Abstract
Aims
Atrial fibrillation (AF) is the most common sustained arrhythmia. Increased body size has been associated with AF, but the relationship is not well understood. In this study, we examined the effect of increased height on the risk of AF and explore potential mediators and implications for clinical practice.
Methods and results
We examined data from 5860 individuals taking part in the Cardiovascular Health Study, a cohort study of older US adults followed for a median of 13.6 (women) and 10.3 years (men). Multivariate linear models and age-stratified Cox proportional hazards and risk models were used, with focus on the effect of height on both prevalent and incident AF. Among 684 (22.6%) and 568 (27.1%) incident cases in women and men, respectively, greater height was significantly associated with AF risk [hazard ratio (HR)women per 10 cm 1.32, confidence interval (CI) 1.16–1.50, P < 0.0001; HRmen per 10 cm 1.26, CI 1.11–1.44, P < 0.0001]. The association was such that the incremental risk from sex was completely attenuated by the inclusion of height (for men, HR 1.48, CI 1.32–1.65, without height, and HR 0.94, CI 0.85–1.20, with height included). Inclusion of height in the Framingham model for incident AF improved discrimination. In sequential models, however, we found minimal attenuation of the risk estimates for AF with adjustment for left ventricular (LV) mass and left atrial (LA) dimension. The associations of LA and LV size measurements with AF risk were weakened when indexed to height.
Conclusion
Independent from sex, increased height is significantly associated with the risk of AF.
Keywords: Atrial fibrillation, Cardiovascular risk factors, Echocardiography, Risk prediction
Introduction
The prevalence of atrial fibrillation (AF) in the population is increasing.1 It is estimated that 2.3 million adults in the USA currently have AF, and that this will increase to 5.6 million by the year 2050.2 Among the numerous risk factors that have been described for AF,3 one of the more complex and poorly understood is that of body size.
A number of studies have noted the association between AF and body size in a range of populations, including in Japanese patients,4 patients with LV dysfunction,5 Scandinavian men,6 European patients,7 Chinese patients,8 and older adults.9 The challenge in these studies is that in most cases, little distinction is made between increased body size reflected in increased body weight, and often obesity, and increased body frame. Nowhere was the complexity of this distinction more evident than in the recent study by Conen et al.,10 which found that in women over 45 years of age followed for 14.5 years, increased birth weight was significantly associated with the development of incident AF. Interestingly, the effect was attenuated when adult height was included in the model. Other studies have found that height, either in addition to or independent of weight, increases the risk of AF.4,6,7,9 However, despite these findings, few risk scores include height as a risk factor for AF,11 and the epidemiological relationship between height and other AF risk factors has not been well described. Further, recent findings from genome-wide association studies that genes near loci associated with increased AF risk, PITX212 and ZFHX3,13 are also associated with growth pathways10,12,13 imply that height may be a result of a pleotropic process that increases the risk of AF.
In this study, we evaluate the hypothesis that increased height is associated with an increased risk of AF in a well-characterized, community-based cohort of older adults followed for over a decade.
Methods
Population
The design and objectives of the Cardiovascular Health Study have been previously described.14 In brief, the Cardiovascular Health Study (CHS) is a longitudinal study of men and women aged 65 years or older, randomly selected from Medicare lists in Pittsburgh, PA; Forsyth County, NC; Sacramento, CA; and Hagerstown, MD. The original cohort of 5201 participants was enrolled in 1989–90; a second cohort of 687 African-Americans was recruited in 1992–93. Except where specified otherwise, both cohorts were used in this analysis, providing a total of 5888 participants. The institutional review board at each centre approved the study, and each participant gave informed consent.
The baseline examination included a standardized questionnaire assessing a variety of risk factors, including smoking, alcohol intake, history of stroke, coronary heart disease, and heart failure, self-reported health status, and medication use on enrolment. Methods of determining prevalent cardiovascular disease were previously validated by Psaty et al.15 The physical examination included measurements of standing height, weight, and seated blood pressure (measured with a random-zero sphygmomanometer),15 as well as a resting 12-lead electrocardiogram (ECG). Fasting laboratory measurements included total cholesterol, high-density lipoprotein cholesterol, glucose, C-reactive protein, serum creatinine,16 and N-terminal-pro-brain natriuretic peptide (NT-proBNP),17 although NT-proBNP was only available in 3464 individuals in the analytic data set (see below).
Of the initial 5888 individuals in the study population, we excluded 11 who were missing height and 17 participants missing education data, leaving a total of 5860 patients for the analysis of prevalent AF (Table 1). For incident AF, we excluded individuals with prevalent AF (n = 157), as well as individuals who were taking digoxin (n = 387) or had a history of stroke (n = 199) due to concerns of undetected prevalent AF in these individuals, leaving 5117 participants.
Table 1.
Baseline characteristics by height (prevalent analysis group)
| Group | Characteristic | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
|---|---|---|---|---|---|---|
| Women (n = 3378) | Height (cm) | ≤150 | 150–155 | 155–160 | 160–165 | >165 |
| Number | 255 | 667 | 1116 | 820 | 520 | |
| Mean height in cm (SD) | 147 (3) | 153 (1) | 158 (1) | 163 (1) | 169 (3) | |
| Mean age in years (SD) | 75.6 (6.2) | 73.8 (5.7) | 72.6 (5.5) | 71.5 (4.9) | 71.0 (4.5) | |
| Black race (%) | 34 (13) | 110 (17) | 189 (17) | 144 (18) | 102 (20) | |
| Income (% below median)† | 160 (63) | 352 (53) | 549 (49) | 358 (44) | 216 (42) | |
| Education (% HS grad or better)† | 162 (64) | 437 (66) | 770 (69) | 627 (76) | 393 (76) | |
| Diabetes (%) | 34 (13) | 88 (13) | 175 (16) | 104 (13) | 84 (16) | |
| CHD (%) | 41 (16) | 133 (20) | 160 (14) | 123 (15) | 65 (13) | |
| Current smoking (%) | 23 (9) | 79 (12) | 136 (12) | 117 (14) | 67 (13) | |
| Treated HTN (%)* | 189 (74) | 489 (73) | 745 (67) | 501 (61) | 328 (63) | |
| CVA (%) | 11 (4) | 24 (4) | 31 (3) | 21 (3) | 17 (3) | |
| Mean BMI (SD) | 27.4 (5.7) | 27.1 (5.3) | 27.1 (5.3) | 26.5 (5.2) | 26.9 (5.3) | |
| Mean SBP in mmHg (SD) | 140 (22) | 140 (22) | 138 (22) | 134 (22) | 135 (23) | |
| Mean creatinine in mg/dL (SD) | 0.94 (0.35) | 0.92 (0.25) | 0.94 (0.28) | 0.94 (0.26) | 0.94 (0.21) | |
| Mean LA diameter in cm (SD) | 3.83 (0.75) | 3.76 (0.65) | 3.77 (0.64) | 3.76 (0.63) | 3.83 (0.67) | |
| Mean NT-proBNP in pg/dL (SD) | 430 (1420) | 271 (728) | 260 (484) | 199 (370) | 256 (569) | |
| Men (n = 2482) | Height (cm) | ≤165 | 165–170 | 170–175 | 175–180 | >180 |
| Number | 280 | 547 | 734 | 578 | 343 | |
| Mean height in cm (SD) | 162 (3) | 168 (1) | 173 (1) | 177 (1) | 184 (3) | |
| Mean age in years (SD) | 75.7 (6.4) | 74.1 (6.0) | 73.1 (5.4) | 72.5 (5.4) | 71.7 (5.1) | |
| Black race (%) | 37 (13) | 67 (12) | 111 (15) | 82 (14) | 48 (14) | |
| Income (% below median)† | 111 (40) | 192 (35) | 244 (33) | 160 (28) | 77 (22) | |
| Education (% HS grad or better)† | 173 (62) | 378 (69) | 518 (71) | 399 (69) | 274 (80) | |
| Diabetes (%) | 50 (18) | 98 (18) | 131 (18) | 124 (21) | 72 (21) | |
| CHD (%) | 78 (28) | 152 (28) | 166 (23) | 144 (25) | 74 (22) | |
| Current smoking (%) | 25 (9) | 69 (13) | 73 (10) | 68 (12) | 37 (11) | |
| Treated HTN (%)* | 195 (70) | 382 (70) | 474 (65) | 359 (62) | 202 (59) | |
| CVA (%) | 22 (8) | 35 (6) | 43 (6) | 23 (4) | 16 (5) | |
| Mean BMI (SD) | 26.6 (4.0) | 26.8 (4.1) | 26.4 (3.6) | 26.3 (3.6) | 26.3 (4.0) | |
| Mean SBP in mmHg (SD) | 140 (23) | 137 (21) | 137 (21) | 134 (22) | 132 (19) | |
| Mean creatinine in mg/dL (SD) | 1.23 (0.33) | 1.24 (0.33) | 1.25 (0.32) | 1.23 (0.29) | 1.19 (0.25) | |
| Mean LA diameter in cm (SD) | 3.96 (0.69) | 4.07 (0.71) | 4.03 (0.66) | 4.04 (0.66) | 4.10 (0.70) | |
| Mean NT-proBNP in pg/dL (SD) | 334 (596) | 392 (871) | 348 (1144) | 291 (752) | 246 (452) |
All tests of heterogeneity had 4 degrees of freedom.
*P < 0.005 (χ2 test).
†P < 0.0001 (χ2 test).
For substudies that included echocardiography variables, we could only include the original cohort of 5201 participants as the subsequent African-American cohort did not undergo echocardiography measurements during initial examination. For these studies, we excluded 281 people who were missing major echocardiographic measurements. In addition to 130 participants who had prevalent AF and 180 with a history of stroke, we excluded 130 participants due to the presence of mitral stenos is or greater than moderate aortic insufficiency or mitral regurgitation to avoid confounding of diastolic measurements, leaving a final study population of 4480 subjects for these substudies.
For sensitivity analyses, NT-proBNP was available in 3464 individuals in the analytic data set, and left atrial (LA) volume measurements were obtained from a second echocardiogram from 1994 to 1995 in which LA volume was measured in a subset of 686 CHS participants.18
Echocardiography
The design of the echocardiography protocol used in CHS has been described in detail elsewhere.19 Echocardiographic parameters included M-mode-based parasternal long-axis LA dimension, left ventricular (LV) dimensions, fractional shortening, and calculated LV mass, as well as Doppler mitral valve inflow, consisting of early and late peak velocities. Left atrial volume measurements were available in a subset of 657 participants.
Determination of incident atrial fibrillation
Participants were contacted every 6 months for follow-up, alternating between a telephone interview and a clinic visit for the first 10 years and by telephone interview only after that. An annual resting ECG was obtained yearly through the ninth year of follow-up, and discharge diagnoses for all hospitalizations were collected. We identified cases of AF in two ways. Annual study ECGs were interpreted by the EPICARE ECG reading centre, where the diagnoses of AF or a trial flutter were verified.9 Hospital discharge diagnoses that included codes for AF and flutter were also included, although AF or flutter diagnoses that were made during the same hospitalization as coronary artery bypass surgery or heart valve surgery were not counted. Prior evaluation in CHS determined the positive predictive value of hospital discharge diagnosis to be 98.6% for diagnosis of AF9 and a Holter substudy identified that only 1 in 819 subjects (0.1%) had persistent or intermittent AF not identified by the above measures.20
Analysis
In studies of prevalent AF, we examined the mean height between participants with and without prevalent AF according to sex using Student's t-test. For the adjusted analysis, we used generalized linear models of height stratified by sex, with adjustment for age, body mass index (BMI), clinic site, highest grade achieved, and race.
For analysis of incident AF, we used the Cox proportional hazards regression modelling with stratification by sex, age-specific hazard functions, and adjustment for BMI, clinic site, highest grade achieved, and race as our base model for all analyses, since we considered these to be the only variables that were conceivably established prior to the attainment of adult height (i.e. ‘upstream’ of height). Time-varying covariates were assessed to check for violation of the proportional hazards assumption and quadratic terms and logarithmic transformations for non-linear effects of height. Analysis was performed using height, per standard deviation, and per unit of measurement (10 cm), as well as using pre-defined cut-points in 5 cm categories. Similarly, we present the Kaplan–Meier curves stratified by sex and age dichotomized at 75.
To examine possible mediators of height-associated incident AF risk, and to assess the independent association of height with AF after adjustment for well-recognized risk factors, we sequentially added potential anatomical and physiological mediators (listed in Table 5) into the base model, as well as inclusion of all variables together. We also repeated all of our base analyses with additional adjustment for systolic blood pressure and the use of antihypertensive agents, given the high burden of AF attributable to hypertension.21 For completeness, we also examined the effect of adjusting for other downstream covariates, including diabetes, kidney function, and the presence of valve disease.
Table 5.
Associations of height with incident AF before and after adjustment for potentially mediating factors
| HR per SD height | CI | P-value | |
|---|---|---|---|
| Women | |||
| Base model | 1.21 | 1.12–1.33 | <0.001 |
| +LA diametera | 1.19 | 1.10–1.30 | <0.001 |
| +LV massa | 1.18 | 1.08–1.29 | <0.001 |
| +LV diastolic dimensiona | 1.21 | 1.11–1.33 | <0.001 |
| +SBP or history of treated hypertension | 1.24 | 1.13–1.35 | <0.001 |
| +Peak E velocity (m/s)a | 1.22 | 1.12–1.33 | <0.001 |
| +Peak A velocity (m/s)a | 1.22 | 1.12–1.33 | <0.001 |
| Combined above (excluding NT-proBNP) | 1.20 | 1.10–1.31 | <0.001 |
| Base model (NT-proBNP available) | 1.23 | 1.12–1.36 | <0.001 |
| + NT-proBNP | 1.23 | 1.12–1.36 | <0.001 |
| Combined (including NT-proBNP) | 1.20 | 1.09–1.33 | <0.001 |
| Men | |||
| Base model | 1.17 | 1.07–1.28 | <0.001 |
| +LA diametera | 1.16 | 1.06–1.27 | 0.002 |
| +LV massa | 1.14 | 1.04–1.25 | 0.005 |
| +LV diastolic dimensiona | 1.15 | 1.05–1.26 | 0.003 |
| +SBP or history of treated hypertension | 1.20 | 1.10–1.32 | <0.001 |
| +Peak E velocity (m/s)a | 1.18 | 1.07–1.29 | <0.001 |
| +Peak A velocity (m/s)a | 1.17 | 1.07–1.28 | <0.001 |
| Combined above (excluding NT-proBNP) | 1.16 | 1.06–1.27 | 0.002 |
| Base model (NT-proBNP available) | 1.24 | 1.11–1.38 | <0.001 |
| +NT-proBNP | 1.27 | 1.14–1.41 | <0.001 |
| Combined (including NT-proBNP) | 1.26 | 1.13–1.42 | <0.001 |
All models adjusted for BMI, clinic site, race, and education; stratified by age.
LA, diameter measured in cm; LV, mass measured in g; LV, diastolic dimension measured in cm; SBP, measured in mmHg, and NT-proBNP measured in pg/dL. All HR are reported per unit of measurement. n for total population = 4480. n for NT-proBNP studies = 3464.
aQuantiles of Peak E velocity, Peak A velocity, LA diameter, LV mass, LV diastolic dimension, PR interval used due to non-linearity; BNP and BNP-squared included due to non-linear effects.
The current guidelines from the Chamber Quantification Writing Group of the American Society of Echocardiography's Guidelines and Standards Committee recommend indexing anthropomorphic measures to body size.22 Recent analyses have demonstrated that in heart failure hospitalizations, cardiovascular mortality, or all-cause mortality, this indexing did not affect predictiveness of these measures.23 To examine the effect on prediction of incident AF of indexing echocardiographic measures to body surface area (BSA), we analysed the associations of LA dimension, LV mass, and LV diastolic dimension on prediction of AF in adjusted and unadjusted models, and with and without adjustment for height, BSA, and weight.
For correlation studies of variables with height, we performed partial Spearman's correlation with adjustment for age and sex. Correlation studies performed on LA volume measures used the same methods as above in a subset of patients in whom these measures were available.
To examine whether height improved standard prediction models of incident AF, we applied the regression model developed from the Framingham Health Study cohort,11 and compared Harrell's c-statistics before and after inclusion of height. We used self-reported diagnosis of any valve disease by a physician in place of ‘any heart murmur’ used in the Framingham score11 because information about physical examination findings by a physician was unavailable for the entire CHS cohort. Confidence intervals (CIs) for this metric were created using bootstrap resampling, with 1000 iterations.
We used SAS, version 9.2, for all analyses except for bootstrapping of the c-statistic, performed in Stata IC10. As previously described, we used singly imputed data for the baseline examination, which was performed in CHS with S-PLUS software (MathSoft, Inc., Seattle, WA, USA).24,25 In the full data set, data were missing on <5% of variables for >85% of the original variables considered for imputation. As described by Arnold et al.,25 the covariate set employed and the model form of each imputed variable were determined individually to maximize the accuracy of imputation. As a consequence, results from single imputation across several outcomes in CHS (including time-to-event) have been found not to differ meaningfully from those using multiple imputation.
Results
Baseline characteristics of the total study population, stratified by sex and separated into pre-defined cut-points of height, are shown in Table 1.
Among 5860 participants, there were 157 cases of prevalent AF, as shown in Table 2. The mean height was higher in prevalent cases than in participants free of AF in both sexes, although the difference was statistically significant only in men. After multivariable adjustment, the mean height was significantly greater among individuals with prevalent AF among both sexes. When inserted in logistic regression models, adjusted for age, BMI, clinic site, race, and education, the OR for prevalent AF was 1.68/cm (CI 1.19–2.37, P = 0.003) for men and 1.61/cm (CI 1.08–2.39, P = 0.02) for women.
Table 2.
Mean height among men and women according to prevalent AF at baseline
| Sex | Group | Unadjusted |
Adjusteda |
||||
|---|---|---|---|---|---|---|---|
| Mean height (cm) | Standard error | P-value | Mean height (cm) | Standard error | P-value | ||
| Women | AF (n = 71) | 159.3 | 0.8 | 0.58 | 160.5 | 0.7 | 0.02 |
| No AF (n = 3300) | 158.9 | 0.1 | 158.8 | 0.1 | |||
| Men | AF (n = 86) | 174.7 | 0.8 | 0.02 | 175.1 | 0.7 | 0.003 |
| No AF (2393) | 173.0 | 0.1 | 173.0 | 0.1 | |||
aAdjusted for age, BMI, clinic site, race, and education.
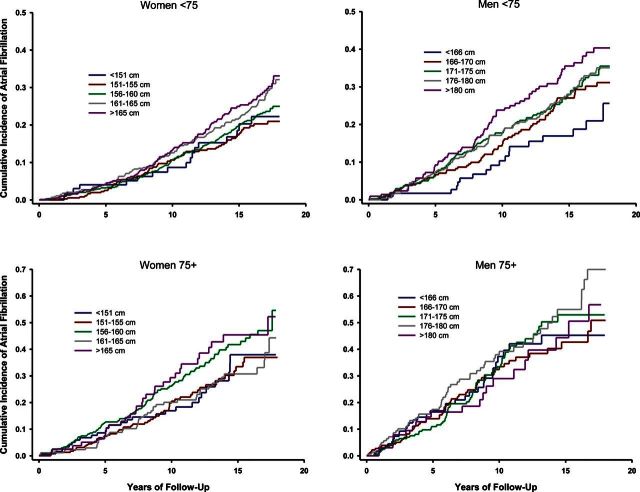
Among 5117 individuals followed for development of incident AF, 1252 (24.5%) developed incident AF; of these, 684 were women (22.6%) and 568 were men (27.1%). The median follow-up was 13.6 years (IQR 8.2–17.2 years) for women and 10.3 years (IQR 5.6–15.5 years) for men. As shown in Figure 1, increased height was associated with increased risk of incident AF in three of four age-sex groups in unadjusted analyses, although the effect did not reach statistical significance in men over 75.
Figure 1.
Kaplan–Meier event curves stratified by sex and age under/over 75 years for each of the pre-determined height cut-points. For all graphs, the abscissa is the years in follow-up and the ordinate is the event fraction (incident AF). P < 0.05 for all curves except men over 75 years old (log-rank test).
After adjustment for BMI, clinic site, race, and highest level of education attained, and stratified by age and sex, height was significantly associated with the development of incident AF (Table 3). There was no evidence of non-linear effects with height and AF in either sex. Moreover, sex was no longer significantly associated with incident AF when height was added to an adjusted model with sex as a covariate: hazard ratio (HR) for male sex 1.48, CI 1.32–1.65 (P < 0.0001) without height included, and HR for male sex 0.94, CI 0.85–1.20 (P = 0.94) with height included.
Table 3.
Relationship of height with incident AF in men and women
| Sex | Group | Height (cm) | AF cases | HRa | CI | Significance |
|---|---|---|---|---|---|---|
| Women (n = 3022) | 1 (n = 255) | ≤150 | 44 | 0.74 | 0.53–1.04 | 0.079 |
| 2 (n = 667) | 150–155 | 113 | 0.76 | 0.60–0.96 | 0.019 | |
| 3 (n = 1116) | 155–160 | 228 | 1 | — | — | |
| 4 (n = 820) | 160–165 | 174 | 1.11 | 0.91–1.35 | 0.326 | |
| 5 (n = 520) | >165 | 125 | 1.31 | 1.05–1.64 | 0.017 | |
| Per SD | 1.19 | 1.10–1.29 | <0.001 | |||
| Per 10 cm | 1.32 | 1.16–1.50 | <0.001 | |||
| Men (n = 2095) | 1 (n = 280) | ≤165 | 50 | 0.73 | 0.52–1.00 | 0.053 |
| 2 (n = 547) | 165–170 | 119 | 0.86 | 0.67–1.10 | 0.220 | |
| 3 (n = 734) | 170–175 | 166 | 1 | — | — | |
| 4 (n = 578) | 175–180 | 144 | 1.10 | 0.87–1.37 | 0.433 | |
| 5 (n = 343) | >180 | 89 | 1.18 | 0.91–1.54 | 0.215 | |
| Per SD | 1.16 | 1.07–1.27 | <0.001 | |||
| Per 10 cm | 1.26 | 1.11–1.44 | <0.001 |
All analyses stratified by age; adjusted for BMI, clinic site, race, and education (highest level attained).
aHR for groups based on cut-points are derived from risk relative to the median height group (Group 3). HR for the bottom two analyses are based on incremental risk relative to the shortest participants for each sex. n = 5117.
Given the large contribution of blood pressure to AF, we repeated all of our analyses with additional adjustment for systolic blood pressure and antihypertensive agent use (see Supplementary material online, Tables); in general, because height tends to be associated with lower blood pressure (Table 5), these adjusted analyses showed slightly stronger risks of AF associated with taller height.
Height was not included in the original Framingham AF risk score,11 although this score has been evaluated in the CHS cohort without height.26 To examine the effect of height on the Framingham model, with the covariates as identified in this study,11 we calculated C-statistics of the Framingham model with and without inclusion of height as an additional variable. The total C-statistic for the Framingham model in this cohort was 0.649, and it increased by 0.010 (CI 0.004–0.017) to 0.659 with the inclusion of height (P < 0.0001). When included in the Framingham model, a 10 cm increment in height was associated with an HR of 1.36 (CI 1.24–1.50).
To examine the impact of indexing echocardiographic measures to body size, we analysed the associations of LA dimension, LV mass, and LV diastolic dimension with the risk of AF with and without adjustment for height, BSA, and weight. As shown in Table 4, inclusion of indexed measures decreased the HR for all measures in both adjusted and unadjusted models, with indexing to BSA causing the greatest decrement. For LV diastolic dimension, indexing to BSA caused this measure to no longer be associated with AF incidence.
Table 4.
Associations of selected echocardiographic parameters with incident AF, before and after adjustment for height or body surface area
| Parameter | Variable | HR (CI) | χ2 | P-value |
|---|---|---|---|---|
| LA diameter (LAD) | ||||
| LAD (per SD) | 1.31 (1.23–1.40) | 72.85 | <0.001 | |
| LAD/height (per SD) | 1.26 (1.18–1.34) | 52.10 | <0.001 | |
| LAD/BSA (per SD) | 1.18 (1.11–1.26) | 24.93 | <0.001 | |
| LV mass (LVM) | ||||
| LVM (per SD) | 1.28 (1.21–1.36) | 68.18 | <0.001 | |
| LVM/height (per SD) | 1.25 (1.18–1.33) | 57.92 | <0.001 | |
| LVM/BSA (per SD) | 1.21 (1.14–1.28) | 41.93 | <0.001 | |
| LV diastolic dimension (LVDD) | ||||
| LVDD (per SD) | 1.18 (1.11–1.25) | 25.42 | <0.001 | |
| LVDD/height (per SD) | 1.11 (1.04–1.18) | 11.00 | <0.001 | |
| LVDD/BSA (per SD) | 1.00 (0.94–1.07) | 0.01 | 0.92 | |
All analyses adjusted for clinic site, race, and education; stratified by age and sex. LA diameter measured in cm, LV mass measured in g, and LV diastolic dimension measured in cm. All HR are reported per unit of measurement. n = 4528.
To explore potential mechanisms of height and increased incidence of AF, we examined the base model (including height after adjustment for BMI, clinic site, race, and education and stratification by age) with inclusion—individually and combined—of various potential mediators of increased AF risk. Table 5 displays results for inclusion of various anatomical and physiological parameters that might be mediators of height-induced increase in AF incidence. Each of these parameters was a significant risk factor for AF incidence. Among the potential mediators, none attenuated the effect of height on increased incident AF. The measure with the strongest association in both men and women was LV mass, which was the most strongly correlated with height in men (Table 6). LA dimension, commonly thought to be the primary mediator of height-induced AF, was not significantly correlated with height, and inclusion did not significantly change the HR for the association of height with incident AF. (Note that the differences in the HR for the base models in Tables 3 and 5 are due to fewer participants included in the analysis of Table 5 due to lack of echocardiography data—see the Methods section for details.). Adjustment for kidney function, diabetes or fasting glucose, and the presence of valve disease had no effect attenuation on the risk of AF with increased height (data not shown).
Table 6.
Partial Spearman's correlations of height with selected cardiovascular parameters
| Sex | Parameter | Spearman correlation coefficient, r (P-value) |
|---|---|---|
| Women | LA dimension (cm) | 0.05 (0.02) |
| LV mass (g) | 0.21 (<0.001) | |
| LV diastolic dimension (cm) | 0.21 (<0.001) | |
| Peak mitral E velocity (m/s) | −0.01 (0.53) | |
| Peak mitral A velocity (m/s) | −0.06 (0.007) | |
| Fractional shortening (%) | −0.04 (0.05) | |
| Quantitative EF (%) | 0.00 (0.88) | |
| NT-proBNP (dg/mL) | 0.01 (0.80) | |
| Average systolic blood pressure (mmHg) | −0.07 (0.001) | |
| Men | LA dimension (cm) | 0.06 (0.02) |
| LV mass (g) | 0.13 (<0.001) | |
| LV diastolic dimension (cm) | 0.10 (<0.001) | |
| Peak mitral E velocity | −0.07 (0.02) | |
| Peak mitral A velocity | −0.10 (<0.001) | |
| Fractional shortening (%) | −0.06 (0.02) | |
| Quantitative EF (%) | −0.03 (0.23) | |
| NT-proBNP (dg/mL) | 0.00 (0.87) | |
| Average systolic blood pressure (mmHg) | −0.11 (<0.001) |
Spearman coefficient (P-value); partial age, BMI, clinic site.
n for total population = 4480. n for NT-proBNP studies = 3464.
To ensure that the lack of association of height with atrial size was not related to its unidimensional measurement, we examined the association of height with LA volume in a subset of participants in whom LA volume measurements were made later during follow-up. In these participants, LA volume still correlated only weakly with height (r = 0.10, P = 0.01) after adjustment for age, sex, and race.
Discussion
In this large cohort of older adults, increased height was associated with incident and prevalent AF after adjustment for confounders of both height and AF, as well as after adjustment for other risk factors of AF. We also demonstrated that height significantly increased the discrimination of standard models of risk prediction, and that indexing anthropomorphic echocardiography measures to body size using either height or the BSA, as is common in clinical practice, lowered their relationship with the risk of AF, and in some cases caused formerly significant predictors to no longer significantly predict AF incidence. Adjustment for multiple physiological and anatomical risk factors, nearly all of which were themselves significantly associated with incident AF, had only limited effect on the association of height with AF; indeed, we were unable to detect any particular measure that appeared to be the mediator of height on increased AF risk.
While height has been found to be associated with AF in other large cohort studies,11,27,28 it has rarely been the focus of investigation. In the Framingham cohort, height was of borderline significance in predicting incident AF, and was thus excluded from the risk score model developed.11 This population, unlike ours, included young participants as well (range 45–95), and thus had a lower cumulative incidence of AF of 10%.11 A similar study in the ARIC cohort, which was composed of individuals under 65 years of age but included African-Americans, had a lower cumulative incidence of AF (3.5%), but found that height was significantly associated with AF.28 These findings suggest that not only is height a parameter that should be considered in any risk factor assessment for AF, but that its impact does not appear to be specific for elderly individuals alone.
An intriguing finding, although one that itself would require further exploration given the multiple confounders of circulating hormone levels and differing body composition, was that when height was included in the model, sex was no longer a significant predictor of incident AF. As other models, such as that used in the Framingham study,11 have included sex, this finding suggests that further research is necessary to truly understand the role of sex in the development of AF.
Increased atrial size as a risk factor for the development of AF has long been recognized,29 and numerous studies have found a positive correlation between increased atrial size and AF.30–32 However, the process of measuring and reporting atrial size with adjustment for body size has been less straightforward.33 The use of indexed measures in echocardiography and other physiological modalities is a common practice, and is recommended by advisory committees such as the American Society of Echocardiography.22 The concept behind this recommendation is that anatomic measures can increase either from pathophysiological processes or from larger body size, and that by adjusting, observed abnormalities will only reflect underlying pathophysiology. When carefully studied, and appropriately applied,34–36 indexing has been shown to improve the predictiveness of the anthropometric variable (especially when the indexed metric, i.e. height or weight, is inversely correlated with the outcome, as is the case for coronary artery disease and height37). However, when body size itself is a risk factor for disease, as observed in this study, these adjustments can be counter-productive. Larger cardiac structure size, be it proportional to natural growth or due to a pathophysiological process, can still results in cardiac diseases. In AF, increased LA size alone theoretically affects the ability of multiple wavelets to reenter and propagate the arrhythmia.38
We were unable to substantiate increased LA dimension or LA volume as an anthropomorphic or physiological mediator of increased AF risk due to height. Left atrial dimension, as well as LA volume that was measured in a subgroup, was only mildly correlated with height, and inclusion in the model did little to attenuate the risk attributable to height. Left ventricular mass, which has been consistently associated with the risk of AF,39–42 was the parameter most strongly correlated with height, yet it attenuated only a small amount of the risk attributable to height. As a result, we are left to speculate that height may have a separate effect on increasing the risk of AF. Among other potential mechanisms for height-mediated increased AF risk, possibilities may lie in the results from recent genome-wide association studies. Among the significant AF-associated genes, PITX212 and ZFHX313 are also associated with growth pathways.12,13 Like these genes themselves, height obviously cannot be modified with intervention, but our findings suggest that it can provide potential clues to other mechanisms of disease. Further evaluation of the interrelationships of height, growth-related genes, and risk of AF in both animal models and human studies may shed light on some such candidate pathways.
One potential limitation to this study is the survival bias inherent in any study of an elderly population. Prior AF studies in younger cohorts have identified height as a risk factor,28 indicating that the risk is likely applicable across populations of varying age. In addition, the consonance of our results from both prevalent and incident AF argues against differential survival as an explanation for our findings.
Another important potential limitation is that because the HRs, and impact on the Framingham risk score, were modest, it is difficult to attribute significance to the observation that increased height is associated with AF. Although we were unable to detect any specific mechanism via which increased height was associated with AF, one potential explanation is that height is simply identifying residual confounding or uncontrolled bias of cohort studies. We chose to include in our base model only variables that could realistically be considered confounders of both AF and height, with further ‘mechanistic’ analysis including insertion of potential downstream mediators of height, such as LA size, and other possible mediators, such as valve disease. None of these analyses found any meaningful attenuation of the height-mediated risk of AF with inclusion of any variable, but further exploration of the mechanisms via which increased height mediates risk of AF is necessary before one can dismiss this potential limitation.
In summary, our results suggest that height is consistently associated with risk of AF, that its inclusion in models of AF risk statistically significantly improves the discrimination of existing prediction models, and that standard echocardiographic measures alone do not appear to mediate its full effect on risk. As a result of these findings, unlike other studies of cardiac disease, we found that indexing anthropometric measures to height actually weakens the associations of cardiac structure size with the risk of AF. Further evaluation of the genetic basis underlying height may provide novel clues to the aetiology of this common arrhythmia.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The research reported in this article was supported by contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grantsHL094555, HL080295, and HL068986 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Conflict of interest: none declared.
Supplementary Material
References
- 1.Majeed A, Moser K, Carroll K. Trends in the prevalence and management of atrial fibrillation in general practice in England and Wales, 1994–1998: analysis of data from the general practice research database. Heart. 2001;86:284–288. doi: 10.1136/heart.86.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P, Lip GY, Van Gelder IC, Bax J, Hylek E, Kaab S, Schotten U, Wegscheider K, Boriani G, Ezekowitz M, Diener H, Heidbuchel H, Lane D, Mont L, Willems S, Dorian P, Vardas P, Breithardt G, Camm AJ. Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options. Executive summary of the report from the 3rd AFNET/EHRA consensus conference. Thromb Haemost. 2011;106:1012–1019. doi: 10.1160/TH11-07-0517. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki S, Yamashita T, Ohtsuka T, Sagara K, Uejima T, Oikawa Y, Yajima J, Koike A, Nagashima K, Kirigaya H, Ogasawara K, Sawada H, Yamazaki T, Aizawa T. Body size and atrial fibrillation in Japanese outpatients. Circ J. 2010;74:66–70. doi: 10.1253/circj.cj-09-0431. [DOI] [PubMed] [Google Scholar]
- 5.Hanna IR, Heeke B, Bush H, Brosius L, King-Hageman D, Beshai JF, Langberg JJ. The relationship between stature and the prevalence of atrial fibrillation in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006;47:1683–1688. doi: 10.1016/j.jacc.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 6.Rosengren A, Hauptman PJ, Lappas G, Olsson L, Wilhelmsen L, Swedberg K. Big men and atrial fibrillation: effects of body size and weight gain on risk of atrial fibrillation in men. Eur Heart J. 2009;30:1113–1120. doi: 10.1093/eurheartj/ehp076. [DOI] [PubMed] [Google Scholar]
- 7.Mont L, Tamborero D, Elosua R, Molina I, Coll-Vinent B, Sitges M, Vidal B, Scalise A, Tejeira A, Berruezo A, Brugada J. Physical activity, height, and left a trial size are independent risk factors for lone atrial fibrillation in middle-aged healthy individuals. Europace. 2008;10:15–20. doi: 10.1093/europace/eum263. [DOI] [PubMed] [Google Scholar]
- 8.Long MJ, Jiang CQ, Lam TH, Xu L, Zhang WS, Lin JM, Ou JP, Cheng KK. Atrial fibrillation and obesity among older Chinese: the Guangzhou Biobank Cohort Study. Int J Cardiol. 2009;148:48–52. doi: 10.1016/j.ijcard.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 10.Conen D, Tedrow UB, Cook NR, Buring JE, Albert CM. Birth weight is a significant risk factor for incident atrial fibrillation. Circulation. 2010;122:764–770. doi: 10.1161/CIRCULATIONAHA.110.947978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB, Sr, Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, Jonasdottir A, Baker A, Thorleifsson G, Kristjansson K, Palsson A, Blondal T, Sulem P, Backman VM, Hardarson GA, Palsdottir E, Helgason A, Sigurjonsdottir R, Sverrisson JT, Kostulas K, Ng MC, Baum L, So WY, Wong KS, Chan JC, Furie KL, Greenberg SM, Sale M, Kelly P, MacRae CA, Smith EE, Rosand J, Hillert J, Ma RC, Ellinor PT, Thorgeirsson G, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin EJ, Rice KM, Arking DE, Pfeufer A, van Noord C, Smith AV, Schnabel RB, Bis JC, Boerwinkle E, Sinner MF, Dehghan A, Lubitz SA, D'Agostino RB, Sr., Lumley T, Ehret GB, Heeringa J, Aspelund T, Newton-Cheh C, Larson MG, Marciante KD, Soliman EZ, Rivadeneira F, Wang TJ, Eiriksdottir G, Levy D, Psaty BM, Li M, Chamberlain AM, Hofman A, Vasan RS, Harris TB, Rotter JI, Kao WH, Agarwal SK, Stricker BH, Wang K, Launer LJ, Smith NL, Chakravarti A, Uitterlinden AG, Wolf PA, Sotoodehnia N, Kottgen A, van Duijn CM, Meitinger T, Mueller M, Perz S, Steinbeck G, Wichmann HE, Lunetta KL, Heckbert SR, Gudnason V, Alonso A, Kaab S, Ellinor PT, Witteman JC. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet. 2009;41:879–881. doi: 10.1038/ng.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty BM, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 15.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 16.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 17.Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, Kronmal RA. N-terminal pro-B-type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation. 2009;120:1768–1774. doi: 10.1161/CIRCULATIONAHA.109.873265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aurigemma GP, Gottdiener JS, Arnold AM, Chinali M, Hill JC, Kitzman D. Left atrial volume and geometry in healthy aging: the Cardiovascular Health Study. Circ Cardiovasc Imaging. 2009;2:282–289. doi: 10.1161/CIRCIMAGING.108.826602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardin JM, Wong ND, Bommer W, Klopfenstein HS, Smith VE, Tabatznik B, Siscovick D, Lobodzinski S, Anton-Culver H, Manolio TA. Echocardiographic design of a multicenter investigation of free-living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 20.Mozaffarian D, Psaty BM, Rimm EB, Lemaitre RN, Burke GL, Lyles MF, Lefkowitz D, Siscovick DS. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368–373. doi: 10.1161/01.CIR.0000138154.00779.A5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Ristow B, Ali S, Na B, Turakhia MP, Whooley MA, Schiller NB. Predicting heart failure hospitalization and mortality by quantitative echocardiography: is body surface area the indexing method of choice? The Heart and Soul Study. J Am Soc Echocardiogr. 2010;23:406–413. doi: 10.1016/j.echo.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold AM, Psaty BM, Kuller LH, Burke GL, Manolio TA, Fried LP, Robbins JA, Kronmal RA. Incidence of cardiovascular disease in older Americans: the cardiovascular health study. J Am Geriatr Soc. 2005;53:211–218. doi: 10.1111/j.1532-5415.2005.53105.x. [DOI] [PubMed] [Google Scholar]
- 25.Arnold AM, Kronmal RA. Multiple imputation of baseline data in the cardiovascular health study. Am J Epidemiol. 2003;157:74–84. doi: 10.1093/aje/kwf156. [DOI] [PubMed] [Google Scholar]
- 26.Schnabel RB, Aspelund T, Li G, Sullivan LM, Suchy-Dicey A, Harris TB, Pencina MJ, D'Agostino RB, Sr, Levy D, Kannel WB, Wang TJ, Kronmal RA, Wolf PA, Burke GL, Launer LJ, Vasan RS, Psaty BM, Benjamin EJ, Gudnason V, Heckbert SR. Validation of an atrial fibrillation risk algorithm in whites and African Americans. Arch Intern Med. 2010;170:1909–1917. doi: 10.1001/archinternmed.2010.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med. 2005;118:489–495. doi: 10.1016/j.amjmed.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 28.Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, Ambrose M, Alonso A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2011;107:85–91. doi: 10.1016/j.amjcard.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schotten U, Neuberger HR, Allessie MA. The role of a trial dilatation in the domestication of atrial fibrillation. Prog Biophys Mol Biol. 2003;82:151–162. doi: 10.1016/s0079-6107(03)00012-9. [DOI] [PubMed] [Google Scholar]
- 30.Leung DY, Chi C, Allman C, Boyd A, Ng AC, Kadappu KK, Leung M, Thomas L. Prognostic implications of left atrial volume index in patients in sinus rhythm. Am J Cardiol. 2010;105:1635–1639. doi: 10.1016/j.amjcard.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 31.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 32.Mathew ST, Patel J, Joseph S. Atrial fibrillation: mechanistic insights and treatment options. Eur J Intern Med. 2009;20:672–681. doi: 10.1016/j.ejim.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Neilan TG, Pradhan AD, Weyman AE. Derivation of a size-independent variable for scaling of cardiac dimensions in a normal adult population. J Am Soc Echocardiogr. 2008;21:779–785. doi: 10.1016/j.echo.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Chirinos JA, Segers P, De Buyzere ML, Kronmal RA, Raja MW, De Bacquer D, Claessens T, Gillebert TC, St John-Sutton M, Rietzschel ER. Left ventricular mass: allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension. 2010;56:91–98. doi: 10.1161/HYPERTENSIONAHA.110.150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brumback LC, Kronmal R, Heckbert SR, Ni H, Hundley WG, Lima JA, Bluemke DA. Body size adjustments for left ventricular mass by cardiovascular magnetic resonance and their impact on left ventricular hypertrophy classification. Int J Cardiovasc Imaging. 2010;26:459–468. doi: 10.1007/s10554-010-9584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lauer MS, Anderson KM, Larson MG, Levy D. A new method for indexing left ventricular mass for differences in body size. Am J Cardiol. 1994;74:487–491. doi: 10.1016/0002-9149(94)90909-1. [DOI] [PubMed] [Google Scholar]
- 37.Kannam JP, Levy D, Larson M, Wilson PW. Short stature and risk for mortality and cardiovascular disease events. The Framingham Heart Study. Circulation. 1994;90:2241–2247. doi: 10.1161/01.cir.90.5.2241. [DOI] [PubMed] [Google Scholar]
- 38.Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J. 1964;67:200–220. doi: 10.1016/0002-8703(64)90371-0. [DOI] [PubMed] [Google Scholar]
- 39.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 40.Verdecchia P, Reboldi G, Gattobigio R, Bentivoglio M, Borgioni C, Angeli F, Carluccio E, Sardone MG, Porcellati C. Atrial fibrillation in hypertension: predictors and outcome. Hypertension. 2003;41:218–223. doi: 10.1161/01.hyp.0000052830.02773.e4. [DOI] [PubMed] [Google Scholar]
- 41.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 42.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–730. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.