Abstract
OBJECTIVES
Surveillance after resection of solitary fibrous tumours of the pleura (SFTP) remains undefined. This study reviews our experience with surgical treatment of SFTP to determine the specific risk factors to predict recurrence.
METHODS
A retrospective review of 59 patients surgically treated for SFTP during the years 1977–2010 was conducted. Clinico-pathological factors for recurrence were analysed by Kaplan–Meier and Cox proportional hazard methods.
RESULTS
The mean age was 57 ± 14 years. There were 32 (54%) men. Among 32 (54%) symptomatic patients, chest pain (22%), cough (19%) and dyspnoea (17%) were most frequent. The mean tumour size was 7.3 ± 6.7 cm, and 14 patients had SFTPs larger than 10 cm. An SFTP was pedunculated in 38 (67%) cases and had a visceral origin in 40 (68%). Paraneoplastic syndromes were observed in 3 (5%) patients. On histopathologic analysis, 4 (7%) presented ≥4 mitosis/10 high-power fields (HPFs), 8 (15%) atypia, 14 (24%) hypercellularity and 6 (10%) necrosis. After a mean follow-up of 8.8 ± 7.0 years, we observed 8 (14%) recurrences; median time to recurrence was 6 years (range 2–16 years). Two (3%) patients received adjuvant therapy. We constructed a predictive score for recurrence by assigning one point to each of the six variables: parietal (vs visceral) pleural origin, sessile (vs pedunculated) morphology, size >10 cm (vs <10 cm), the presence of hypercellularity, necrosis and mitotic activity ≥4/HPF (vs <4). A score of ≥3 best predicted recurrence (sensitivity: 100%, specificity: 92%, area under receiver operating characteristic curve = 0.966, P < 0.0001). With a score of ≥3, recurrence-free survival was 80%, 69, 23 and 23% at 3, 5, 10 and 15 years, whereas a score of <3 was 100% up to 15 years. Our scoring system was superior in predicting malignant behaviour and recurrence compared with England's criteria or de Perrot staging.
CONCLUSIONS
The proposed scoring system is simple, easily obtained from existing pathological description and reliably predicts recurrence in this patient population harbouring SFTP. The SFTP score may stratify patient risk and guide postoperative surveillance. We recommend validation in additional clinical series.
Keywords: Solitary fibrous tumour, Pleura, Recurrence
INTRODUCTION
Solitary fibrous tumours of the pleura (SFTP) are infrequent neoplasms of mesenchymal origin that are thought to arise from submesothelial connective tissue [1]. In most cases, these tumours have a benign biological behaviour, where primary surgical resection remains the mainstay of curative treatment. However, there is a subpopulation of patients who develop pleural recurrence, or even more rarely, metastasize and die. Factors associated with a malignant phenotype have been recognized for years, based on a 1989 landmark Armed Forces Institute of Pathology study by England et al. [2]. In 223 SFTPs, factors associated with aggressive behavior included origin from the parietal pleura, absence of a stalk, size >10 cm, presence of hypercellularity, pleomorphism, tumour necrosis or haemorrhage and ≥4 mitosis/10 high-power fields (HPFs).
Despite recognition in the early 1990s of histopathological risk factors for predicting malignant behaviour of resected SFTP, more contemporary surgical series [3–10] have combined clinical and morphological criteria to classify tumours as ‘malignant’, making comparisons across few studies difficult. Furthermore, no study, to date, has defined radiographical follow-up and surveillance after complete resection. In the absence of consensus regarding optimal postoperative surveillance for recurrence (in both benign and malignant phenotypes), various radiographical studies and follow-up intervals are observed after resection despite benign behaviour in most cases [11].
The objective of the present study is to review our experience with the surgical treatment of SFTP and tumour recurrence. We evaluate the ordered use of clinical and pathological features associated with recurrence by proposing a predictive scoring system. The proposed scoring system aims to classify the patients according to their risk of recurrence to inform postoperative surveillance. A reliable scoring system potentially reduces unnecessary radiation exposure from imaging studies and relaxes observation protocol in patients at low risk of recurrence while reserving a stricter follow-up protocol among patients at high risk.
MATERIALS AND METHODS
The study population included consecutive patients undergoing surgical treatment for SFTP at the Massachusetts General Hospital between 1st January 1977 and 31st December 2010. Patients were identified in a prospectively organized pathology and surgery database, and retrospectively analysed. Demographics, preoperative, intraoperative and postoperative data as well as long-term outcome were recorded. The Institutional Review Board approved this retrospective chart review, including subject selection and confidentiality, and waived the need for patient consent; they also granted permission to contact patients by telephone when feasible.
All patients underwent diagnostic chest computed tomography and occasionally, magnetic resonance imaging (MRI). Tissue to confirm a diagnosis was obtained before resection in 39% of patients. Available histological slides were reviewed by a single pulmonary pathologist (M.M.K.) to determine the factors known to be associated with recurrence and/or malignant behaviour [2]. The presence of hypercellularity, pleomorphism and nuclear atypia, necrosis or haemorrhage and the mitotic index (the number of mitotic figures per 10 HPFs) were recorded. Histological slides available for 38 of the 59 (64%) patients were individually stained for a Ki67 proliferation index. Immunostaining of CD34, pan-cytokeratin and vimentin was not routinely performed for SFTP until 1998 at our institution.
Long-term follow-up data were obtained primarily from the patient's medical record. Where feasible, patients were contacted about their disease and follow-up visits, specifically about the results of the last imaging study of the chest. The Social Security Death Index (SSDI) was consulted to determine the vital status of the patients. The primary endpoint of this study is tumour recurrence. Secondary endpoints analysed were postoperative morbidity, in-hospital or 30-day mortality and overall and disease-free survival.
Statistical analysis
Data were recorded in a database designed in Microsoft Office Excel 2007 (Microsoft, Redmond, WA, USA) and later exported to Stata/SE 10.1 statistical software (StataCorp LP, College Station, TX, USA). Variables were analysed as proportions, means or medians according to their nature. Overall survival was defined as the time from surgery to death from any cause based on SSDI data. Disease-free survival was defined as the time from surgery to tumour recurrence or to death from any cause based on data from medical records only. Recurrence was defined as the time from surgery to the date of first evidence of tumour recurrence, censoring patients who died without experiencing recurrence based on medical records. Potential risk factors for recurrence were analysed by the Kaplan–Meier and Cox proportional hazard methods. A multivariate model including six morphological and histopathological variables was elaborated based on univariate analysis results and literature findings. Harrell's C-statistic was calculated to assess the predictive power, and its 95% confidence interval (CI) was calculated by bootstrapping with 1000 repetitions. A predictive scoring system was constructed with variables included in this model in order to predict recurrence. Receiver operating characteristic (ROC) curves were plotted for the scoring system and were compared with ROC curves constructed from previously described criteria for malignancy of SFTP, namely England et al.'s criteria [2] (the presence of hypercellularity, pleomorphism/atypia, necrosis/haemorrhage or a mitotic count ≥4 mitosis/10 HPFs) and de Perrot's classification [11] (England's criteria plus the presence of stalk). For all comparisons, statistical significance was considered with P-value of <0.05.
RESULTS
Fifty-nine patients underwent surgical treatment of primary SFTP during the study period. Baseline characteristics and presenting symptoms are described in Table 1. Tumours were located in the right chest in 30 (51%) patients. At least 6 (10%) patients harboured an associated pleural effusion. Preoperative tumour diagnosis was obtained in 23 (39%) patients; 17 (29%) of these underwent fine-needle aspiration (FNA) biopsy, 4 (7%) had a core needle biopsy and 2 (3%) open incisional biopsy. Percutaneous needle biopsies were often unsuccessful: a preoperative diagnosis was reached in only 6 of all 21 patients (29%), and specifically in only 3 of 17 (18%) FNA and 3 of 4 (75%) core needle biopsies.
Table 1:
Baseline characteristics and presenting symptoms among 59 patients undergoing surgical treatment of SFTP from 1977 to 2010
| Characteristic | Result |
|---|---|
| Age | |
| Median (range) | 60 (28–79) |
| Mean ± SD | 57.1 ± 13.6 |
| Sex, n (%) | |
| Male | 32 (54) |
| Female | 27 (46) |
| Toxic exposures, n (%) | |
| Tobacco | 25 (42) |
| Pack-year, mean ± SD | 39.8 ± 28.4 |
| Asbestos | 5 (8) |
| Comorbidities, n (%) | |
| Hypertension | 16 (27) |
| Coronary artery disease | 3 (5) |
| Diabetes | 2 (3) |
| Chronic obstructive pulmonary disease | 2 (3) |
| Asthma | 2 (3) |
| Atrial fibrillation | 1 (2) |
| History of neoplasia, n (%) | 10 (17) |
| Schwannoma | 3 (5) |
| Breast | 2 (3) |
| Lung | 1 (2) |
| Thyroid | 1 (2) |
| Melanoma | 1 (2) |
| Endometrium | 1 (2) |
| Prostate | 1 (2) |
| Previous surgery, n (%) | 20 (34) |
| Ongoing pregnancy, n (%) | 1 (4) |
| Presence of symptoms, n (%) | 32 (54) |
| Duration of symptoms (months) | |
| Median (range) | 4.5 (0.25–60) |
| Symptoms, n (%) | |
| Chest pain | 13 (22) |
| Cough | 11 (19) |
| Dyspnoea | 10 (17) |
| Flu-like | 6 (10) |
| Weight loss | 4 (7) |
| Abdominal pain | 1 (2) |
| Paraneoplastic syndromes, n (%) | |
| Hypertrophic osteoarthropathy | 2 (3) |
| Hypoglycaemia | 1 (2) |
This study includes different surgical approaches. Thoracotomy was preferred in 41 (69%) patients, followed by video assisted thoracoscopic surgery in 14 (24%), of whom 3 required conversion to thoracotomy. Median sternotomy and a thoracoabdominal incision were used in 2 (3%) patients each. A complete (R0) resection was performed in all patients. Wedge resection of the lung was required in 43 (73%) cases, lobectomy in 4 (7%) and bilobectomy and segmentectomy in 1 (2%) each; en bloc chest wall resections were required in 5 (8%) cases.
Postoperative course
All patients had a chest tube placed at the end of surgery. The median duration of chest drainage was 3 days (range 1–15 days). Seven (12%) patients experienced postoperative complications: 4 (7%) had atelectasis while haematuria, pulmonary oedema and postoperative bleeding requiring re-exploration occurred in 1 (2%) each. No patients died in-hospital or within 30 days after surgery.
Tumour characteristics and histopathology
SFTP originated from the visceral pleura in 40 (68%) cases and the parietal pleura in 16 (27%), and were intrapulmonary in 3 (5%). Tumours originated from the left upper lobe in 8 (14%) patients, the left lower lobe in 14 (24%), the right upper lobe in 8 (14%), the right middle lobe in 1 (2%), the right lower lobe in 11 (19%), the chest wall in 9 (16%), the mediastinum in 5 (9%) and the diaphragm in 2 (3%). A stalk was observed in 38 (67%) cases. The median tumour size in the long axis was 5 (range 0.7–33 cm); 14 (25%) patients had SFTPs larger than 10 cm.
Histopathological review identified hypercellularity in 14 (24%) cases, pleomorphism or atypia in 8 (14%), necrosis or haemorrhage in 6 (10%), and the median number of mitosis per 10 HPFs was 1 (range 0–12), with only 4 patients having ≥4 mitosis/10 HPFs. Ki67 staining of 38 tumour specimens showed a median proliferative index of 5.2% (range 0.5–13.5%); seven (18%) tumours had a Ki67 of ≥10%.
Long-term follow-up and overall survival
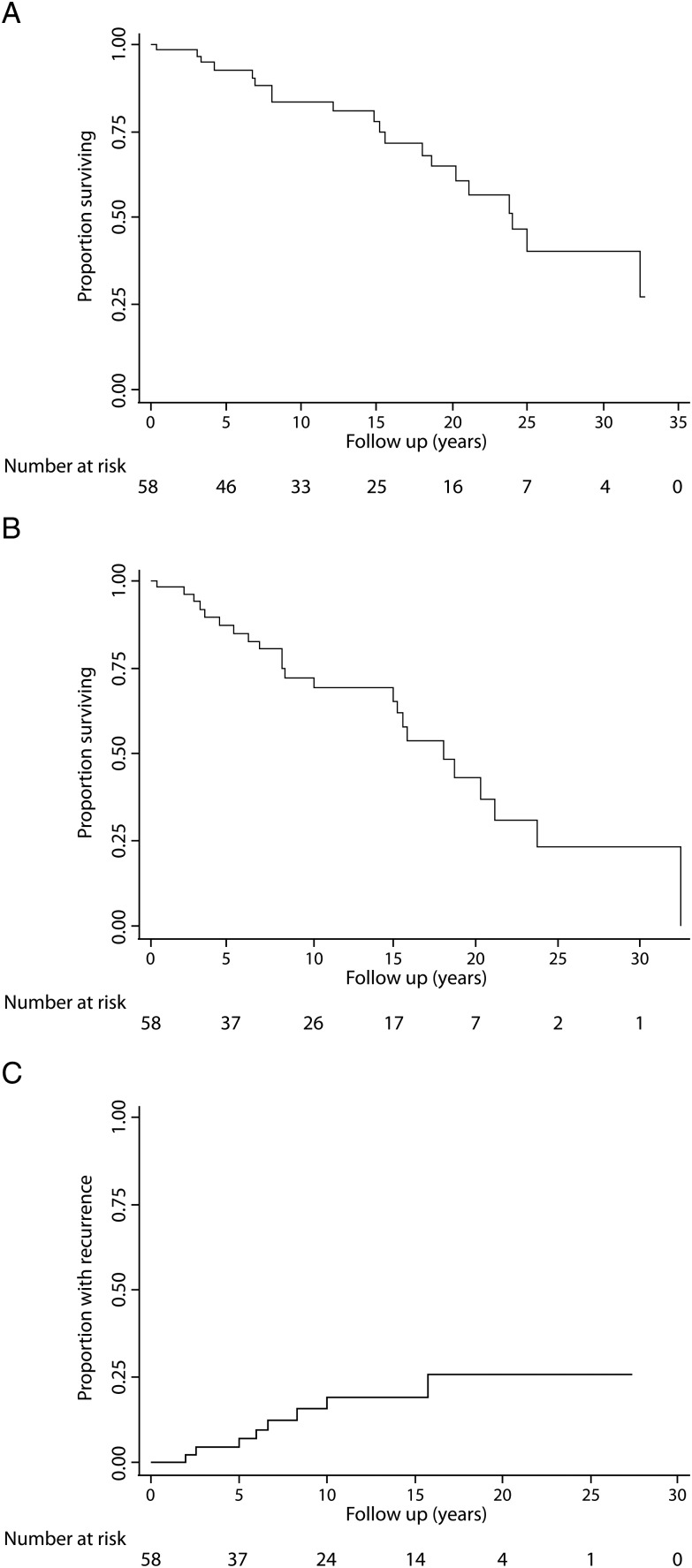
No patient received adjuvant therapy after surgical resection. Cohort median overall survival was 24 years after a median follow-up of 12.9 years (range 1 month–32.8 years). Overall survival (standard error; 95% CI) at 1, 3, 5, 10, 15 and 20 years was 98.2 (1.8%; 88.0–99.8%), 98.2 (1.8%; 88.0–99.8%), 92.4 (3.7%; 81.0–97.1%), 83.4 (5.4%; 69.5–91.4%), 77.7 (6.4%; 62.1–87.5%) and 64.5% (8.0%; 46.5–77.8%), respectively (Fig. 1A). After a median follow-up based on medical records of 8.1 years (range 1 month–32.5 years), the calculated disease-free survival (standard error; 95% CI) at 1, 3, 5, 10, 15 and 20 years was 98.2 (1.8%; 87.6–99.7%), 94.0 (3.4%; 82.4–98.0%), 87.2 (4.9%; 73.7–94.1%), 72.1 (7.0%; 55.8–83.2%), 65.5 (7.8%; 48.0–78.3%) and 42.9% (9.8%; 23.7–60.8%), respectively (Fig. 1B). Tumour recurrence (standard error; 95% CI) at 1, 3, 5, 10, 15 and 20 years was 0 (NA; NA), 4.3 (3.0%; 1.1–16.0%), 4.3 (3.0%; 1.1–16.0%), 15.2 (5.8%; 7.1–31.1%), 18.8 (6.6%; 9.3–35.9%) and 25.5% (8.8%; 12.5–47.8%) (Fig. 1C). There were no differences in overall survival when comparing patients classified as high or low risk based on the proposed scoring system for recurrence (P = 0.218), by England's criteria (P = 0.107) or de Perrot's classification (P = 0.314).
Figure 1:
Overall (A) and disease-free (B) survival, and time to recurrence (C) after surgery for SFTP.
Risk factor analysis for recurrence: development of a scoring system
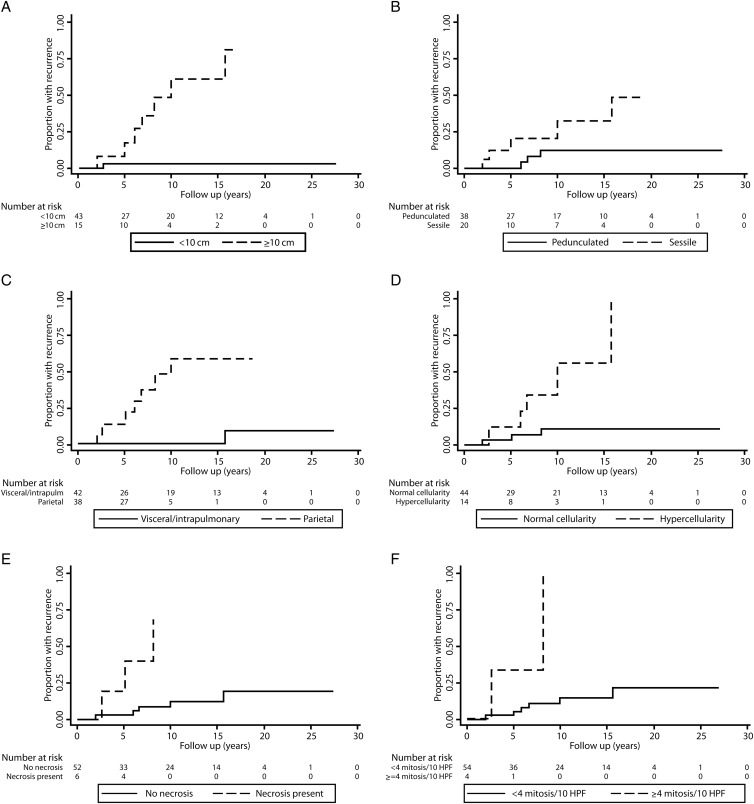
After a median follow-up of 8 years (range 1 month–27 years), there have been 8 (14%) recurrences. Median cohort recurrence has not been achieved. Median time to recurrence was 6 years (range 2–16 years), and the latest recurrence observed was ∼16 years after resection. All recurrences were ipsilateral to the original tumour without extrathoracic disease. Two patients required chest wall resection to achieve an R0 resection. On univariate analysis, the greatest impact on survival was observed for tumour size, presence of a pleural effusion, hypercellularity, necrosis and ≥4 mitosis/HPFs. Symptoms at presentation, sessile morphology and pleomorphism were not significant for recurrence among the parameters listed in Table 2. Factors found to be most associated with recurrence-free survival of SFTP are shown in Fig. 2. A multivariate model was constructed with six morphological and histopathological parameters that harboured P < 0.100 (i.e. pleural origin, presence of stalk, size, hypercellularity, presence of necrosis and mitotic count). Pleural effusion was omitted from the scoring system due to the low frequency rate in our series and the lack of accurate description of this finding in the reviewed medical records. Harrell's C-statistic was calculated for this model, being 0.953 (95% CI 0.909–0.997). The single variable significant on multivariate analysis was tumour size ≥10 cm.
Table 2:
Risk factor analysis for SFTP recurrence
| Variable | Univariate |
Multivariate |
|||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | Coefficient | HR (95% CI) | P-value | |
| Symptoms at presentation | 6.04 (0.74–49.12) | 0.093 | |||
| Pleural effusion | 11.53 (2.88–46.17) | 0.001 | |||
| Origin from parietal pleura vs visceral/intrapulmonary | 25.49 (2.99–217.18) | 0.003 | 3.359 | 28.76 (0.52–1586.3) | 0.101 |
| Sessile morphology | 4.01 (0.95–16.90) | 0.058 | 0.441 | 1.55 (0.19–12.83) | 0.682 |
| Diameter ≥10 cm | 25.74 (3.14–210.66) | 0.002 | 2.405 | 11.08 (1.07–114.80) | 0.044 |
| Hypercellularity | 8.32 (1.94–35.74) | 0.004 | 2.376 | 10.77 (0.69–168.87) | 0.091 |
| Cellular pleomorphism | 1.10 (0.13–9.24) | 0.928 | |||
| Necrosis | 10.44 (2.08–52.40) | 0.004 | 3.134 | 22.99 (0.47–1118.1) | 0.114 |
| Mitosis ≥4/10 HPFs | 12.13 (2.16–68.33) | 0.005 | −0.252 | 0.78 (0.03–20.18) | 0.879 |
| Ki67 ≥10% | 14.02 (1.44–136.17) | 0.023 | |||
The numbers in bold represent a P-value that are statistically significant.
Figure 2:
Kaplan–Meier time to recurrence curves for factors found to be associated with SFTP recurrence.
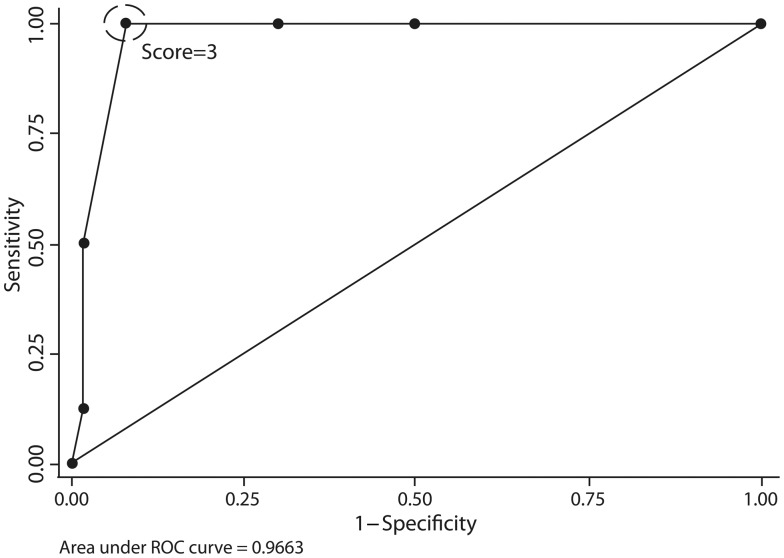
A scoring system was constructed assigning one point to each of the six tumour characteristics, as shown in Table 3. The median score in our cohort was 1 (range 0–5). Scores had the following distribution: 0 in 25 (42%) patients, 1 in 11 (19%), 2 in 11 (19%), 3 in 7 (12%), 4 in 3 (5%), 5 in 2 (3%) and 6 points in no patients. ROC curves were plotted for this scoring system as shown in Fig. 3 and compared with previously established criteria for malignancy. The area under the ROC curve was 0.966 (0.924–1.000). The scoring system was most predictive for tumour recurrence when using a score of three points or greater as cut-off, yielding a sensitivity of 100%, specificity of 92%, a positive likelihood ratio of 12.5 and a negative likelihood ratio of <0.0001. The proposed scoring system's area under the ROC curve was significantly greater than that obtained with England's criteria [0.808 (0.670–0.945); P = 0.020] and de Perrot's classification [0.855 (0.755–0.955); P = 0.016].
Table 3:
Proposed scoring system for SFTP recurrence
| Tumour characteristic | Points |
|---|---|
| Pleural origin | |
| Visceral/intrapulmonary | 0 |
| Parietal | 1 |
| Morphology | |
| Pedunculated | 0 |
| Sessile | 1 |
| Size (long axis) | |
| <10 cm | 0 |
| ≥10 cm | 1 |
| Hypercellularity | 1 |
| Presence of necrosis or haemorrhage | 1 |
| Number of mitosis (per 10 HPFs) | |
| <4 | 0 |
| ≥4 | 1 |
Minimum score: 0; maximum score: 6.
Figure 3:
ROC curve for the proposed scoring system for SFTP recurrence. Best diagnostic performance is using a cut-off at score of 3.
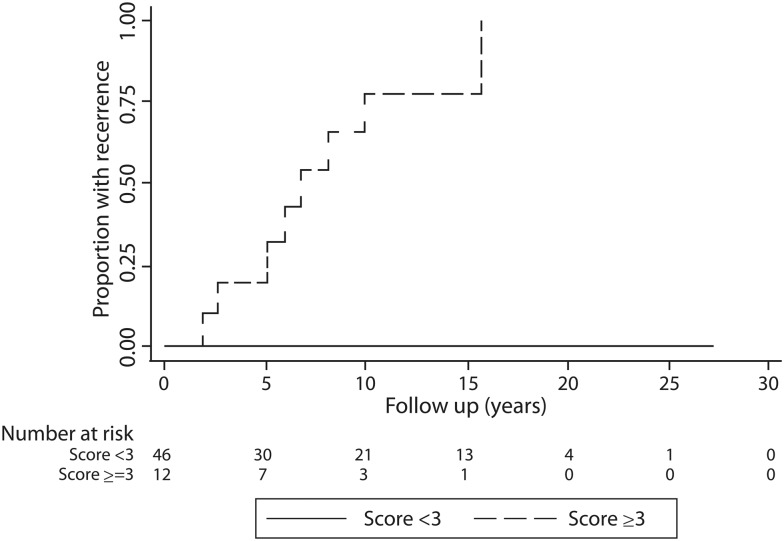
Accordingly, 12 (20%) patients were then classified at high risk for recurrence (≥3 points), while the remaining 47 (80%) were classified at low risk (<3 points). The contribution of clinical and histopathological parameters to the patient score is listed in Table 4. Patients with scores ≥3 often underwent more aggressive surgery (i.e. lobectomy or chest wall resection). SFTP recurrence among groups was significantly lower in patients classified as low risk: 0% at 15 years compared with 77% in patients considered at high risk (P < 0.0001) as shown in Fig. 4. No recurrences occurred in the group classified as low risk (<3 points). Tumour recurrences were ipsilateral to the previously resected tumour in all cases. A solitary recurrent lesion was found in 4 patients, while multiple lesions were observed in 4. Four recurrent cases were seen to arise from the parietal pleura, while in the other 4 it was found to be invading both the visceral and parietal pleura. No recurrent tumour was pedunculated. In 1 case, a solitary recurrent tumour was observed to have a benign histology without mitotic activity; among the other seven recurrent tumours, the mitotic count ranged from 1 mitosis to 17 mitosis/HPFs with five tumours having a mitotic count greater than 4/HPF.
Table 4:
Tumour characteristics, surgical resection and recurrence-free survival stratified by the proposed scoring system
| Variable, n (%) | Score ≥3 (n = 12) | Score <3 (n = 47) | P-value |
|---|---|---|---|
| Presence of symptoms | 9 (75) | 23 (49) | 0.193 |
| Pleural effusion | 5 (42) | 1 (2) | 0.001 |
| Sessile morphology | 9 (75) | 12 (26) | 0.002 |
| Visceral pleura/intrapulmonary origin | 2 (17) | 41 (87) | <0.001 |
| Size ≥10 cm | 9 (75) | 6 (13) | <0.001 |
| Presence of necrosis | 4 (33) | 2 (4) | 0.013 |
| Hypercellularity | 8 (67) | 6 (13) | <0.001 |
| Mitosis ≥4/10 HPFs | 3 (25) | 1 (2) | 0.024 |
| Type of surgery | 0.064 | ||
| Local excision only | 1 (8) | 4 (9) | |
| Sublobar resection | 6 (50) | 38 (81) | |
| Lobectomy/bilobectomy | 2 (17) | 3 (6) | |
| Chest wall resection | 3 (25) | 2 (4) | |
| Recurrence | <0.0001 | ||
| 3 years | 20% | 0% | |
| 5 years | 20% | 0% | |
| 10 years | 77% | 0% |
Figure 4:
Time to recurrence curve according to the risk based on the proposed scoring system.
DISCUSSION
Solitary fibrous tumours are rare pleural neoplasms with benign histopathology in ∼80% of cases. Despite benign characteristics, some of these tumours have the propensity to recur via malignant transformation— likely through an undefined molecular pathway of tumourigenesis. A few studies have attempted to crystallize features that portend malignant behaviour and recurrence. Early reports focused on histopathological characteristics (such as necrosis, mitosis, hypercellularity and pleomorphism) [2]. Later, p53 expression [5], sessile tumour morphology (inverted tumours) [11] and patient symptoms [3] were correlated with malignant potential. While no single factor has been elucidated to help predict the biological behaviour of SFTP, we found that tumour size alone was a sole predictor (hazard ratio, HR = 11.1) of recurrence-free survival on multivariate analysis of all clinical and pathological criteria.
Molecular studies [5, 12] have contributed to the differentiation of localized fibrous tumours from malignant mesothelioma, but still fall short in predicting recurrence. Localized tumours typically lack the expression of cytokeratin, but can express vimentin (a mesenchymal marker), CD34 (a haematopoietic stem cell marker) and CD99 (a spindle cell tumour marker) [12, 13]. The expression of Bcl-2 (an apoptosis regulator and proto-oncogene) can be useful in CD34-negative tumours to confirm SFTP [11, 14]. In a study that included the examination of several actionable mutations (among them c-kit, BRAF, platlet-derived growth factor receptor, c-met and epidermal growth factor receptor) in 88 patients diagnosed with SFTP, only high expression of p53 and tumour necrosis were observed to influence disease-free survival [5]. Of 14 resected tumours evaluated for CD34 in our case series, CD34 expression was positive in all but one and is not a predictor of recurrence (data not shown). Similar to previous published series, we did not routinely perform immunohistochemistry on all cases. The immunohistochemical characterization of SFTP is not standardized across institutions: in the current era, a consensus of opinion should be organized to unify criteria and make comparisons feasible among future publications on SFTP.
Given the slowly progressive and sometimes unpredictable biological nature of SFTP, a challenging issue in these patients is appropriate surveillance. Recurrence after complete resection is infrequent in the majority of patients. Single-institution case series report recurrence in 10–15% of resected tumours [3–7, 10]. We observed recurrence in 14% of our study cohort with 37.5% (3 of 8) of the cases having a histologically ‘bland’ or benign pathology. Moreover, our median time to recurrence of 6 years and latest recurrence at 16 years were long. The proposed scoring system as applied to our patients enabled us to identify patients without recurrence in more than 20 years (score <3). Since there is increasing concern about cumulative radiation exposure and cost from surveillance imaging [15], we recommend that patients with the lowest risk of recurrence undergo a less-aggressive surveillance imaging strategy.
After reviewing the literature for SFTP, the use of diagnostic FNA biopsy has a disappointing yield from 20 to 39%. We and others have found improved yields and better tumour characterization with the use of a core needle biopsy. Diagnostic biopsy of these lesions may not always be necessary, but 75% of the core samples were diagnostic in our study. We also found that preoperative percutaneous embolization of feeding arteries in large tumours (>20 cm) can contribute to reduced intraoperative blood loss. The use of a thoracoabdominal approach to SFTP was very helpful to extricate large tumours.
Our specific goal was to develop a reliable prediction of recurrence to influence the use of surveillance chest imaging. The standard imaging is still chest computed tomography, but chest MRI is a non-radiation alternative with improving visualization. Based on our risk classification, 12 patients in our study cohort would require a strict follow-up with chest imaging, while 20 based on England et al. criteria, and 30 based on de Perrot's classification would require this kind of surveillance (even though de Perrot et al. [11] recommend at least yearly radiological examination for all patients). Our proposed score and classification are simple and easy to implement in clinical practise. Additionally, this scoring system could standardize future literature on SFTP, thus allowing direct comparisons or pooling of data. Nevertheless, this model must be validated and refined in other patient populations of resected SFTP.
Our study has obvious limitations by virtue of a small sample size and the retrospective nature of data collection. The analysis could be strengthened using more current immunohistochemical interrogation of all tumours. As decades have elapsed since this study, paraffin sections of some older tumours were unavailable. The proposed scoring system for SFTP recurrence will clearly need further validation in larger and different populations; specifically, the proposed simple scoring system (assigning one point to each variable, some of which were not significant on multivariate analysis, but were included because of the consistent association reported in the literature) needs to be enhanced by performing comparisons with a weighted score based on the multivariate recurrence-free survival model coefficients derived from larger samples. There is also the question of defining an intermediate-risk group, which can only be achieved by combining single-centre populations. Interestingly, the role of Ki67 staining seems promising as a predictive variable for tumour recurrence. Seven of the eight recurrences had Ki67 ≥10%. We were unable to include this variable in our multivariate model for recurrence due to the small number of slides available to perform this staining.
CONCLUSIONS
Surgical treatment of SFTP remains the primary curative therapy with low perioperative morbidity and mortality. A new scoring system constructed on common clinical and histological characteristics is easy to compute, correlates well with recurrence and predicted all recurrences when retrospectively applied to our study cohort. The score may be used to assess prognosis and to guide postoperative surveillance. If validated in a larger population, selected patients with excellent prognosis may not require surveillance imaging. In contrast, patients with scores predictive of disease recurrence may be selected for the study of adjuvant therapy and frequent surveillance imaging.
Funding
This work was supported by the Division of Thoracic Surgery at the Massachusetts General Hospital. The statistical analysis of this work was conducted with support from Harvard Catalyst; The Harvard Clinical and Translational Science Center; National Center for Research Resources and the National Center for Advancing Translational Sciences; National Institutes of Health Award [8UL1TR000170-05] and financial contributions from Harvard University and its affiliated academic health care centres. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centres, or the National Institutes of Health.
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
We acknowledge our data manager and research coordinator, Sheila Cann and Diane Davies, respectively, for their diligence and dedication towards compiling and maintaining the Thoracic Surgery Database.
REFERENCES
- 1.Abu Arab W. Solitary fibrous tumors of the pleura. Eur J Cardiothorac Surg. 2012;41:587–97. doi: 10.1093/ejcts/ezr009. doi:10.1093/ejcts/ezr009. [DOI] [PubMed] [Google Scholar]
- 2.England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol. 1989;13:640–58. doi: 10.1097/00000478-198908000-00003. doi:10.1097/00000478-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Cardillo G, Carbone L, Carleo F, Masala N, Graziano P, Bray A, et al. Solitary fibrous tumors of the pleura: an analysis of 110 patients treated in a single institution. Ann Thorac Surg. 2009;88:1632–7. doi: 10.1016/j.athoracsur.2009.07.026. doi:10.1016/j.athoracsur.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 4.Harrison-Phipps KM, Nichols FC, Schleck CD, Deschamps C, Cassivi SD, Schipper PH, et al. Solitary fibrous tumors of the pleura: results of surgical treatment and long-term prognosis. J Thorac Cardiovasc Surg. 2009;138:19–25. doi: 10.1016/j.jtcvs.2009.01.026. doi:10.1016/j.jtcvs.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schirosi L, Lantuejoul S, Cavazza A, Murer B, Yves Brichon P, Migaldi M, et al. Pleuro-pulmonary solitary fibrous tumors: a clinicopathologic, immunohistochemical, and molecular study of 88 cases confirming the prognostic value of de Perrot staging system and p53 expression, and evaluating the role of c-kit, BRAF, PDGFRs (alpha/beta), c-met, and EGFR. Am J Surg Pathol. 2008;32:1627–42. doi: 10.1097/PAS.0b013e31817a8a89. doi:10.1097/PAS.0b013e31817a8a89. [DOI] [PubMed] [Google Scholar]
- 6.Rosado-de-Christenson ML, Abbott GF, McAdams HP, Franks TJ, Galvin JR. From the archives of the AFIP: localized fibrous tumor of the pleura. Radiographics. 2003;23:759–83. doi: 10.1148/rg.233025165. doi:10.1148/rg.233025165. [DOI] [PubMed] [Google Scholar]
- 7.Sung SH, Chang JW, Kim J, Lee KS, Han J, Park SI. Solitary fibrous tumors of the pleura: surgical outcome and clinical course. Ann Thorac Surg. 2005;79:303–7. doi: 10.1016/j.athoracsur.2004.07.013. doi:10.1016/j.athoracsur.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Magdeleinat P, Alifano M, Petino A, Le Rochais JP, Dulmet E, Galateau F, et al. Solitary fibrous tumors of the pleura: clinical characteristics, surgical treatment and outcome. Eur J Cardiothorac Surg. 2002;21:1087–93. doi: 10.1016/s1010-7940(02)00099-4. doi:10.1016/S1010-7940(02)00099-4. [DOI] [PubMed] [Google Scholar]
- 9.Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, Lewis JJ, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002;94:1057–68. doi:10.1002/cncr.10328. [PubMed] [Google Scholar]
- 10.Lahon B, Mercier O, Fadel E, Ghigna MR, Petkova B, Mussot S, et al. Solitary fibrous tumor of the pleura: outcomes of 157 complete resections in a single center. Ann Thorac Surg. 2012;94:394–400. doi: 10.1016/j.athoracsur.2012.04.028. doi:10.1016/j.athoracsur.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 11.de Perrot M, Fischer S, Bründler MA, Sekine Y, Keshavjee S. Solitary fibrous tumors of the pleura. Ann Thorac Surg. 2002;74:285–93. doi: 10.1016/s0003-4975(01)03374-4. doi:10.1016/S0003-4975(01)03374-4. [DOI] [PubMed] [Google Scholar]
- 12.Van de Rijn M, Lombard CM, Rouse RV. Expression of CD34 by solitary fibrous tumors of the pleura, mediastinum, and lung. Am J Surg Pathol. 1994;18:814–20. doi: 10.1097/00000478-199408000-00008. doi:10.1097/00000478-199408000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez-Gil Y, González MA, Carcavilla CB, Santamaría JS. Lines of cell differentiation in solitary fibrous tumor: an ultrastructural and immunohistochemical study of 10 cases. Ultrastruct Pathol. 2009;33:274–85. doi: 10.3109/01913120903352177. doi:10.3109/01913120903352177. [DOI] [PubMed] [Google Scholar]
- 14.Carretta A, Bandiera A, Melloni G, Ciriaco P, Arrigoni G, Rizzo N, et al. Solitary fibrous tumors of the pleura: immunohistochemical analysis and evaluation of prognostic factors after surgical treatment. J Surg Oncol. 2006;94:40–4. doi: 10.1002/jso.20562. doi:10.1002/jso.20562. [DOI] [PubMed] [Google Scholar]
- 15.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. doi:10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]