melanocytes were more similar to adult than neonatal primary melanocytes (Figure S6), although ES-melanocytes differed from both populations in their lack of Hox gene expression. It is possible that patterning conditions for BE-derived melanocytes specify a more anterior population of melanocytes as compared to primary cells isolated from posterior regions such as breast, abdominal, and foreskin tissues. Caudal melanocytes expressing HoxB2 and HoxB4 could however be established from precursors treated with RA during the early stages of differentiation (Figure S6). ES-derived melanocytes also uniquely upregulated genes associated with protein localization and transport (Figure S6) perhaps reflecting the increased melanin production observed when compared to primary melanocytes.
Temporal analysis of Sox10::GFP and KIT expression during NC induction showed that Sox10::GFP expressing cells could be detected by day 6 of differentiation (Figure 1D) while Sox10::GFP, c-kit coexpressing cells did not emerge before day 10 (Figure S4). To test the competency of Sox10 positive cells to give rise to c-kit+ melanoblasts, we isolated Sox10::GFP and KIT single positive cells (GFP+/cKit− and GFP−/cKit+, respectively) as well as double positive and double negative cells, by flow cytometry and replated each in maturation media. Cells were again assayed for Sox10::GFP and c-kit expression after 5 and 12 days. As predicted, GFP+/cKit− cells gave rise to GFP+/cKit+ and GFP−/cKit+ progeny, while GFP- cells lacked this plasticity (Figure S5). More importantly, GFP+/cKit− and GFP- /cKit+ populations eventually differentiated into MITF expressing pigmented cells (Figure S5), although GFP+/cKit− cells did so with delayed kinetics. Meanwhile the GFP−/cKit− population never gave rise to pigmented cells and could not be propagated in our maturation media. These data illustrate that, even without sorting for SOX10 or KIT, BE-treated cells can efficiently generate melanocytes using our defined maturation conditions, greatly facilitating studies in hESC lacking a SOX10 reporter or in disease specific iPSCs.
Modeling Melanosome Formation and Trafficking Defects Using iPS-Derived Melanocytes
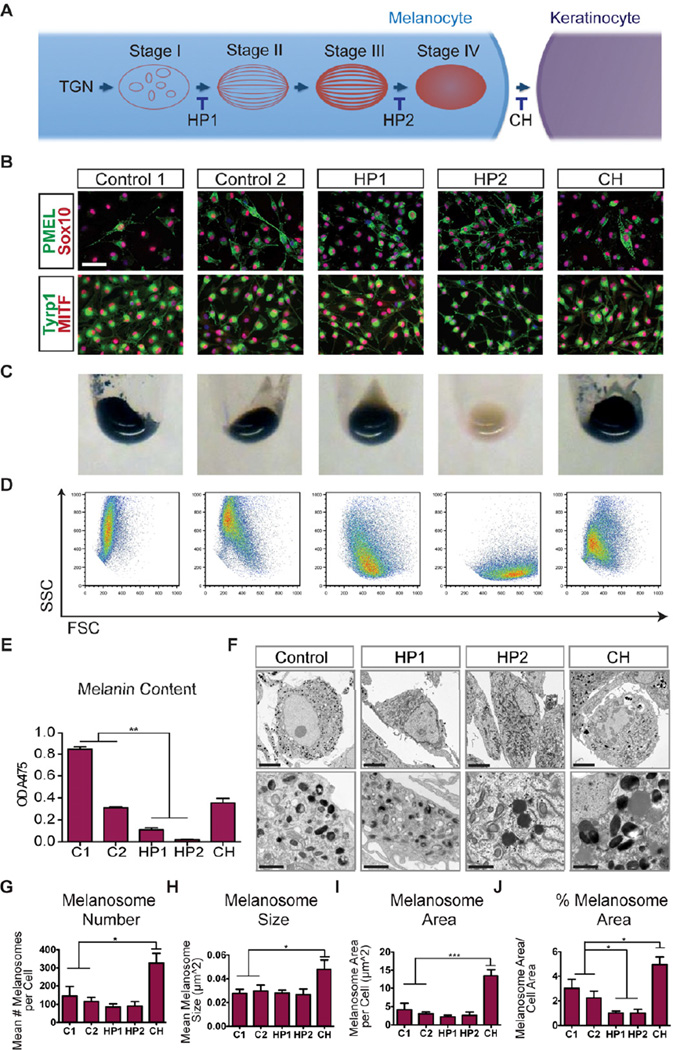
We next determined whether pluripotent stem cell-derived melanocytes could be used to model genetic pigmentation defects using induced pluripotent stem cells (iPSCs). We selected two subgroups of patients with defects in melanosome vesicle formation and trafficking: Hermansky-Pudlak Syndrome (HP) encompasses a collection of eight autosomal recessive disorders defined by deficiencies in the ACCEPTED MANUSCRIPT biogenesis of lysosome-related organelles, including melanosomes (Oh et al., 1996; Wei, 2006) (Figure 6A). Patients present with a range of symptoms including oculocutaneous albinism, platelet storage disease, and, depending on disease subtype, with immune deficiency and pulmonary fibrosis. One of the patients used in this study had a mutation in the HPS1 gene (HP1) while the other patient was deficient in AP3B1 (HP2) (Figure S7). Chediak-Higashi Syndrome (CH) is caused by a mutation in the lysosomal trafficking regulator (LYST) gene which results in the persistence of abnormally large lysosome-related organelles (Karim et al., 1997; Nagle et al., 1996) (Figure S7). Due to their enlarged size, melanosomes in CH patients cannot be efficiently transferred to neighboring keratinocytes which leads to hypopigmentation (Introne et al., 1999) (Figure 6A).
Figure 6. iPS-derived melanocytes recapitulate disease-associated pigmentation defects.
(A) Hermansky-Pudlak Syndrome (HP) and Chediak Higashi (CH) Syndrome are disorders with defects in melanosome biogenesis and trafficking amenable for iPS-based disease modeling. (B) Melanocytes derived from patient-specific and control (C1, C2) iPSCs express melanocyte-associated transcription factors and melanosomal proteins. Scale bar represents 50µm. (C) Cell pellets of HP2-derived melanocytes exhibit a near lack of pigmentation while HP1-derived melanocytes exhibit a more subtle defect. (D) Pigmentation levels of patient-specific melanocytes are correlated to granularity (SSC) in flow cytometric analysis. (E) Melanin content was determined from the absorbance of cell lysates at 475nm. (F) HP1- and HP2-associated pigmentation defects can be observed in electron micrographs while CH-derived melanocytes exhibit disease-typical enlarged melanosomes. Scale bars represent 5µm (top row) and 1µm (bottom row). (G–J) Stereological quantification of melanosome phenotype observed in electron micrographs. Error bars represent the s.e.m. from melanocytes derived from three independent iPS lines for each disease (2 lines were derived from donor C2). See also Figure S7.
Three independent iPS lines were established from each patient and control fibroblast donor using a single polycistronic lentiviral vector expressing Oct4, Klf4, Sox2, and c-Myc (Papapetrou et al., 2011) (Table S2). The resulting iPSCs exhibited hESC-like morphology and expressed the pluripotent transcription factors Oct4 and Nanog as well as cell surface markers Tra-1-60, SSEA3, and SSEA4 (Figure S7). Mature melanocytes expressing PMEL, SOX10, TYRP1, and MITF were derived at comparable efficiencies from each of the patient and control iPSC clones using the BE differentiation protocol (Figure 6B, S7) further illustrating the robustness of our differentiation protocol. Due to the selective growth of melanocytes in our maturation media, it was not necessary to select cells for KIT or SOX10 expression at the melanoblast stage to obtain essentially pure populations of iPSC-derived melanocytes (Figure S5). All HP2 iPS-derived melanocytes exhibited a near complete loss of pigmentation both at the macroscopic level and when quantified after cell lysis, while HP1 melanocytes exhibited a more subtle phenotype detectable only after quantification (Figures 6C, D). CH melanocytes exhibited pigmentation levels lower than those observed with an African American control (Control 1) but comparable to a Caucasian control (Control 2) (Figures 6C, E), compatible with the hypothesis that CH affects melanosome transfer rather than production. Differences in pigmentation could also be assayed by FACS analysis, with higher levels of pigmentation correlating to higher side scatter (SSC) values (Figure 6D, S7). All disease-related phenotypes were consistent among melanocytes derived from each of three independently derived iPS clones, indicating that disease behavior reflects genome-specific differences rather than iPS clonal variability.
The nature of the individual pigmentation defects could be further analyzed at the EM level, with HP1 and HP2-derived melanocytes exhibiting a notable reduction in mature melanosomes (Figure 6F) as quantified using computer-assisted image analysis (Figure 6G–J). There was a significant decrease in the ratio of melanosome to total cell area when compared to both C1 and C2 controls (Figure 6F,J). At higher magnification numerous dark punctae that may correspond to free melanin in the cytoplasm were also discernible in HP2 cells (Figure 6F). In contrast, CH melanocytes exhibited the most striking phenotype with significant increases in both melanosome number and size (Figures 6G–J). Increased melanosome number and size could be further corroborated by immunofluorescence staining for the early melanosomal marker PMEL (Figure 6B). High magnification EM analysis revealed that CH-derived melanocytes exhibit mature but strikingly enlarged melanosomes not seen in the melanocytes derived from any of the other disease or control lines (Figure 6F). Global gene expression analyses in which control- and disease-derived melanocytes were compared revealed few differences at the transcript level (Figure S7). This likely reflects the biological nature of the diseases in which downstream processes are disrupted with little opportunity for transcriptional feedback. These data also again highlight the robust nature of our differentiation protocol in which clones derived independently from control- and disease lines clustered together with high degrees of similarity.
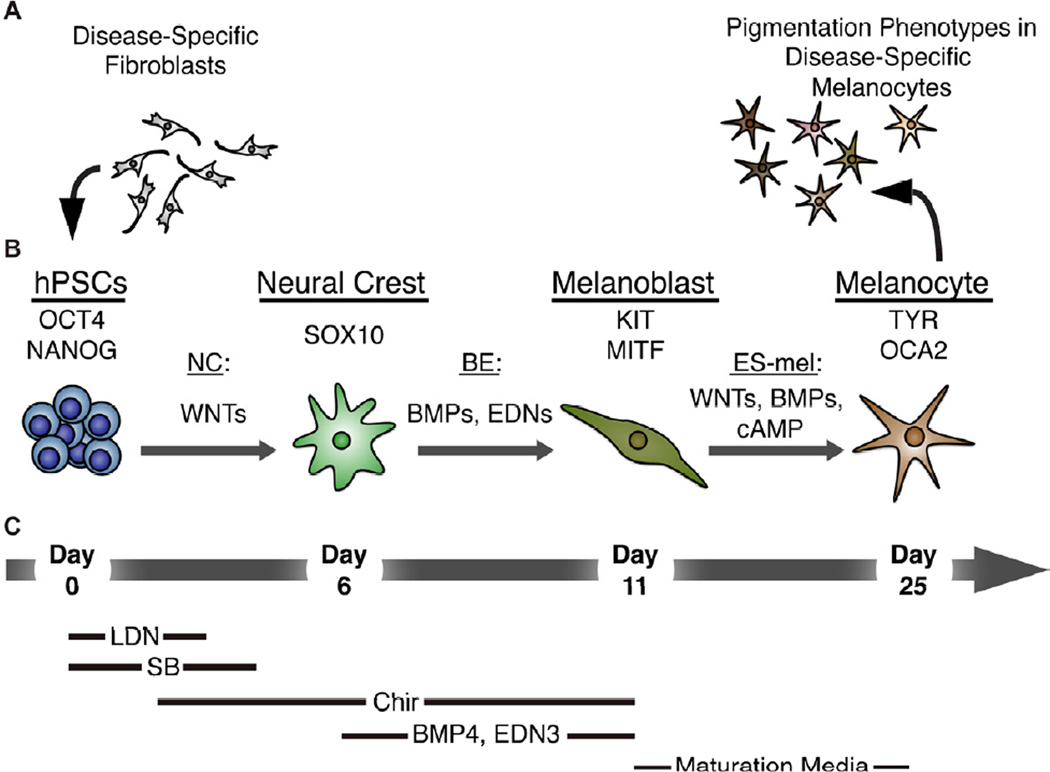
Taken together, our data establish the derivation of melanocytes from human pluripotent stem cells as a robust protocol to study lineage specification of neural crest and melanocyte lineages and as a platform to study pigmentation related disorders in patient specific pluripotent stem cells (Figure 7).
Figure 7. Disease-specific melanocytes that faithfully recapitulate pigmentation defects can be derived from human pluripotent stem cells using a stepwise differentiation paradigm.
(A) Disease specific fibroblasts from HP- and CH- donors were reprogrammed to establish hPSCs. (B) Exposure of Oct4 and Nanog expressing hPSCs to the WNT-activating NC protocol resulted in the emergence of a Sox10-positive NC population by day 6. Subsequent additional treatment with BMP4 and EDN3 (BE) skewed the specification along the melanocytic lineage to allow for the establishment of a melanoblast progenitor population expressing KIT and MITF at day 11. Further maturation under ES-melanocyte (ES-mel) conditions in the presence of WNTs, BMP4, and cAMP supported the induction of late melanocyte markers tyrosinase (TYR) and oculocutaneous albinism II (OCA2). Mature melanocytes were used to model the disease-specific pigmentation defects of HP and CH. (C) Growth conditions supporting each stage of differentiation are summarized below.
DISCUSSION
The specification of NC during mouse and chick development is dependent on both WNT and intermediate levels of BMP signaling generated by neighboring non-neural ectoderm and mesodermal tissues (García-Castro et al., 2002; Kléber et al., 2005; LaBonne and Bronner-Fraser, 1998; Marchant et al., 1998; Patthey et al., 2008). The requirement for BMP signaling is however preceded in Xenopus models by an early need for BMP inhibition that promotes robust acquisition of neural plate identity that establishes the competence to respond to subsequent NC inductive cues (LaBonne and Bronner- Fraser, 1998; Steventon et al., 2009). While we were able to confirm a similar requirement for early BMP inhibition in the induction of human NC, we surprisingly did not find that continued BMP inhibition negatively impacted NC induction even though studies in chick and xenopus embryos have implicated BMP signaling during both NC induction and maintenance (Kléber et al., 2005; Marchant et al., 1998; Selleck et al., 1998). Our data are also distinct from previous observations in p75/HNK-1 expressing NC precursors derived from hESC via a rosette intermediate (Lee et al., 2007). Under those conditions there was a significant decrease in the percentage of putative NC precursors following treatment with the BMP inhibitor noggin and an increase following exposure to BMP4. These discrepancies may reflect inherent differences in rosette- versus non-rosette derived NC, with the former reflecting the stage of NC delamination whereas the accelerated NC derivation conditions presented here may represent an early NC induction stage prior to the presence of a distinct neuroepithelial intermediate. Alternatively, as Sox10::GFP expression and p75/HNK-1 coexpression appear to identify overlapping but distinct NC populations, it is possible that these two subtypes of NC may exhibit different requirements for BMP signaling. It should be noted that a recent immunohistochemical study of Carnegie Stage 12 to 18 human embryos found that HNK-1 only labeled a small subset of migrating NC cells, while p75 expression also broadly labeled non-NC populations (Betters et al., 2010). Future studies will be required to dissect the exact contribution of BMP pathway manipulations during hESC differentiation to NC and to define whether differential requirements reflect distinct developmental NC stages or subtypes.
The use of Chir in our protocol offers a simple and cost-effective strategy to activate WNT signaling during NC induction. While we were able to demonstrate robust induction of Sox10::GFP upon exposure to high doses of WNT3a (Figure S1), the concentrations of WNT3a required were costprohibitive and not practical for routine NC induction. Chir treatment triggers rapid activation of WNT signaling pathway molecules and robust induction of a TCF::luciferase reporter (Figure S1). The critical role for WNT signaling in our NC induction conditions is consistent with findings in avian systems which have demonstrated that WNT signaling is necessary and sufficient for NC induction (García-Castro et al., 2002). Similar requirements for WNT signaling for the induction of a p75, HNK-1 co-expressing human NC population have also been reported (Lee et al., 2007; Menendez et al., 2011). Our study is however unique in addressing the temporal requirements for WNT signaling during human NC specification. The very narrow window during which WNT signaling promoted Sox10::GFP induction was unexpected and offers a powerful tool to mechanistically define competency factors acting together with WNT in NC specification. One interesting hypothesis is that loss of competency correlates with the time course of Dkk1 induction and anterior CNS fate specification observed during DSi (Fasano et al., 2010). This is supported by our own observations that the negative WNT regulator SIX3 continues to be expressed when Chir treatment is not initiated until day 4. It is tempting to speculate that knockdown of SIX3 may restore NC-competence to cells at this late timepoint. Furthermore, it has recently been reported that sequential activation of WNT signaling in the mouse embryo affects NC lineage specification, particularly along sensory neuronal and melanocyte lineages (Hari et al., 2012). It will be interesting to define whether SOX10::GFP NC cells induced following a Chir pulse treatment are functionally distinct from those generated upon long-term Chir treatment, particularly in conditions when endogenous WNT signaling was further suppressed by treatment with XAV.
Our study has also established a readily scalable, defined protocol that generates pure populations of mature melanocyte with great efficiency. Our cells recapitulate all structural features of wild-type melanocytes, making them suitable for a wide range of applications and future studies. In particular, the remarkable fidelity of our cells in capturing the different levels of pigmentation in Caucasian- and African American-derived melanocytes highlights the potential utility of these cells in other fields including the cosmetic industry. Furthermore, to our knowledge this is the first protocol that allows for the stage-specific isolation of melanocyte progenitors and mature melanocytes. This will be of particular interest in studies investigating melanocyte development and malignant transformation. We also demonstrate that the axial levels of NC and melanocyte precursor populations can be readily patterned using caudalizing FGF and RA cues. The ability to derive Hox-positive versus Hox-negative human NC cells is particularly intriguing as it will enable studies on the unique plasticity reported for Hox-negative NC in the avian system (Le Douarin et al., 2004).
A recent study has also indentified Schwann cells as an additional source of melanocytes (Adameyko et al., 2009) while EDN3 has been shown to induce the reversion of melanocytes to a bipotent progenitor (Dupin et al., 2000). It will be interesting to further explore this close association between glial and melanocyte fates although the reported expression of Schwann cell associated markers in murine melanoblasts (Colombo et al., 2011) highlights the need for a thorough and careful analysis to differentiate between the origin of melanocytes from a bipotent glial-melanocyte progenitor and the derivation of melanocytes from Schwann cell precursors.
The derivation of pluripotent stem cells from human fibroblasts (Park et al., 2008a; Takahashi et al., 2007; Yu et al., 2007) has been successfully applied for modeling human disease (Dimos et al., 2008; Ebert et al., 2009; Park et al., 2008b), including disorders of the neural crest such as familial dysautonomia, (Lee et al., 2009). In the current study we present the first example of modeling melanocyte-specific disorders, deriving patient-specific iPSCs from three distinct genetic syndromes. In each case we were able to successfully generate patient specific melanocytes and to define pigmentation defects characteristic of each disorder in vitro. Previous iPS-based disease modeling studies have suffered greatly from variability observed between iPS lines. Therefore the unusual degree of fidelity observed between melanocyte clones derived from multiple iPS lines illustrates the highly robust nature of our melanocyte induction protocol. This establishes the framework for high throughput screening studies to further explore disease biology or to identify novel candidate drugs affecting melanocytes specification or levels of pigmentation. A particularly attractive strategy for the future might be the use of disease-specific melanocytes in the identification of drugs that reverse easily screened pigmentation defects associated with HP1 as a surrogate assay for compounds that may also correct lysosomal function in other disease relevant cell types such as pulmonary pneumocytes.
Our study has offered novel insights into the precise signaling requirements for early neural crest induction, melanocyte lineage specification, and melanocyte maturation. These can now be harnessed to establish unlimited numbers of melanocytes suitable for a broad range of applications including new model system for the study of melanocyte pathologies and malignant melanoma origination.
EXPERIMENTAL PROCEDURES
Neural Crest and Melanocyte Differentiation
Neural induction using the dual SMAD inhibition protocol was performed as previously described (Chambers et al., 2009; Chambers et al., 2011; Lee et al., 2010). Briefly, dissociated hESCs and iPSCs were plated on matrigel at a density of 18,000–25,000 cells/cm2 in MEF-conditioned hES medium containing 10 ng/ml FGF2 and 10µM ROCK-inhibitor (Y-27632). Cells were allowed to reach 70–80% confluence over 3 days. Differentiation was initiated by switching to a media that included knockout serum replacement media with 500nM LDN193189 (Stemgent) and 10µM SB431542 (Tocris). Beginning at day 4 the knockout serum replacement media was gradually replaced with increasing amounts of N2 media. For NC differentiation, the DSi protocol was adapted by treating with 3µM CHIR99021 (Stemgent) beginning day 2 and withdrawing LDN193189 and SB431542 at days 3 and 4 respectively. BE-derived cells were additionally treated with 25ng/ml BMP4 and 100nM EDN3 beginning at day 6 of differentiation. Cells were collected on day 11 for analysis or passaging.
Melanocyte Maturation and Maintenance
Day 11 dissociated BE-derived cells were replated on poly-ornithine, laminin, and fibronectin coated plates in NB/Mel media. NB/Mel media was prepared by replacing the WNT3A-CM component in Mel-1 media (Fang et al., 2006) with Neurobasal (Invitrogen) medium containing 2% B27 supplement (Invitrogen), 3µM Chir, 25 ng/ml BMP4, and 500 µM dbcAMP. Cells were fed every other day and passaged weekly for maintenance and expansion.
Pigmentation Quantification
Melanin content was quantified using previously described methods (Friedmann and Gilchrest, 1987; Wen-Jun et al., 2008). Briefly, 2.5×105 melanocytes were collected and pelleted at 16,000xg for 30 seconds. Cells were washed twice with PBS and then dissolved in 250µl 1M NaOH for 40 minutes at 37°C. 100 µl of cell lysate were transferred in duplicate to 96-well plates and the OD475 measured using a PerkinElmer EnSpire plate reader.
Organotypic Skin Reconstruct
Organotypic skin reconstructs were established as previously described (Meier et al., 2000). Briefly, an artificial dermal layer was established by seeding human dermal fibroblasts (Invitrogen) in collagen in transwell permeable supports (Corning). After several days, human epidermal keratinocytes and primary (control) or ES-derived melanocytes were introduced on top of the dermal layer and allowed to expand and stratify at the air-liquid interphase for an additional two weeks. Skin reconstructs were collected, fixed in 10% formalin and embedded in paraffin for sectioning and immunohistochemical processing.
Supplementary Material
HIGHLIGHTS.
Narrow window of WNT activation efficiently induces neural crest from hESCs
BMP and EDN3 bias NC fate towards KIT+ melanocyte lineage
Melanocytes are functional and recreate different human pigmentation levels
Melanocytes from patient-specific iPSCs model ultrastructural disease features
ACKNOWLEDGEMENTS
We would like to thank J. Hendrikx (SKI Flow Cytometry Core), A. Viale (SKI Genomics Core), K. Manova-Todorova (SKI Molecular Cytology Core) and the Rockefeller University Electron Microscopy Resource Center for excellent technical support. This work was supported in part by CO26446 and CO26447 from NYSTEM to L.S and C026399 to SMC. Y.M. was supported by an award from the Joanna M. Nicolay Melanoma Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession code for gene expression data. GEO: xxxx.
REFERENCES
- Adameyko I, Lallemend F, Aquino JB, Pereira JA, Topilko P, Müller T, Fritz N, Beljajeva A, Mochii M, Liste I, et al. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell. 2009;139:366–379. doi: 10.1016/j.cell.2009.07.049. [DOI] [PubMed] [Google Scholar]
- Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Betters E, Liu Y, Kjaeldgaard A, Sundström E, García-Castro MI. Analysis of early human neural crest development. Developmental biology. 2010;344:578–592. doi: 10.1016/j.ydbio.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun MM, Etheridge A, Bernard A, Robertson CP, Roelink H. Wnt signaling is required at distinct stages of development for the induction of the posterior forebrain. Development. 2003;130:5579–5587. doi: 10.1242/dev.00685. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Developmental biology. 1986;115:44–55. doi: 10.1016/0012-1606(86)90226-5. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Mica Y, Studer L, Tomishima MJ. Converting human pluripotent stem cells to neural tissue and neurons to model neurodegeneration. Methods Mol Biol. 2011;793:87–97. doi: 10.1007/978-1-61779-328-8_6. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Qi Y, Mica Y, Lee G, Zhang X-J, Niu L, Bilsland J, Cao L, Stevens E, Whiting P, et al. Combined small molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol. 2012 doi: 10.1038/nbt.2249. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo S, Champeval D, Rambow F, Larue L. Transcriptomic Analysis of Mouse Embryonic Skin Cells Reveals Previously Unreported Genes Expressed in Melanoblasts. J Invest Dermatol. 2011 doi: 10.1038/jid.2011.252. [DOI] [PubMed] [Google Scholar]
- Creuzet S, Couly G, Le Douarin NM. Patterning the neural crest derivatives during development of the vertebrate head: insights from avian studies. J Anat. 2005;207:447–459. doi: 10.1111/j.1469-7580.2005.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimos J, Rodolfa K, Niakan K, Weisenthal L, Mitsumoto H, Chung W, Croft G, Saphier G, Leibel R, Goland R, et al. Induced Pluripotent Stem Cells Generated from Patients with ALS Can Be Differentiated into Motor Neurons. Science. 2008 doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Dunn KJ, Williams BO, Li Y, Pavan WJ. Neural crest-directed gene transfer demonstrates Wnt1 role in melanocyte expansion and differentiation during mouse development. Proc Natl Acad Sci USA. 2000;97:10050–10055. doi: 10.1073/pnas.97.18.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin E, Glavieux C, Vaigot P, Le Douarin NM. Endothelin 3 induces the reversion of melanocytes to glia through a neural crest-derived glial-melanocytic progenitor. Proc Natl Acad Sci USA. 2000;97:7882–7887. doi: 10.1073/pnas.97.14.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin E, Le Douarin NM. Development of melanocyte precursors from the vertebrate neural crest. Oncogene. 2003;22:3016–3023. doi: 10.1038/sj.onc.1206460. [DOI] [PubMed] [Google Scholar]
- Ebert AD, Yu J, Rose FF, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkabetz Y, Panagiotakos G, Al Shamy G, Socci ND, Tabar V, Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008;22:152–165. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson CA, Goins TL. Avian neural crest cells can migrate in the dorsolateral path only if they are specified as melanocytes. Development. 1995;121:915–924. doi: 10.1242/dev.121.3.915. [DOI] [PubMed] [Google Scholar]
- Fang D, Leishear K, Nguyen TK, Finko R, Cai K, Fukunaga M, Li L, Brafford PA, Kulp AN, Xu X, et al. Defining the conditions for the generation of melanocytes from human embryonic stem cells. Stem Cells. 2006;24:1668–1677. doi: 10.1634/stemcells.2005-0414. [DOI] [PubMed] [Google Scholar]
- Fasano CA, Chambers SM, Lee G, Tomishima MJ, Studer L. Efficient derivation of functional floor plate tissue from human embryonic stem cells. Cell Stem Cell. 2010;6:336–347. doi: 10.1016/j.stem.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Friedmann PS, Gilchrest BA. Ultraviolet radiation directly induces pigment production by cultured human melanocytes. J Cell Physiol. 1987;133:88–94. doi: 10.1002/jcp.1041330111. [DOI] [PubMed] [Google Scholar]
- Fujimura N, Vacik T, Machon O, Vlcek C, Scalabrin S, Speth M, Diep D, Krauss S, Kozmik Z. Wnt-mediated down-regulation of Sp1 target genes by a transcriptional repressor Sp5. The Journal of biological chemistry. 2007;282:1225–1237. doi: 10.1074/jbc.M605851200. [DOI] [PubMed] [Google Scholar]
- García-Castro MI, Marcelle C, Bronner-Fraser M. Ectodermal Wnt function as a neural crest inducer. Science. 2002;297:848–851. doi: 10.1126/science.1070824. [DOI] [PubMed] [Google Scholar]
- Hari L, Miescher I, Shakhova O, Suter U, Chin L, Taketo M, Richardson WD, Kessaris N, Sommer L. Temporal control of neural crest lineage generation by Wnt/β-catenin signaling. Development. 2012 doi: 10.1242/dev.073064. [DOI] [PubMed] [Google Scholar]
- Huizing M, Scher CD, Strovel E, Fitzpatrick DL, Hartnell LM, Anikster Y, Gahl WA. Nonsense mutations in ADTB3A cause complete deficiency of the beta3A subunit of adaptor complex-3 and severe Hermansky-Pudlak syndrome type 2. Pediatr Res. 2002;51:150–158. doi: 10.1203/00006450-200202000-00006. [DOI] [PubMed] [Google Scholar]
- Introne W, Boissy RE, Gahl WA. Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol Genet Metab. 1999;68:283–303. doi: 10.1006/mgme.1999.2927. [DOI] [PubMed] [Google Scholar]
- Karim MA, Nagle DL, Kandil HH, Bürger J, Moore KJ, Spritz RA. Mutations in the Chediak-Higashi syndrome gene (CHS1) indicate requirement for the complete 3801 amino acid CHS protein. Hum Mol Genet. 1997;6:1087–1089. doi: 10.1093/hmg/6.7.1087. [DOI] [PubMed] [Google Scholar]
- Kléber M, Lee H-Y, Wurdak H, Buchstaller J, Riccomagno MM, Ittner LM, Suter U, Epstein DJ, Sommer L. Neural crest stem cell maintenance by combinatorial Wnt and BMP signaling. The Journal of Cell Biology. 2005;169:309–320. doi: 10.1083/jcb.200411095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriks S, Shim J-W, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisada T, Yoshida H, Yamazaki H, Miyamoto A, Hemmi H, Nishimura E, Shultz LD, Nishikawa S, Hayashi S. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development. 1998;125:2915–2923. doi: 10.1242/dev.125.15.2915. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Neural crest induction in Xenopus: evidence for a twosignal model. Development. 1998;125:2403–2414. doi: 10.1242/dev.125.13.2403. [DOI] [PubMed] [Google Scholar]
- Lagutin OV, Zhu CC, Kobayashi D, Topczewski J, Shimamura K, Puelles L, Russell HRC, McKinnon PJ, Solnica-Krezel L, Oliver G. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 2003;17:368–379. doi: 10.1101/gad.1059403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav R, Ziller C, Dupin E, Le Douarin NM. Endothelin 3 promotes neural crest cell proliferation and mediates a vast increase in melanocyte number in culture. Proc Natl Acad Sci USA. 1996;93:3892–3897. doi: 10.1073/pnas.93.9.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A, Lagutin OV, Oliver G. Six3 inactivation causes progressive caudalization and aberrant patterning of the mammalian diencephalon. Development. 2008;135:441–450. doi: 10.1242/dev.010082. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- Lee G, Chambers SM, Tomishima MJ, Studer L. Derivation of neural crest cells from human pluripotent stem cells. Nat Protoc. 2010;5:688–701. doi: 10.1038/nprot.2010.35. [DOI] [PubMed] [Google Scholar]
- Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, Tabar V, Studer L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468–1475. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- Lee G, Papapetrou EP, Kim H, Chambers SM, Tomishima MJ, Fasano CA, Ganat YM, Menon J, Shimizu F, Viale A, et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461:402–406. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R, Gao J, Wehrle-Haller B, Henion PD. Molecular identification of distinct neurogenic and melanogenic neural crest sublineages. Development. 2003;130:321–330. doi: 10.1242/dev.00213. [DOI] [PubMed] [Google Scholar]
- Marchant L, Linker C, Ruiz P, Guerrero N, Mayor R. The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev Biol. 1998;198:319–329. [PubMed] [Google Scholar]
- Meier F, Nesbit M, Hsu MY, Martin B, Van Belle P, Elder DE, Schaumburg-Lever G, Garbe C, Walz TM, Donatien P, et al. Human melanoma progression in skin reconstructs : biological significance of bFGF. The American Journal of Pathology. 2000;156:193–200. doi: 10.1016/S0002-9440(10)64719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L, Flajolet M, Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol Sci. 2004;25:471–480. doi: 10.1016/j.tips.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Menendez L, Yatskievych TA, Antin PB, Dalton S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19240–19245. doi: 10.1073/pnas.1113746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle DL, Karim MA, Woolf EA, Holmgren L, Bork P, Misumi DJ, McGrail SH, Dussault BJ, Perou CM, Boissy RE, et al. Identification and mutation analysis of the complete gene for Chediak-Higashi syndrome. Nat Genet. 1996;14:307–311. doi: 10.1038/ng1196-307. [DOI] [PubMed] [Google Scholar]
- Nissan X, Larribere L, Saidani M, Hurbain I, Delevoye C, Feteira J, Lemaitre G, Peschanski M, Baldeschi C. Functional melanocytes derived from human pluripotent stem cells engraft into pluristratified epidermis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14861–14866. doi: 10.1073/pnas.1019070108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Bailin T, Fukai K, Feng GH, Ho L, Mao JI, Frenk E, Tamura N, Spritz RA. Positional cloning of a gene for Hermansky-Pudlak syndrome, a disorder of cytoplasmic organelles. Nat Genet. 1996;14:300–306. doi: 10.1038/ng1196-300. [DOI] [PubMed] [Google Scholar]
- Papapetrou EP, Lee G, Malani N, Setty M, Riviere I, Tirunagari LMS, Kadota K, Roth SL, Giardina P, Viale A, et al. Genomic safe harbors permit high β-globin transgene expression in thalassemia induced pluripotent stem cells. Nat Biotechnol. 2011;29:73–78. doi: 10.1038/nbt.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I-H, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008a;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Park I, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch M, Cowan C, Hochedlinger K, Daley G. Disease-Specific Induced Pluripotent Stem Cells. Cell. 2008b doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthey C, Gunhaga L, Edlund T. Early development of the central and peripheral nervous systems is coordinated by Wnt and BMP signals. PLoS ONE. 2008;3:e1625. doi: 10.1371/journal.pone.0001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedy MV, Faraco CD, Erickson CA. The delayed entry of thoracic neural crest cells into the dorsolateral path is a consequence of the late emigration of melanogenic neural crest cells from the neural tube. Dev Biol. 1998;200:234–246. doi: 10.1006/dbio.1998.8963. [DOI] [PubMed] [Google Scholar]
- Reid K, Nishikawa S, Bartlett PF, Murphy M. Steel factor directs melanocyte development in vitro through selective regulation of the number of c-kit+ progenitors. Dev Biol. 1995;169:568–579. doi: 10.1006/dbio.1995.1170. [DOI] [PubMed] [Google Scholar]
- Reid K, Turnley AM, Maxwell GD, Kurihara Y, Kurihara H, Bartlett PF, Murphy M. Multiple roles for endothelin in melanocyte development: regulation of progenitor number and stimulation of differentiation. Development. 1996;122:3911–3919. doi: 10.1242/dev.122.12.3911. [DOI] [PubMed] [Google Scholar]
- Ring DB, Johnson KW, Henriksen EJ, Nuss JM, Goff D, Kinnick TR, Ma ST, Reeder JW, Samuels I, Slabiak T, et al. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes. 2003;52:588–595. doi: 10.2337/diabetes.52.3.588. [DOI] [PubMed] [Google Scholar]
- Selleck MA, García-Castro MI, Artinger KB, Bronner-Fraser M. Effects of Shh and Noggin on neural crest formation demonstrate that BMP is required in the neural tube but not ectoderm. Development. 1998;125:4919–4930. doi: 10.1242/dev.125.24.4919. [DOI] [PubMed] [Google Scholar]
- Steel KP, Davidson DR, Jackson IJ. TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development. 1992;115:1111–1119. doi: 10.1242/dev.115.4.1111. [DOI] [PubMed] [Google Scholar]
- Steventon B, Araya C, Linker C, Kuriyama S, Mayor R. Differential requirements of BMP and Wnt signalling during gastrulation and neurulation define two steps in neural crest induction. Development. 2009;136:771–779. doi: 10.1242/dev.029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takeda K, Yasumoto K, Takada R, Takada S, Watanabe K, Udono T, Saito H, Takahashi K, Shibahara S. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J Biol Chem. 2000;275:14013–14016. doi: 10.1074/jbc.c000113200. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Erickson CA. The making of a melanocyte: the specification of melanoblasts from the neural crest. Pigment cell & melanoma research. 2008;21:598–610. doi: 10.1111/j.1755-148X.2008.00506.x. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Mochii M, Vogel KS, Weston JA. Avian neural crest-derived neurogenic precursors undergo apoptosis on the lateral migration pathway. Development. 1998;125:4205–4213. doi: 10.1242/dev.125.21.4205. [DOI] [PubMed] [Google Scholar]
- Wei ML. Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19:19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Weidinger G, Thorpe CJ, Wuennenberg-Stapleton K, Ngai J, Moon RT. The Sp1-related transcription factors sp5 and sp5-like act downstream of Wnt/beta-catenin signaling in mesoderm and neuroectoderm patterning. Curr Biol. 2005;15:489–500. doi: 10.1016/j.cub.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Wen-Jun L, Hai-Yan W, Wei L, Ke-Yu W, Rui-Ming W. Evidence that geniposide abrogates norepinephrine-induced hypopigmentation by the activation of GLP-1R-dependent c-kit receptor signaling in melanocyte. J Ethnopharmacol. 2008;118:154–158. doi: 10.1016/j.jep.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane T, Hayashi S, Mizoguchi M, Yamazaki H, Kunisada T. Derivation of melanocytes from embryonic stem cells in culture. Dev Dyn. 1999;216:450–458. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<450::AID-DVDY13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Kunisada T, Kusakabe M, Nishikawa S, Nishikawa SI. Distinct stages of melanocyte differentiation revealed by anlaysis of nonuniform pigmentation patterns. Development. 1996;122:1207–1214. doi: 10.1242/dev.122.4.1207. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.