Abstract
Anergy is induced in T cells as a consequence of a partial or suboptimal stimulation. Anergic T cells become unresponsive and fail to proliferate and produce cytokines. We had previously shown that in anergic CD4+ T cells, Ikaros participates in the transcriptional repression of the Il2 gene by recruiting histone deacetylases that cause core histone deacetylation at the Il2 promoter. Here we show that deacetylation at the Il2 promoter is the initial step in a process that leads to the stable silencing of the Il2 gene transcription in anergic T cells. We have found that anergy-induced deacetylation of the Il2 promoter permits binding of the histone methyl-transferase Suv39H1, which trimethylates lysine-9 of histone-3 (Me3H3-K9). Furthermore, the establishment of the Me3H3-K9 mark allows the recruitment of the heterochromatin protein HP-1, allowing the silenced Il2 loci to reposition close to heterochromatin-rich regions. Our results indicate that silencing of Il2 transcription in anergic T cells is attained through a series of epigenetic changes that involve the establishment of repressive marks and the subsequent nuclear repositioning of the Il2 loci, which become juxtaposed to transcriptionally silent regions. This mechanism may account for the stable nature of the inhibition of IL-2 production in anergic cells.
Keywords: Anergy, CD4+ T cells, Epigenetic, Histone methylation, Heterochromatin
Introduction
In order to limit autoimmune responses, mechanisms of peripheral tolerance exist to prevent the activation of self-reactive T cells that may have escaped thymic selection. This is achieved mainly through two different processes: a dominant form of suppression mediated by regulatory T cells; and a cell intrinsic program of inactivation termed anergy. Anergy is established in T cells in response to a suboptimal stimulus. Classically, clonal anergy has been shown to occur when T cells engage their antigen receptors in the absence of co-stimulation [1, 2]. A more complex picture has been unveiled in the last few years, when it has become evident that other signals integrated by T cells also contribute to determine their functional fate [3].
Under conditions that deliver an anergizing stimulus, T cells become unresponsive and fail to proliferate and produce IL-2 when re-stimulated even in the presence of co-stimulatory signals [2, 4]. Although initially the molecular characterization of anergic cells identified a defect in Ras/MAPK pathway activation [5, 6], it has been in the last ten years when the molecular mechanisms that are responsible for the induction and maintenance of the anergic state have started to be fully characterized [7–9].
Upon receiving an anergizing stimulus, T cells upregulate the expression of a series of genes that encode proteins that acting at different levels inhibit new responses to antigen [7, 10]. This anergy-inducing program of gene expression is activated by calcium signaling and depends on the activity of NFAT transcription factors [10, 11]. Most proteins, expressed in anergic T cells, including several E3 ubiquitin ligases, caspase 3 or diacylglycerol kinase alpha, target components of the TCR signaling cascade to induce their degradation or functional inactivation, thus blocking signal transduction downstream of the TCR [12–16].
Recent evidence has unveiled that a second level of repression occurs in anergic T cells that directly inhibits the expression of the Il2 gene [17–19]. Epigenetic modifications have been shown to underlie the differential expression of cytokine genes in T cells and contribute to establish the patterns of cytokine expression that determine lineage commitment and T cell differentiation [20, 21]. Regarding the Il2 gene, increases in the levels of histone (H) acetylation have been shown to correlate with the ability of T cells to express this cytokine. Accordingly, naive cells present low levels of H3 and H4 acetylation at the Il2 promoter, which increase upon conversion into effector cells [17, 22]. Further hyperacetylation occurs following activation in a CD28-dependent manner [23]. In anergic T cells, the transcription factor Ikaros binds to the Il2 promoter and recruits histone deacetylases (HDAC), inducing changes in the acetylation status on H3 and H4, which result in direct silencing of Il2 transcription [17, 18].
The establishment of epigenetic modifications on the Il2 promoter may underlie the long-lasting nature of the unresponsive state typical of anergic T cells. However, even though it is clear that core histones at the Il2 promoter undergo deacetylation, this is a modification with a relatively fast turnover that can be easily reversed [24]. Little is known about the possibility that other mechanisms may also contribute to ensure long-term silencing of the expression of Il2 in anergic cells by inducing more stable epigenetic modifications.
In this study we aim at identifying the mechanisms that contribute to the stable epigenetic silencing of the expression of the Il2 gene in anergic effector T helper cells. We find that the chromatin at the Il2 promoter is not only marked by histone deacetylation but that additional silencing marks, namely trimethylation of lysine 9 of histone 3 (Me3H3-K9), are also present. Furthermore, H3-K9 methylation leads to recruitment of the heterochromatin binding protein HP-1 to the Il2 promoter and the redistribution of the Il2 locus to the proximity of heterochromatin region in the nucleus. These modifications, which underlie the re-structuring and nuclear repositioning of the Il2 locus, may be responsible for the maintenance of long-term silencing of the Il2 gene expression in anergic T cells.
Results
The Il2 locus is hypoacetylated and methylated at H3-K9 in anergic T cells
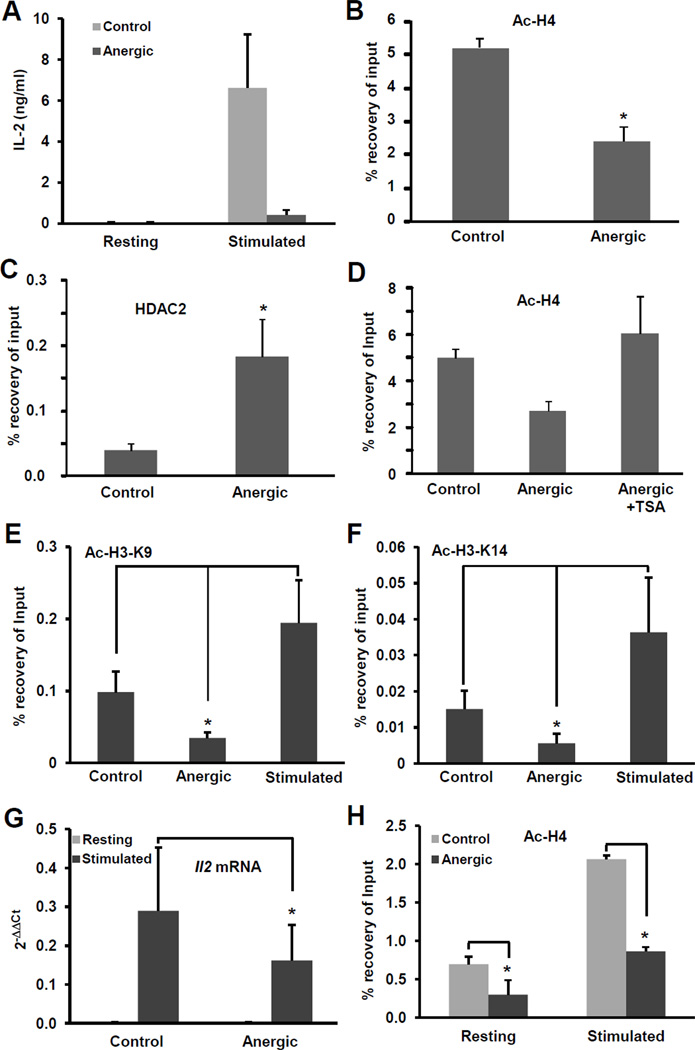
Epigenetic mechanisms regulate the Il2 promoter activity in anergic T cells. We and others have previously shown that in anergic cells the transcription factor Ikaros binds to the Il2 promoter and recruits HDACs that cause deacetylation of core histones H3 and H4, contributing to the silencing of the Il2 gene [17, 18]. Interestingly, in concordance with the stable nature of the unresponsive state in anergic T cell, histone deacetylation of the Il2 promoter was maintained even when anergic cells were re-stimulated under the same conditions that were able to induce increased histone acetylation in naïve cells [17]. Histone acetylation is an epigenetic modification that has been described to have a rapid turnover. Given that clonal anergy imposes a relatively stable state of functional unresponsiveness, we explored the possibility that Ikaros-mediated deacetylation of histones at the Il2 promoter could just represent the initial epigenetic modification that would allow for further changes to occur in order to ensure a more stable silencing of the expression of the Il2 gene. As we had previously reported [17], primary CD4+ cells primed and differentiated into Th1 cells that received an anergizing stimulus became unresponsive to re-stimulation, failing to produce IL-2 in response to TCR and CD28 engagement (Fig 1A). This effect correlated with a marked decrease in the levels of histone acetylation at the Il2 promoter (Fig.1B), which was due to active recruitment of HDACs to the Il2 promoter, and could be blocked by the use of HDAC-inhibitors such as TSA (Fig.1C–D). To characterize further the changes in histone acetylation that occur at the Il2 promoter in anergic T cells, we analyzed two epigenetic marks that have been shown to mark actively transcribed gene: acetylation at H3-K9 and H3-K14 [25, 26]. We found that the degree of acetylation in both positions was decreased in T cells that received an anergizing stimulus, in clear contrast with the marked increase in H3-K9 and H3-K14 acetylation observed in fully stimulated cells (Fig. 1E–F). To corroborate that histone deacetylation was also taking place in anergic T cells in vivo, DO11.10 mice, which express a transgenic TCR that recognizes the OVA323–339 peptide restricted to MHC-II, were tolerized with a single oral dose of OVA [15]. One week later, CD4+ T cells were isolated and activated ex vivo with T-cell depleted splenocytes loaded with OVA323–339 peptide. As expected, cells from mice that received OVA produced significantly lower amounts of Il2 mRNA than cells from control mice (Fig.1G). Furthermore the Il2 promoter also showed reduced H4 acetylation that was not restored upon re-stimulation (Fig.1H).
Figure 1. The Il2 promoter is hypoacetylated in anergic T cells.
A. Primary mouse Th1 cells were left resting (control) or anergized by treating them with 1µM ionomycin for 16 hours (anergic). Cells were then washed, left to recover for 2–4 hours and re-stimulated for 24 hours with plate bound antiCD3 and antiCD28. IL-2 production was determined by ELISA. B–F. Histone acetylation at the Il2 promoter was assessed in in vitro differentiated primary mouse Th1 cells by chromatin immunoprecipitation to detect: acetylated histone H4 (B and D), occupancy by HDAC2 (C) or acetylation of H3-K9 and H3-K14 (E and F) in cells anergized with ionomycin or plate-bound anti-CD3 in the presence or absence of the HDAC inhibitor TSA and control untreated cells (D). G and H. DO11.10 mice were administered a single dose of OVA in PBS (anergic) or PBS alone (control). CD4+ T cells were isolated one week later and activated with OVA323–339-loaded splenocytes for 24 hours. Il2 expression was determined by real-time PCR (G) and H4 acetylation at the Il2 promoter by ChIP (H). Results are shown as mean+SEM from three to five independent experiments. *p< 0.05.
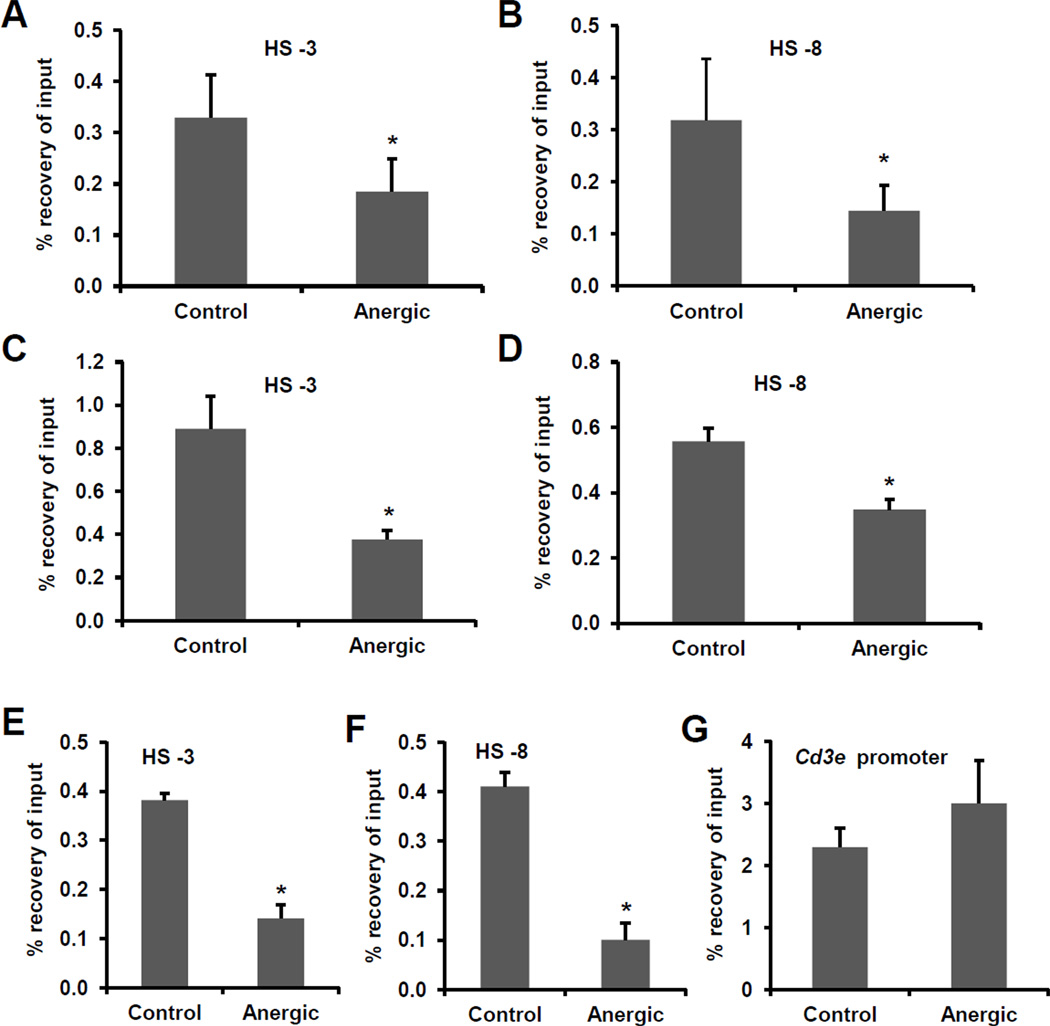
The expression of the Il2 gene is also regulated by control elements located upstream of the Il2 promoter. Previous studies using DNase-I hypersensitivity and histone acetylation analyses identified different regions distal to the proximal Il2 promoter that were modified in T cells in response to activation [27, 28]. To determine if histone deacetylation would extend to those elements, we analyzed two of the regions that showed more marked changes in activated cells [27, 28]. We also detected H4 hypoacetylation in regions located -3Kb and -8Kb upstream of the Il2 promoter in cells anergized in vitro with ionomycin (Fig.2A–B) or anti-CD3 (Fig.2C–D), and in cells isolated form orally tolerized mice (Fig.2E–F). These modifications appeared specifically in the Il2 locus, as H4 acetylation was conserved in the Cd3e promoter, whose expression is not affected in anergic cells (Fig.2G).
Figure 2. The Il2 locus is hypoacetylated in anergic T cells.
A–D. Histone acetylation at two Il2 distal DNase-I hypersensitivty sites (HS -3Kb and HS -8Kb) was assessed in in vitro differentiated primary mouse Th1 cells by ChIP for acetylated histone H4 in untreated resting cells (control) or cells anergized with ionomycin (A–B) or plate-bound anti-CD3 (C–D). E–G. DO11.10 mice were administered a single dose of OVA in PBS (anergic) or PBS alone (control). CD4+ T cells were isolated one week later and H4 acetylation at two DNase-I hypersensitivity sites (HS) located upstream of the Il2 promoter and the Cd3e promoter was assessed by ChIP. Results are shown as mean+SEM from three independent experiments. *p< 0.05.
As Me3H3-K9 has been associated with the recruitment of loci to heterochromatin-rich silent regions [29], we carried out experiments to find if the Il2 locus could also undergo H3-K9 methylation in anergic T cells. In vitro differentiated mouse primary Th1 cells were either left resting or treated with ionomycin for 16 hours to induce anergy. ChIPs performed with anti-Me3H3-K9 antibodies revealed that the Il2 promoter was indeed significantly enriched for Me3H3-K9 in anergic cells (Fig.3A). Similar results were obtained when cells were anergized with anti-CD3 (signal 1) in the absence of co-stimulation (Fig.3B). Methylation of H3-K9 at the two distal Il2 DNase hypersensitivity sites, HS -3Kb and HS -8Kb, was also analyzed and found to be increased in anergic cells (Fig.3C–D). However, these changes appeared to be restricted to the Il2 locus as they did not extend to Adad1, the immediately downstream located gene (approx. 9Kb) at chromosome 3 (Fig. 3E).
Figure 3. H3-K9 methylation at the Il2 locus in anergic T cells is stable and requires prior histone deacetylation.
A–F. H3-K9 methylation at the Il2 promoter (A and B), the HS -3Kb (C) and HS -8Kb (D) distal Dnase-I hypersensitivity sites and the Adad1 gene (E) was measured by ChIP using anti-Me3H3-K9 antibodies in Th1 cells that were anergized with ionomycin (A, C, D, E and F) or anti-CD3 (B) and compared to control resting cells or cells stimulated with plate bound antiCD3 and antiCD28. The effect of re-stimulation of anergic cells on Me3H3-K9 at the Il2 promoter was also analyzed (G). H and I. Me3H3-K9 was measured by ChIP at the HS -3Kb and HS- 8Kb distal DNase-I hypersensitivity sites in control resting, stimulated, anergic or re-stimulated anergic Th1 cells J. Th1 cells were anergized by ionomycin or plate-bound anti-CD3 and levels of M3H3-K9 at the Il2 promoter were compared to similarly anergized cells in the presence of TSA. Results are shown as mean+SEM from three independent experiments. *p< 0.05.
H3-K9 methylation cannot be reversed by re-stimulation in anergic T cells and depends on previous deacetylation
One of the main characteristics of anergic T cells is that they remain unresponsive to subsequent antigenic challenges even when these may occur in the presence of co-stimulatory signals. To explore if Me3H3-K9 remained in place in anergic cells following re-stimulation, we first compared Me3H3-K9 at the Il2 promoter in anergic and stimulated T cells. ChIP experiments confirmed that Me3H3-K9 accumulated at the Il2 promoter in anergic cells. However, fully stimulated T cells with antiCD3 and antiCD28 showed levels of Me3H3-K9 that were even slightly lower than control resting cells (Fig.3F). To assess the stability of this epigenetic modification, anergic cells were re-stimulated with plate bound anti-CD3 and anti-CD28. Me3H3K9 at the Il2 promoter was maintained even after anergic cells were re-stimulated in the presence of co-stimulation (Fig.3G). Similar results were obtained when the HS -3Kb and HS -8Kb distal DNase hypersensitivity sites were analyzed in those cells (Fig.3H–I).
Combinations of different epigenetic modification in one or more core histones occur frequently in regulatory regions to ensure a fine control of gene expression. In many cases an initial modification creates a binding site for the recruitment of enzymes that will introduce subsequent modifications. In an attempt to establish if prior histone deacetylation could be required to allow for subsequent H3-K9 methylation, we determined the effect of an HDAC inhibitor on the levels of Me3H3-K9 at the Il2 promoter. We had previously shown that the use of trichostatin A (TSA) prevented full induction of anergy in Th1 cells, which resulted from defective histone deacetylation at the Il2 promoter ([17] and Fig.1C). Interestingly, the increase in the levels of Me3H3-K9 in anergic cells was prevented when cells were anergized in the presence of TSA (Fig.3J), indicating that deacetylation of the Il2 promoter was probably a prerequisite for the establishment of the Me3H3-K9 mark, and supporting that active epigenetic modulatory mechanisms in the form of histone deacetylation and H3-K9 methylation worked together to silence the Il2 gene in anergic T cells.
The histone methyl transferase Suv39H1 is recruited to the Il2 promoter in anergic T cells
Our results showed that the Il2 promoter was methylated at H3-K9 in anergic cells. A number of histone methyl transferases (HMT) have been identified in mammalian systems. Suv39H1 has been characterized as a major HMT responsible for Me3H3-K9, and it has been involved in silencing of endogenous and viral genes in T cells [30–32]. We explored the possibility that Suv39H1 could be recruited to the Il2 promoter in anergic T cells. ChIP experiments showed enrichment of Suv39H1 binding to the Il2 promoter in anergic T cells compared to control resting or activated cells, where very low levels of this HMT were detected (Fig.4A). Suv39H1 recruitment to the Il2 promoter in anergic cells was specific: whereas this promoter exhibited clear enrichment in Suv39H1 binding, the promoter of the Cd3e gene, which does not undergo silencing during anergy, showed minimal basal levels of Suv39H1 binding that were not increased in anergic cells (Fig.4B).
Figure 4. The histone methyltransferase Suv39H1 is recruited to the Il2 promoter in anergic T cells.
A Th1 cells were left resting (control), stimulated with plate bound antiCD3 and antiCD28 or anergized with ionomycin and ChIPs were performed with anti-Suv39H1 antibodies. Enrichment at the Il2 promoter was assessed. Results are shown as mean+SEM from four independent experiments. *p< 0.05. B Similar ChIP experiments were performed to compare binding of Suv39H1 to the Il2 promoter and the Cd3e promoter.
HP1 binds the Il2 promoter in anergic T cells
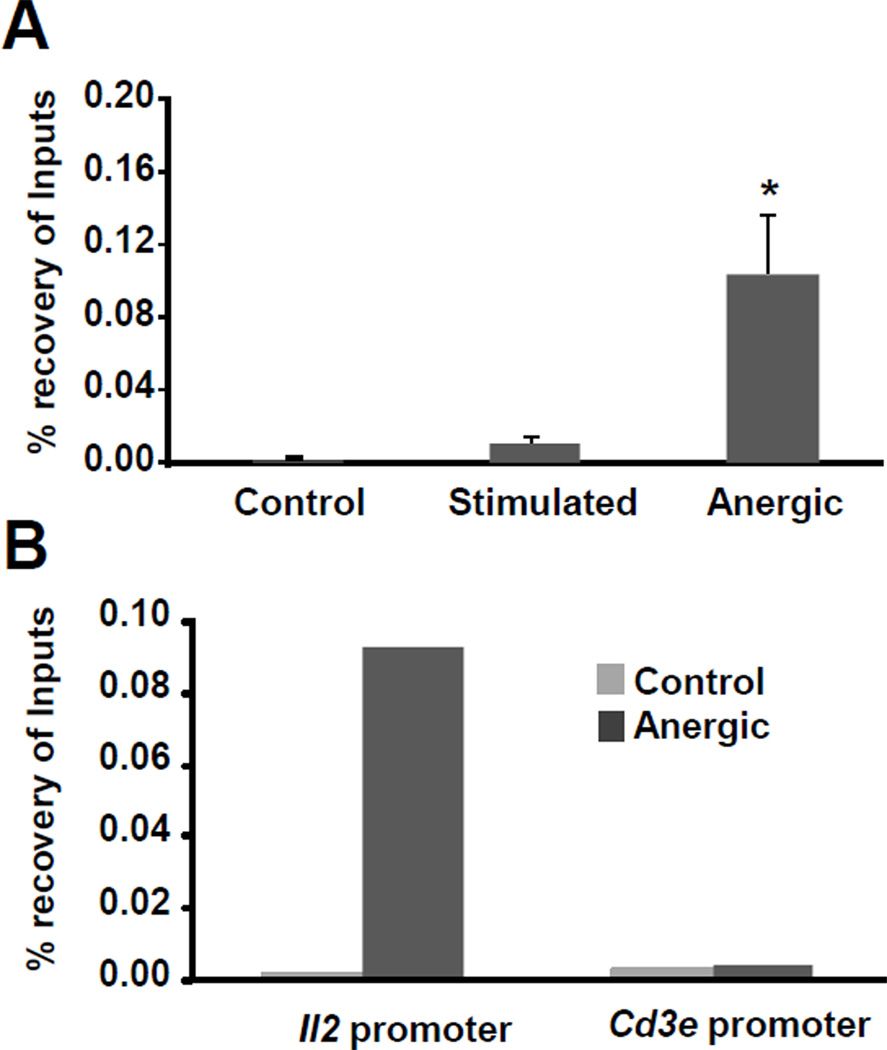
The experiments shown above indicated that the Il2 promoter was targeted by Suv39H1 to undergo Me3H3-K9. It has been shown that Suv39H1 mediated tri-methylation of H3-K9 creates a docking pocket that allows HP1 binding [33]. For many silenced loci, HP-1 binding not only initiates processes required for the locus to re-localize to heterochromatin-rich domains in the nucleus but also helps stabilize H3-K9 methylation leading to the establishment of a stable chromatin structure [34]. This prompted us to investigate if the Il2 locus could be a target for HP1 in anergic cells by ChIP assays using anti-HP1α antibodies. Remarkably, we observed a >10 fold increase in HP1 enrichment at the Il2 locus in anergic cells compared to control cells (Fig.5A). To establish specificity, we analyzed in parallel HP1 binding to the Cd3e promoter. HP1 binding to the Cd3e promoter was negligible in both control and anergic cells, while the Il2 promoter was clearly enriched for HP1 binding in anergic cells (Fig.5B).
Figure 5. HP-1 is recruited to the Il2 promoter in anergic T cells.
A and B Th1 cells were left resting untreated (control) or anergized by treatment with 1µM ionomycin and ChIP assays were carried out with anti-HP1α antibody. Enrichment of HP1 binding at the Il2 promoter was compared between control and anergic cells (A), and also between similarly treated cells at the Il2 and Cd3e promoters (B). C and D Th1 cells were anergized by ionomycin (C) or plate-bound anti-CD3 (D) in the presence or absence of the HDAC inhibitor TSA and ChIP assays were performed using an anti-HP1α antibody. Results are shown as mean+SEM from three to five independent experiments. *p< 0.05.
As we had seen that Me3H3-K9 at the Il2 promoter was dependent upon preexisting deacetylation (Fig.3G) and Me3H3-K9 has been shown to determine HP1 docking [33], we further reasoned that all these events could be connected causally in a sequential manner. To explore this possibility, we determine HP1 binding in cells anergized in presence or absence of TSA. These experiments revealed that the increased HP1 recruitment to the Il2 promoter during anergy was prevented when HDAC activity was inhibited (Fig.5C), supporting our previous studies that showed that inhibition of HDAC activity prevented silencing of Il2 expression in anergic cells [17]. We observed similar results when anti-CD3 stimulation was used to induce anergy (Fig.5D).
Taken together, these results indicate that a series of chromatin modifications occur at the Il2 locus in anergic T-cells to stabilize the silencing of the expression of this gene. Histone deacetylation favors Me3H3-K9 which, in turn, enables HP1 binding to the Il2 promoter.
IL-2 reverses the epigenetic silencing of the Il2 gene in anergic T cells
Signaling through the IL-2 receptor breaks anergy in T cells, which regain the ability to produce IL-2 upon re-stimulation [35], which could follow reversal of epigenetic silencing of the Il2 gene. To test this hypothesis we analyzed H4 acetylation, H3-K9 trimethylation and HP-1 binding at the Il2 promoter in cells that were treated with IL-2 eight hours after receiving an anergizing stimulus. Addition of exogenous IL-2 reversed the anergy-induced H4 deacetylation and H3-K9 methylation and prevented HP-1 binding at the Il2 promoter (Fig. 6A–C).
Figure 6. IL-2 reverses silencing epigenetic changes at the Il2 promoter.
A–C: H4 acetylation (A), H3-K9 methylation (B) and HP-1 recruitment (C) to the Il2 promoter was determined by ChIP using in vitro differentiated primary Th1 cells that were left resting untreated (control) or anergized with 1 µM ionomycin for 16 hours in the presence (Anergic+IL-2) or absence (Anergic) of 10 U/ml of recombinant IL-2 for the last 8 hours or the treatment.
The Il2 locus associates with heterochromatin-rich regions in the nuclei of anergic T cells
HP1 recruitment to the Il2 promoter could indicate that, in anergic T cells, the Il2 locus might relocate to heterochromatin-rich domains in the nucleus. The condensed state of the chromatin in these regions renders the resident genes very refractory to the transcriptional machinery. Generally, genes located in heterochromatin exhibit various silencing epigenetic marks and are targeted by a slew of transcription factors and chromatin remodeling proteins associated with gene silencing [36].
We had earlier shown that Ikaros targeted the Il2 promoter to recruit HDACs and cause histone deacetylation [17]. To further elucidate the chain of molecular events at the Il2 locus during anergy induction, first, we investigated whether Ikaros would co-localize with the DAPI dense, hence compacted, and transcriptionally more refractory, regions of the nucleus in anergic cells. We stained resting, anergic and activated T cells with an anti-Ikaros antibody and counterstained the nuclei with DAPI. Ikaros staining in resting and activated cells exhibited a low intensity staining. In contrast, Ikaros staining in anergic cells adopted an intense speckled pattern that largely co-localized with the DAPI dense regions (Fig.7A). These results further supported the notion that the Il2 locus, which is a target for Ikaros in anergic T cells [23, 37], might associate with heterochromatin-rich regions in anergic cells. We had previously shown that Ikaros expression was upregulated in anergic T (refs). To confirm that the upregulation of Ikaros expression could account for the increased staining detected in anergic T cells, we assessed Ikaros protein by western blot, which revealed a clear increase in the total levels of Ikaros in anergic T cells when compared to resting or stimulated cells (Fig. 7B). Histone deacetylation at the Il2 promoter is dependent of Ikaros in anergic T cells and inhibition of this protein prevents HDAC recruitment, deacetylation of H3 and H4 at this locus and silencing of the Il2 gene [23, 37]. Supporting that H3-K9 methylation was dependent on histone deacetylation and the role of Ikaros in this process, inhibition of this transcriptional repressor using a dominant negative IK6 protein [37] also prevented methylation of H3K9 at the Il2 promoter (Fig. 7C). Further supporting the colocalization of Ikaros with silenced chromatin regions in anergic cells, we found that the increased levels of Ikaros present in anergic T cells colocalized with methylated H3-K9 and HP-1 positive regions in the nucleus (Fig. 7D). As we had previously reported (ref), IL-2 signaling inhibited Ikaros expression in anergic T cells which resulted in decreased nuclear Ikaros signal in anergic cells treated with IL-2 (Fig. 7D).
Figure 7. The Il2 locus co-localizes with heterochromatin-rich regions in the nucleus in anergic T cells.
A. Th1 cells were left resting, activated with plate-bound anti-CD3 without (anergic) or with (stimulated) anti-CD28, respectively, and immunostained with an anti-Ikaros antibody (green). DAPI was used to stain the nuclei. Images (630x) were acquired as a Z-stack and subjected to 3D deconvolution. B Levels of Ikaros protein were determined by western blot in Th1 cells treated as in A. C. Th1 cells were transfected with plasmids expressing GFP or an Ikaros dominant negative IK6 protein+GFP. Cells were sorted and left resting or anergized with 1 µM ionomycin. Methylation of H3-K9 at the Il2 promoter was determined by ChIP. The data represent mean+SEM from two independent experiments D. Th1 cells were left resting or anergized with 1 µM ionomycin in the presence or absence of IL-2. Expression and localization of Ikaros (green), HP-1(yellow) and K9-methylated H3 (red) was asses by immunofluorescence (630x). E. FISH experiments were carried out in Th1 cells that were treated as in A (left panels) or B (middle panels) using a blue aqua-labeled gamma-satellite probes and either a spectrum orange-labeled Il2 locus specific probe (left panels) or a Texas-red-labeled Il2 locus specific probe and an Alexa fluor-488-labeled Gapdh locus specific probe (middle panels). Images (630x) were acquired as a Z-stack and subjected to 3D deconvolution or using structured illumination optical sectioning. Colocalization of the Il2 or Gapdh probes and gamma satellite positive regions (blue) was counted for each experimental condition over more than 10 fields per experiment. Il2 (red) probe or Gapdh (green) probe specific dots are indicated by arrows (white: no colocalization; yellow: colocalized with gamma satellite). The data represent mean+SEM from two independent experiments. *p< 0.05.
We then assessed the possibility that following HP-1 binding (Fig. 5) the Il2 locus could relocate to heterochromatin-rich regions by FISH using a gamma satellite (commonly associated with heterochromatin [38]) and an Il2 locus probe. A significantly higher percentage of Il2 loci (red dots) colocalized with the heterochromatin-rich regions stained by the gamma satellite probe (blue) in anergic cells than in resting or activated cells (Fig.7E). This effect could be reversed by IL-2 signaling and was specific for the Il2 gene as ionomycin treatment did not cause any changes in the position of a housekeeping gene, Gapdh (Fig. 7E).
Starting from the recruitment of Ikaros to the Il2 promoter, a key cascade of events would occur at the Il2 locus that would include histone deacetylation, Me3H3-K9, HP-1 recruitment and relocalization to heterochromatin-rich domains in the cell nucleus.
Discussion
Anergy is a mechanism of peripheral tolerance that in T helper cells results from suboptimal stimulation. The hallmarks that characterize anergic T cells are diminished proliferative responses and decreased IL-2 production in response to new antigen encounter [2, 39]. The establishment of the anergic phenotype is caused by the action of several anergy-inducing proteins. The function of many of these proteins has been recently characterized and shown to be responsible for interfering with different signaling events downstream of the TCR. For instance, Grail is essential for the induction of anergy in T cells, where it targets different substrates for degradation, including CD3 chains of the TCR complex [13, 40, 41]. Similar effects have been described for other E3 ubiquitin ligases, such as Cbl-b and Itch, as well as for other enzymes such as DGK α or caspase 3 [12, 14–16, 42]. The expression of most of the genes that encode for these anergy-inducing proteins appears to be dependent on calcium signaling through the activation of NFAT [10, 11]. NFAT can directly activate their expression or upregulate other transcription factors that in turn induce the expression of anergy-associated genes, as it has been shown for Egr transcription factors and Cbl-b [43]. Recent evidence indicates though that interference with TCR signaling is not the only mechanism involved in inhibiting activation-induced cytokine expression in anergic T cells. Epigenetic modifications at the promoters of effector cytokines have been described in anergic cells, indicating that, as it is the case with many other pathways of T cell differentiation, stable changes in the ability of anergic T cells to produce effector cytokines may be regulated through epigenetic silencing [19].
Decreased levels of histone acetylation and increased levels of DNA methylation at the Il2 promoter have been reported in T cells anergized either in vitro, using ionomycin or by stimulation of the TCR without engagement of costimulatory receptors, or in vivo, by viral superantigen or through oral administration of antigen [17–19]. We and others have identified Ikaros as the transcription factor responsible for the silencing of the expression of Il2 in anergic T cells [17, 18]. In mature lymphocytes, Ikaros is frequently found associated with the chromatin remodeling complex NURD [44]. Upon binding to its target motifs, Ikaros can recruit this complex, which contains HDAC1 and HDAC2 [45]. In fact, Ikaros binding to the Il2 promoter in anergic T cells leads to HDAC recruitment and core histone deacetylation [17]. The concept of a histone code or, in a broader sense, a nucleosome code, implies that initial posttranslational modifications in histone tails may create the docking motifs for new enzymes to add new modifications to the same histone, another histone on the same nucleosome or even histones in adjacent nucleosomes, that contribute to establish a complete epigenetic signature that determines the regulation of the expression of a given gene. This situation is favored by the fact that multiple histone modifying enzymes are frequently found associated in the same chromatin remodeling complex [46, 47]. Our results indicate that previous histone deacetylation, including H3-K9 deacetylation, appears to allow for subsequent addition of the Me3H3-K9 repressive modification, as inhibition of HDAC activity with TSA prevents methylation of H3K9 at the Il2 promoter. Supporting this concept, we have found that recruitment of Suv39H1 to the Il2 promoter is also dependent on prior histone deacetylation. Suv39H1-mediated silencing of gene expression has been reported in the regulation of different aspects of T cell biology such as the maintenance of HIV-1 latency and the silencing of Cd4 during thymocyte development [31, 32]. Our results indicate that this HMT may also play a key role in repressing Il2 expression in anergic T helper cells. Nevertheless, we cannot rule out that other HMTs that have been shown to mediate H3-K9 methylation in T cells, such as those from the G9a or ESET families [48, 49], may also be involved in this process. Our data indicates that H3-K9 methylation occurs similarly in T cells anergized with anti-CD3 or ionomycin. Furthermore, inhibition of Ikaros activity prevents the establishment of this silencing epigenetic mark in the Il2 promoter. These results support the notion that this process is calcium-dependent and results from the NFAT-mediated induction of the expression of Ikaros in anergic cells [10,17].
Partial acetylation of H3 and H4 in naïve T cells should allow transcription of this cytokine upon first encounter with antigen [22]. We have seen that antigen experienced Th1 cells that have the ability to produce greater amounts of IL-2, present higher levels of histone acetylation at the Il2 promoter [17]. The loss of those acetyl groups in the Il2 promoter would prevent Il2 transcription in anergic cells. In fact, cells that received anergizing stimuli when HDAC activity was inhibited became refractory to those stimuli and recovered the ability to produce IL-2 when the TCR-blockade was bypassed [17]. Turnover of histone tail acetylation is a rapid process with average half- lives of only a few minutes [50]. Inhibition of Il2 expression is however a long-term phenotype in anergic T cells [2]. As opposed to histone acetylation, methylation of histone tails is a more stable posttranslational modification, and repressive marks associated with gene silencing that involve histone methylation, such as Me3H3-K9, have a much slower turnover rate [51]. Furthermore, methylation of H3-K9 creates a binding site for HP-1 [33], a chromobox protein, involved in heterochromatin formation. HP-1 can then lead to further recruitment of more Suv39H1 and the propagation of the methylation of H3-K9 and further recruitment of HP-1 [34]. These two proteins have also been shown to have the ability to recruit DNA methyl transferases [52], which could account for the increased DNA methylation that has been found in the Il2 promoter in anergic T cells [19].
Following HP-1 binding, our data shows that the Il2 locus translocates to a subnuclear localization in close proximity to heterochromatin regions identified by the presence of gamma satellite repeats. Position effect variegation may lead to the inactivation of the expression of a gene through juxtaposition to silenced chromatin [53]. Our results support that the Il2 locus gets repositioned in anergic T cells in a way that it may cause stable repression of the expression of this cytokine gene. Repositioning of loci to heterochromatin-rich regions in the nucleus has been proposed to play important roles in gene silencing during development, however, this mechanism can also regulate gene expression during T cell differentiation. For instance, the Gata3 locus, which is silenced in Th1 cells, gets repositioned to a pericentromeric localization during Th1 differentiation [54]. Interestingly, Ikaros regulates the expression of lineage-specific genes during lymphoid and myeloid development through a process that involves loci repositioning and heterochromatin formation [55, 56]. In anergic cells, Ikaros would bind to its target sites on the Il2 promoter, recruiting HDACs and causing core histone deacetylation. Deacetylation of the Il2 promoter would favor recruitment of Suv39H1, leading to H3-K9 tri-methylation, recruitment of HP-1 and repositioning of the Il2 locus. It would be interesting to find whether similar mechanisms apply to other cytokines, as silencing epigenetic modifications have also been described in the Ifng promoter and a new role for Ikaros has been recently reported in the silencing of the expression of Tbet and IFNγ during Th2 cell differentiation [19, 57].
Recently, it has been shown that cytokine expression in anergic cells can also be regulated at the posttranslational level. Decreased translation of several effector cytokines, including IL-2, was shown to occur in anergic T cells [58]. These results may indicate that cytokine expression may be controlled in anergic cells through complementary mechanisms or, alternatively, that distinct mechanisms may be activated in T cells to inhibit cytokine expression in response to different anergizing stimuli (e.g. clonal anergy, oral tolerance or superantigen-induced anergy vs. soluble self-peptide) that may induce specific forms of T cell anergy.
Anergy can be reversed in T cells through IL-2R signaling [35]. Anergic cells grown in the presence of IL-2 regain their ability to proliferate and produce cytokines when reactivated. Although chromatin-based mechanisms of silencing may guarantee a stable long-lasting inhibition of cytokine expression they may nevertheless still allow for reversal of the gene repression [59]. Reversibility of changes in the structure of chromatin can provide the basis for cell plasticity. It is, in many cases, the regulation of the levels of transcription factors or components of a chromatin remodeling complexes that determines whether a given locus may become active in response to certain stimuli [38]. For instance balance between the presence of Ikaros and Mi2β determine whether the Cd4 gene is transcribed or silenced during thymocyte development [60]. These factors could allow for the recruitment of histone demethylases that would then initiate the remodeling of a locus to induce nuclear repositioning and reactivation [61, 62]. We have reported that IL-2R signaling in T cells prevent anergy through the induction of AP-1 activity that prevent the expression of anergy-associated genes [63]. The presence of increased AP-1 and maybe other IL-2R dependent transcription factors may tilt the balance and transform the Il2 locus from a repressed to an active state.
Our results show that epigenetic changes contribute to the inhibition of Il2 expression in anergic T cells. Stable silencing of Il2 transcription would add an extra level of regulation added to the mechanisms that inhibit TCR signaling in anergic T cells that would ensure a long-lasting inhibition of Il2 expression.
Materials and methods
Mice
Four to eight-week old C57BL6/J and DO11.10 mice were purchased from the Jackson Laboratories and were maintained in a pathogen-free animal care facility. All animal work was performed according to the approved guidelines set by the Albert Einstein College of Medicine Institutional Animal Care and Use Committee.
Cell culture
Primary CD4+ T cells were isolated from spleen and lymph nodes from 4 to 8-week old mice using anti-CD4 coupled magnetic beads (Invitrogen). Isolated CD4+ T cells were stimulated with plate bound anti-mouse CD3ε and anti-mouse CD28 (BD Biosciences) at 0.5 µg/ml and differentiated for 7 days to the Th1 phenotype in media containing 10 ng/ml of IL-12 (Cell Sciences), 10 µg/ml anti-IL-4 and 10 u/ml of human recombinant IL-2 (obtained from the NCI-BRB preclinical repository). T cells were cultured in DMEM supplemented with 10% FBS, 2 mM L-glutamine, non-essential amino acids and essential vitamins (Cambrex), and 50 µM 2-mercaptoethanol.
Induction of anergy
Th1 cells were treated with 1 µM Ionomycin (Calbiochem) for 16 hours to induce anergy. As an alternative method, cells were stimulated for 20 hours with 2 µg/ml plate-bound anti-CD3. Following these treatments, cells were detached from the wells, washed and rested for 4 or 48 hours, respectively, before being re-stimulated. In relevant experiments, the global histone acetylase inhibitor TSA (Upstate Biotechnology) was added at a final concentration of 10nM 1 hour before anergy induction and allowed to remain in the culture medium during the induction of anergy. Oral tolerance was induced by the administration of a single intragastric dose of 25mg of OVA in PBS to DO11.10 mice. After 7 days CD4+ T cells were isolated from orally tolerized and PBS-fed control mice and analyzed.
ChIP
Histone acetylation, methylation and binding of HDAC2, Suv39H1 and HP1 α were assayed using a ChIP assay kit (Upstate Biotechnology), following the manufacturer’s protocol. Briefly, precleared nuclear lysates from 2×106 to 107 paraformaldehyde fixed T cells were subjected to overnight immunoprecipitation with the relevant ChIP-grade antibodies. For HDAC2 and Suv39H1, cells were pretreated for 10 minutes with 10 mM dimethyl-adipimidate-dihydrochloride before fixing with freshly prepared paraformaldehyde at 1% for 10 minutes at room temperature. Following immune complex collection, DNA fragments were recovered and subjected to real time PCR using primers designed to specifically amplify the proximal region of the Il2 promoter [17], and two regions distal to the proximal Il2 promoter/enhancer: HS -3Kb F: 5’-GTCTGAACTGAAAGCCAAACAAC, R:5’-ATGGTCCACTCATGCAAACTACT, and HS -8Kb F:5’-GCCAGAAAAGGACATCTGTATTG, R:5’-CATAAAAAGCTGGGTATGACTGC, the Cd3e promoter F:5’-TTCCTGCCTCCGCTGGAGGG, R:5’-GGCAGAAGCCTCCGCCTTGG, or the Adad1 gene promoter F:5’-CGGGCGAGGACAAGCAGCCACAT, E: 5’-GCGCCCTATGCGCGTGGGCT. Specific enrichment was calculated (after subtraction of values obtained using an isotoype control antibody) and expressed as the percent recovery of input in each experiment.
Fluorescence Microscopy
T cells were fixed in 4% paraformaldehyde, blocked, permeabilized and incubated with an anti-Ikaros primary antibody (Santa Cruz Biotechnology) and/or an anti-Me3H3K9 antibody (Abcam) and/or a anti HP1a (Millipore) and secondary antibodies conjugated with FITC, Texas-red or Alexa fluor-680. The nuclei were stained with DAPI. Immunostained cells were visualized with a fluorescence microscope. Z-stacks were acquired and processed using deconvolution software. Alternatively, images were acquired using structured illumination optical sectioning.
FISH
Cells under each experimental condition were fixed in methanol/acetic acid and dropped onto glass slides. DNA isolation and labeling and hybridization and detection were performed as previously described [64]. Aqua-labeled 2×4 tandem repeated gamma satellite probe (a gift from N. Dillon, Imperial College, London) [38], a spectrum orange- or Texas red-labeled BAC library clone for the mouse Il2 locus or a Alexa fluor 488-labeled BAC library clone for the mouse Gapdh2 locus (Clone RP23-361B19 and RP24-358H24, respectively, in a pBACe3.6 backbone, obtained from Bac/Pac Resources Children’s Hospital of Rhode Island). Slides were mounted and observed under a fluorescence microscope. Z-stacks from several individual fields from each condition were acquired per slide. Deconvoluted images were analyzed. Colocalization was scored as positive when the signals from the Il2 locus overlapped or were continuous to (in the XY, YZ and XZ planes) the nuclear regions that stained positive with the gamma satellite probe.
Statistical analysis
Differences between multiple groups were analyzed by ANOVA with a Tukey post-test. Comparisons between specific pairs of groups were analyzed with a t test. Statistical significance was defined as p<0.05.
Acknowledgements
We would like to thank Dr. N. Dillon for the generous gift of the plasmid containing the gamma satellite probe. The mouse anti-Ikaros antibody (clone 4E9) used for western blot, was a kind gift from Katia Georgopoulos This work was supported by National Institutes of Health Grant AI059738 (F.M.).
Abbreviations
- H
histone
- HDAC
histone deacetylase
- HMT
histone methyl-transferase
- TSA
trichostain A
Footnotes
Conflict of interest
None
References
- 1.Jenkins MK, Chen CA, Jung G, Mueller DL, Schwartz RH. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J. Immunol. 1990;144:16–22. [PubMed] [Google Scholar]
- 2.Schwartz RH. T cell anergy. Annu. Rev. Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 3.Chappert P, Schwartz RH. Induction of T cell anergy: integration of environmental cues and infectious tolerance. Curr. Opin. Immunol. 2010;22:552–559. doi: 10.1016/j.coi.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandyopadhyay S, Soto-Nieves N, Macian F. Transcriptional regulation of T cell tolerance. Semin. Immunol. 2007;19:180–187. doi: 10.1016/j.smim.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fields PE, Gajewski TF, Fitch FW. Blocked Ras activation in anergic CD4+ T cells. Science. 1996;271:1276–1278. doi: 10.1126/science.271.5253.1276. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Whaley CD, Mondino A, Mueller DL. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 7.Baine I, Abe BT, Macian F. Regulation of T-cell tolerance by calcium/NFAT signaling. Immunol. Rev. 2009;231:225–240. doi: 10.1111/j.1600-065X.2009.00817.x. [DOI] [PubMed] [Google Scholar]
- 8.Wells AD. New insights into the molecular basis of T cell anergy: anergy factors, avoidance sensors, and epigenetic imprinting. J. Immunol. 2009;182:7331–7341. doi: 10.4049/jimmunol.0803917. [DOI] [PubMed] [Google Scholar]
- 9.Fathman CG, Lineberry NB. Molecular mechanisms of CD4+ T-cell anergy. Nat. Rev. Immunol. 2007;7:599–609. doi: 10.1038/nri2131. [DOI] [PubMed] [Google Scholar]
- 10.Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 11.Soto-Nieves N, Puga I, Abe BT, Bandyopadhyay S, Baine I, Rao A, Macian F. Transcriptional complexes formed by NFAT dimers regulate the induction of T cell tolerance. J. Exp. Med. 2009;206:867–876. doi: 10.1084/jem.20082731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heissmeyer V, Macian F, Im SH, Varma R, Feske S, Venuprasad K, Gu H, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat. Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 13.Nurieva RI, Zheng S, Jin W, Chung Y, Zhang Y, Martinez GJ, Reynolds JM, et al. The E3 ubiquitin ligase GRAIL regulates T cell tolerance and regulatory T cell function by mediating T cell receptor-CD3 degradation. Immunity. 2010;32:670–680. doi: 10.1016/j.immuni.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat. Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 15.Puga I, Rao A, Macian F. Targeted cleavage of signaling proteins by caspase 3 inhibits T cell receptor signaling in anergic T cells. Immunity. 2008;29:193–204. doi: 10.1016/j.immuni.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, Praveen K, et al. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat. Immunol. 2006;7:1166–1173. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- 17.Bandyopadhyay S, Dure M, Paroder M, Soto-Nieves N, Puga I, Macian F. Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells. Blood. 2007;109:2878–2886. doi: 10.1182/blood-2006-07-037754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas RM, Chunder N, Chen C, Umetsu SE, Winandy S, Wells AD. Ikaros enforces the costimulatory requirement for IL2 gene expression and is required for anergy induction in CD4+ T lymphocytes. J. Immunol. 2007;179:7305–7315. doi: 10.4049/jimmunol.179.11.7305. [DOI] [PubMed] [Google Scholar]
- 19.Thomas RM, Saouaf SJ, Wells AD. Superantigen-induced CD4+ T cell tolerance is associated with DNA methylation and histone hypo-acetylation at cytokine gene loci. Genes Immun. 2007;8:613–618. doi: 10.1038/sj.gene.6364415. [DOI] [PubMed] [Google Scholar]
- 20.Taniuchi I, Ellmeier W, Littman DR. The CD4/CD8 lineage choice: new insights into epigenetic regulation during T cell development. Adv. Immunol. 2004;83:55–89. doi: 10.1016/S0065-2776(04)83002-5. [DOI] [PubMed] [Google Scholar]
- 21.Avni O, Lee D, Macian F, Szabo SJ, Glimcher LH, Rao A. T(H) cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nat. Immunol. 2002;3:643–651. doi: 10.1038/ni808. [DOI] [PubMed] [Google Scholar]
- 22.Ward SB, Hernandez-Hoyos G, Chen F, Waterman M, Reeves R, Rothenberg EV. Chromatin remodeling of the interleukin-2 gene: distinct alterations in the proximal versus distal enhancer regions. Nucl. Acids Res. 1998;26:2923–2934. doi: 10.1093/nar/26.12.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas RM, Gao L, Wells AD. Signals from CD28 induce stable epigenetic modification of the IL-2 promoter. J. Immunol. 2005;174:4639–4646. doi: 10.4049/jimmunol.174.8.4639. [DOI] [PubMed] [Google Scholar]
- 24.Barth TK, Imhof A. Fast signals and slow marks: the dynamics of histone modifications. Trends Biochem. Sci. 2010;35:618–626. doi: 10.1016/j.tibs.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, McMahon S, et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–181. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Kametani Y, Katano I, Habu S. T-cell specific enhancement of histone H3 acetylation in 5' flanking region of the IL-2 gene. Biochem. Biophys. Res. Commun. 2005;331:589–594. doi: 10.1016/j.bbrc.2005.03.216. [DOI] [PubMed] [Google Scholar]
- 28.Yui MA, Hernandez-Hoyos G, Rothenberg EV. A new regulatory region of the IL-2 locus that confers position-independent transgene expression. J. Immunol. 2001;166:1730–1739. doi: 10.4049/jimmunol.166.3.1730. [DOI] [PubMed] [Google Scholar]
- 29.Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- 30.Peters AH, O'Carroll D, Scherthan H, Mechtler K, Sauer S, Schofer C, Weipoltshammer K, et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 31.du Chene I, Basyuk E, Lin YL, Triboulet R, Knezevich A, Chable-Bessia C, Mettling C, et al. Suv39H1 and HP1gamma are responsible for chromatin-mediated HIV-1 transcriptional silencing and post-integration latency. EMBO J. 2007;26:424–435. doi: 10.1038/sj.emboj.7601517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed-Inderbitzin E, Moreno-Miralles I, Vanden-Eynden SK, Xie J, Lutterbach B, Durst-Goodwin KL, Luce KS, et al. RUNX1 associates with histone deacetylases and SUV39H1 to repress transcription. Oncogene. 2006;25:5777–5786. doi: 10.1038/sj.onc.1209591. [DOI] [PubMed] [Google Scholar]
- 33.Lachner M, O'Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 34.Lomberk G, Wallrath L, Urrutia R. The Heterochromatin Protein 1 family. Genome Biol. 2006;7:228. doi: 10.1186/gb-2006-7-7-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boussiotis VA, Barber DL, Nakarai T, Freeman GJ, Gribben JG, Bernstein GM, D'Andrea AD, et al. Prevention of T cell anergy by signaling through the gamma c chain of the IL-2 receptor. Science. 1994;266:1039–1042. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]
- 36.Grewal SI, Jia S. Heterochromatin revisited. Nat. Rev. Genet. 2007;8:35–46. doi: 10.1038/nrg2008. [DOI] [PubMed] [Google Scholar]
- 37.Bandyopadhyay S, Dure M, Paroder M, Soto-Nieves N, Puga I, Macian F. Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells. Blood. 2006 doi: 10.1182/blood-2006-07-037754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundgren M, Chow CM, Sabbattini P, Georgiou A, Minaee S, Dillon N. Transcription factor dosage affects changes in higher order chromatin structure associated with activation of a heterochromatic gene. Cell. 2000;103:733–743. doi: 10.1016/s0092-8674(00)00177-x. [DOI] [PubMed] [Google Scholar]
- 39.Macian F, Im SH, Garcia-Cozar FJ, Rao A. T-cell anergy. Curr. Opin. Immunol. 2004;16:209–216. doi: 10.1016/j.coi.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Anandasabapathy N, Ford GS, Bloom D, Holness C, Paragas V, Seroogy C, Skrenta H, et al. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–547. doi: 10.1016/s1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 41.Kriegel MA, Rathinam C, Flavell RA. E3 ubiquitin ligase GRAIL controls primary T cell activation and oral tolerance. Proc. Natl. Acad.Sci. U S A. 2009;106:16770–16775. doi: 10.1073/pnas.0908957106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, et al. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Safford M, Collins S, Lutz MA, Allen A, Huang CT, Kowalski J, Blackford A, et al. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat. Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 44.Sridharan R, Smale ST. Predominant interaction of both Ikaros and Helios with the NuRD complex in immature thymocytes. J. Biol. Chem. 2007;282:30227–30238. doi: 10.1074/jbc.M702541200. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Sif S, Jones B, Jackson A, Koipally J, Heller E, Winandy S, et al. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity. 1999;10:345–355. doi: 10.1016/s1074-7613(00)80034-5. [DOI] [PubMed] [Google Scholar]
- 46.Lee JS, Smith E, Shilatifard A. The language of histone crosstalk. Cell. 2010;142:682–685. doi: 10.1016/j.cell.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nature reviews. Mol. Cel. Biol. 2007;8:983–994. doi: 10.1038/nrm2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schultz DC, Ayyanathan K, Negorev D, Maul GG, Rauscher FJ., 3rd SETDB1: a novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev. 2002;16:919–932. doi: 10.1101/gad.973302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tachibana M, Sugimoto K, Fukushima T, Shinkai Y. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J. Biol. Chem. 2001;276:25309–25317. doi: 10.1074/jbc.M101914200. [DOI] [PubMed] [Google Scholar]
- 50.Chestier A, Yaniv M. Rapid turnover of acetyl groups in the four core histones of simian virus 40 minichromosomes. Proc. Natl. Acad.Sci. U S A. 1979;76:46–50. doi: 10.1073/pnas.76.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zee BM, Levin RS, Xu B, LeRoy G, Wingreen NS, Garcia BA. In vivo residue-specific histone methylation dynamics. J. Biol. Chem. 2010;285:3341–3350. doi: 10.1074/jbc.M109.063784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuks F, Hurd PJ, Deplus R, Kouzarides T. The DNA methyltransferases associate with HP1 and the SUV39H1 histone methyltransferase. Nucl. Acids Res. 2003;31:2305–2312. doi: 10.1093/nar/gkg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallrath LL, Elgin SC. Position effect variegation in Drosophila is associated with an altered chromatin structure. Genes Dev. 1995;9:1263–1277. doi: 10.1101/gad.9.10.1263. [DOI] [PubMed] [Google Scholar]
- 54.Hewitt SL, High FA, Reiner SL, Fisher AG, Merkenschlager M. Nuclear repositioning marks the selective exclusion of lineage-inappropriate transcription factor loci during T helper cell differentiation. Eur. J. Immunol. 2004;34:3604–3613. doi: 10.1002/eji.200425469. [DOI] [PubMed] [Google Scholar]
- 55.Brown KE, Guest SS, Smale ST, Hahm K, Merkenschlager M, Fisher AG. Association of transcriptionally silent genes with Ikaros complexes at centromeric heterochromatin. Cell. 1997;91:845–854. doi: 10.1016/s0092-8674(00)80472-9. [DOI] [PubMed] [Google Scholar]
- 56.Cobb BS, Morales-Alcelay S, Kleiger G, Brown KE, Fisher AG, Smale ST. Targeting of Ikaros to pericentromeric heterochromatin by direct DNA binding. Genes Dev. 2000;14:2146–2160. doi: 10.1101/gad.816400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas RM, Chen C, Chunder N, Ma L, Taylor J, Pearce EJ, Wells AD. Ikaros silences T-bet expression and interferon-gamma production during T helper 2 differentiation. J. Biol. Chem. 2010;285:2545–2553. doi: 10.1074/jbc.M109.038794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villarino AV, Katzman SD, Gallo E, Miller O, Jiang S, McManus MT, Abbas AK. Post-transcriptional silencing of effector cytokine mRNA underlies the anergic phenotype of self-reactive T cells. Immunity. 2011;34:50–60. doi: 10.1016/j.immuni.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonasio R, Tu S, Reinberg D. Molecular signals of epigenetic states. Science. 2010;330:612–616. doi: 10.1126/science.1191078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naito T, Gomez-Del Arco P, Williams CJ, Georgopoulos K. Antagonistic interactions between Ikaros and the chromatin remodeler Mi-2beta determine silencer activity and Cd4 gene expression. Immunity. 2007;27:723–734. doi: 10.1016/j.immuni.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 61.Klose RJ, Yamane K, Bae Y, Zhang D, Erdjument-Bromage H, Tempst P, Wong J, et al. The transcriptional repressor JHDM3A demethylates trimethyl histone H3 lysine 9 and lysine 36. Nature. 2006;442:312–316. doi: 10.1038/nature04853. [DOI] [PubMed] [Google Scholar]
- 62.Whetstine JR, Nottke A, Lan F, Huarte M, Smolikov S, Chen Z, Spooner E, et al. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell. 2006;125:467–481. doi: 10.1016/j.cell.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 63.Dure M, Macian F. IL-2 signaling prevents T cell anergy by inhibiting the expression of anergy-inducing genes. Mol Immunol. 2009;46:999–1006. doi: 10.1016/j.molimm.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Montagna C, Lyu MS, Hunter K, Lukes L, Lowther W, Reppert T, Hissong B, et al. The Septin 9 (MSF) gene is amplified and overexpressed in mouse mammary gland adenocarcinomas and human breast cancer cell lines. Cancer Res. 2003;63:2179–2187. [PubMed] [Google Scholar]