Abstract
Exposure to thirdhand smoke (THS) is a newly described health risk. Evidence supports its widespread presence in indoor environments. However, its genotoxic potential, a critical aspect in risk assessment, is virtually untested. An important characteristic of THS is its ability to undergo chemical transformations during aging periods, as demonstrated in a recent study showing that sorbed nicotine reacts with the indoor pollutant nitrous acid (HONO) to form tobacco-specific nitrosamines (TSNAs) such as 4-(methylnitrosamino)-4-(3-pyridyl)butanal (NNA) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). The goal of this study was to assess the genotoxicity of THS in human cell lines using two in vitro assays. THS was generated in laboratory systems that simulated short (acute)- and long (chronic)-term exposures. Analysis by liquid chromatography–tandem mass spectrometry quantified TSNAs and common tobacco alkaloids in extracts of THS that had sorbed onto cellulose substrates. Exposure of human HepG2 cells to either acute or chronic THS for 24h resulted in significant increases in DNA strand breaks in the alkaline Comet assay. Cell cultures exposed to NNA alone showed significantly higher levels of DNA damage in the same assay. NNA is absent in freshly emitted secondhand smoke, but it is the main TSNA formed in THS when nicotine reacts with HONO long after smoking takes place. The long amplicon–quantitative PCR assay quantified significantly higher levels of oxidative DNA damage in hypoxanthine phosphoribosyltransferase 1 (HPRT) and polymerase β (POLB) genes of cultured human cells exposed to chronic THS for 24h compared with untreated cells, suggesting that THS exposure is related to increased oxidative stress and could be an important contributing factor in THS-mediated toxicity. The findings of this study demonstrate for the first time that exposure to THS is genotoxic in human cell lines.
Introduction
Thirdhand smoke (THS) has gained wide attention in the last few years as a previously overlooked source of exposure to toxicants arising from tobacco use. THS includes the pollutants from tobacco smoke that remain on surfaces and in dust after active smoking has ceased, as well as THS constituents that are reemitted back into the gas phase and/or react with environmental oxidants to produce secondary toxicants (1–5). Secondhand smoke (SHS, a mixture of ~85% diluted side stream and ~15% exhaled mainstream smoke) is the precursor of THS. THS is found in enclosed spaces where habitual smoking occurs, such as residences and automobiles. Compounds in THS have been identified on carpets, walls and furniture, in house dust, as well as on the clothing, hair and skin of smokers (1). Exposure to THS can occur through inhalation, ingestion and dermal contact, and THS has become an increasing public health concern. However, little is known about its toxicity.
A critical question is what toxicants are present in THS and in what quantities. Cigarette smoke is a rich source of exogenous mutagens and carcinogens such as polycyclic aromatic hydrocarbons (PAHs), N-nitrosamines, aromatic amines, aldehydes and other inorganic and organic compounds (6–8). Mainstream smoke contains over 60 carcinogens classified by the International Agency for Research on Cancer (IARC) (8) and many more suspected toxicants, and SHS contains many of the same species, such as nicotine, PAHs, N-nitrosamines, aldehydes and benzene (9). During the last decade, researchers have shown that indoor surfaces adsorb semi-volatile organic compounds (SVOCs) from SHS, such as nicotine, 3-ethenylpyridine, PAHs, naphthalene and phenol, which are slowly reemitted into the air (10–15), thus increasing exposure risk for non-smokers.
One important characteristic of THS is that surface-adsorbed residues can undergo chemical transformations to produce secondary toxicants by interacting with atmospheric compounds. Recent studies at Lawrence Berkeley National Laboratory (LBNL) showed that nicotine reacts with ozone (O3) to yield irritant by-products (2,10) and ultrafine, asthmagenic particles (3) and with nitrous acid (HONO) to form tobacco-specific nitrosamines (TSNAs) (4). HONO is a common atmospheric species that can arise from direct emission from indoor combustion appliances and smoking, as well as from surface conversion of NO2 and NO. TSNAs are formed in tobacco through N-nitrosation of nicotine and several structurally related alkaloids during tobacco curing and processing (16). These include 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N-nitrosonornicotine (NNN). It has been shown that NNK concentrations increase as SHS ages and becomes THS (17). 4-(Methylnitrosamino)-4-(3-pyridyl)butanal (NNA) is absent in freshly emitted tobacco smoke, but it was identified as the major TSNA product in THS in laboratory studies that showed that NNA resulted from the reaction of nicotine with HONO (4). Appreciable levels of surface-bound TSNAs, including NNA and NNK, were also detected inside a smoker’s vehicle in our recent study (4), highlighting their relevance to human exposure and health.
Genotoxicity is one of the critical mechanisms responsible for many types of cancer caused by smoking and SHS exposure and the global genotoxic potential of THS has not yet been tested. THS contains known mutagens and carcinogens. The questions this study was designed to address are: what levels of THS mutagens and carcinogens can be achieved through realistic exposure scenarios and would these levels be high enough to cause genotoxic or other adverse effects in exposed humans? We chose both acute and chronic THS samples for DNA damage studies as the two conditions vary in both the identity and quantity of component toxicants. If unrepaired, DNA damage caused by THS chemicals may block replication and transcription. DNA damage can either activate checkpoint signalling pathways leading to cell cycle arrest or induce apoptosis. Most importantly, persistence of DNA adducts, such as those formed by the tobacco carcinogens PAHs and N-nitrosamines, plays a central role in tobacco-induced carcinogenesis. THS is expected to contain fewer compounds than its precursor SHS, undergo chemical conversion of compounds to more or less toxic derivatives and possibly possess new chemical constituents with unknown biological implications. For example, although NNK and NNN have been extensively studied and both are believed to play important roles in tobacco carcinogenesis (18), NNA is poorly understood in this regard. To the best of our knowledge, only one report has described its mutagenic potential using a 6-thioguanine mutagenicity assay, showing that NNA was able to induce concentration-dependent increase in mutant fractions (19). However, the finding that bioassays of its tumorigenic activity in rodents were not significantly positive was attributed to its aldehyde group and ability to reach cellular targets (20). Nevertheless, more studies are needed to improve understanding of the mutagenic and enduring nature of THS, including its important constituents such as NNA. These compounds also exert other biological effects. For example, Rehan et al. (21) recently revealed the deleterious effects of NNA and NNK on the developing lung. Such studies support the important role of nicotine and its TSNA derivatives in other areas of cigarette smoke-induced pathogenesis.
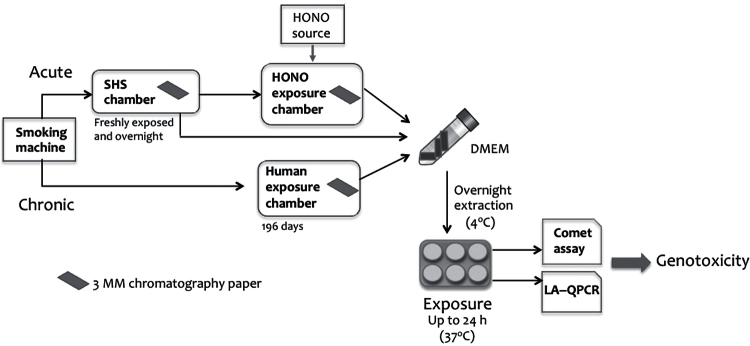
This study investigated the genotoxicity of THS constituents by measuring DNA strand breaks and oxidative DNA damage in exposed human cell lines (Figure 1). We first applied the Comet assay (also known as the single cell gel electrophoresis assay) to examine whether THS samples or pure NNA were able to induce DNA strand breaks and, if so, to what extent. This is a well-established assay for quantitating DNA damage at the single cell level, with a sensitivity of ~50 strand breaks per diploid mammalian cell (22–24). The Comet assay has already established the genotoxicity of tobacco smoke condensates, mainstream smoke and SHS (25–27). The results from the Comet assay reflect the potential of the mixture of all the genotoxic compounds in THS for causing strand breaks under the experimental conditions used.
Fig. 1.
Schematic representation of the main laboratory procedures used in this work. This includes SHS/THS generation with or without HONO treatment, extraction procedure, cellular exposure and two bioassays for testing DNA damage. See Materials and methods for detailed description.
A central causal role of free radicals and reactive oxygen species (ROS) in smoke-induced oxidative stress and carcinogenesis has been established (28,29). The base mutations in DNA that are caused by reaction with free radicals are associated with many disorders, particularly cancer (30,31). The next part of the study investigated oxidative DNA damage in two genes of human lung epithelial cells after exposure to THS using the long amplicon–quantitative PCR (LA–QPCR) assay (32,33). This technique is highly sensitive to oxidative DNA damage in specific genes when coupled with the use of specific DNA repair enzymes. Most recently, M. Sleiman, H. Destaillats, and L. A. Gundel (submitted for publication) measured ROS in THS samples and found that ROS persists for many hours. Whether exposure to THS leads to increased levels of oxidative DNA damage should be an important consideration in future toxicological assessments.
Materials and methods
Materials
d0- and d8-nicotelline, d0- and d9-cotinine (COT) and d0- and d4-pseudooxynicotine (4-(methylamino)-1-(3-pyridinyl)-1-butanone) were synthesised in the UCSF Clinical Pharmacology Laboratory (manuscript in preparation). d0- and d3-NNA, d0- and d4-NNK, d0- and d4-NNN, d0- and d4-bipyridine (2,3′-bipyridyl, 2,3′-dipyridyl) and N-formylnornicotine (N-formyl-2-[3-pyridyl]pyrrolidine) were procured from Toronto Research Chemicals (Ontario, Canada). Pentafluorophenylhydrazine (PFPH, 97%) was obtained from Sigma–Aldrich (St Louis, MO, USA). All other reagents and HPLC grade solvents were from Fisher Scientific (Walham, MA, USA).
Generation of smoke samples
Smoke samples were generated in two laboratory systems to simulate acute and chronic THS exposure, as described below.
Generation of SHS and acute THS.
A Teflon-lined cubic chamber constructed from wallboard (thickness 1.25cm) was used to generate SHS and THS. The total internal volume was 0.19 m3, and the chamber was mounted inside a laboratory fume hood. The front surface of the chamber had a sliding Plexiglas window that allowed access to the interior and to a row of six access ports near the lower front of the chamber. The ports were outfitted with brass Swagelok fittings for tubing with 0.25 outer diameter.
Using an approach developed in our previous work (4), cellulose (paper) substrates were used as surrogates for indoor surfaces, onto which fresh SHS gases could adsorb and SHS particles deposit. Strips of 3MM Whatman chromatography paper of 1.3×17.4cm were mounted vertically in ethanol-cleaned test-tube racks on the floor of the chamber. The papers and the interior of the chamber were sterilised by ethanol vapour and irradiation from a germicidal UV lamp for 4h prior to smoke generation.
One port of the chamber was connected to a three-stage Teflon holder that held two 47-mm Teflon-coated fibreglass filters that had been pre-weighed on a Sartorius SE2-F microbalance. The filters were connected to a pump (Gilian AirCon2) that operated at 4.8 l/min to sample particulate SHS. The other ports were used to support five cigarettes of a leading brand (Marlboro Reds) pointing towards the interior of the chamber, and a 50ml syringe was used on the exterior to withdraw 35ml puffs of about 2 s each. The cigarettes were smoked in 20min by taking one puff on each one, exhausting about 20ml of the mainstream smoke into the chamber to mix with the smoke inside, then repeating with the next cigarette and continuing the cycle until about 0.8cm of each tobacco rod remained unsmoked. The particulate concentration averaged 123mg/m3 during the smoking period [SHS] and dropped to 1.6mg/m3 by 17h after smoking ceased [THS].
Immediately after smoking, half of the cellulose substrates were placed in gas-tight sterile containers and stored in a freezer at −20°C (SHS sample). The other half of the strips were left inside the sealed chamber for an additional period of 15h and then placed in gas-tight sterile containers and stored in a freezer at −20°C (acute THS sample). In addition, each of these samples was split into two subgroups, one of which was subsequently exposed to high levels of HONO (gas) at room temperature in a separate experimental setup, as described below (to prepare acute THS + HONO samples).
Exposure of acute THS samples to HONO.
HONO was produced by mixing aqueous solutions of NaNO2 and H2SO4. A countercurrent airflow flushed the HONO (gas) into the reaction vessel. A total airflow of 400–560ml/min was generated by mixing equal flows of HONO-enriched clean air (zero grade) and moisture-saturated air. The concentrations of the aqueous solutions and the airflow were adjusted to achieve the desired steady-state HONO concentration, which was determined by absorption into impingers containing NaOH (aqueous). HONO was quantitatively captured as nitrite and analysed by ion chromatography. Further details of the HONO generation and exposure systems are presented in our previous study (4).
Samples of acute THS on cellulose were placed in a gas-tight glass flow cell through which 610±70 ppb HONO in humidified air (50% relative humidity) circulated continuously. The substrates were exposed to HONO over periods of 40–150min, then immediately moved to gas-tight sterile containers and stored at −20°C.
Chronic THS.
The chronic THS samples were generated by exposing the cellulose strips to cigarette smoke in a 6-m3 stainless steel chamber for a total of 258h over 196 days. During smoking, the SHS concentration varied between 100 and 1500 µg/m3 and a total of 1896mg of total particulate material was introduced into the steel chamber. Between experiments, totally ~35h of ventilation flushed through the chamber to remove desorbing compounds. Assuming that half of the amount of SHS introduced into the chamber (1896mg) deposited on its interior surfaces (35), the surface loading of SHS–THS on the cellulose substrates was roughly 3 µg/cm2.
THS extract preparation and chemical analysis
Both THS-laden and blank paper strips were weighed and cut into small pieces with scissors in a hood under sterile conditions and were then immersed in serum-free Dulbecco’s Modified Eagle’s Medium (DMEM) in 15ml Falcon centrifuge tubes at a ratio of 0.1g paper to 2ml medium. After vortexing, the tubes were placed in a 4°C refrigerator overnight. Prior to use, the samples were vortexed again thoroughly for 2min, and spun at 2000rpm for 2min at room temperature to remove the strips. For chemical analysis, 0.8ml of media were taken out and stored at 4°C until use.
Chemical analysis of THS samples.
Liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis of DMEM extracts of SHS and THS: Targeted compounds in the THS-exposed DMEM samples were analysed by LC–MS/MS. As for NNA measurement, a newly developed method based on PFPH derivatisation was employed (manuscript in preparation). Stock solutions of d0- and d3-labelled NNA were prepared in 0.02 N HCl and stored at −20°C. Fresh NNA and internal standards were diluted before the use in DMEM and 0.2 N HCl, respectively, and added separately from the other standards. All other standards and internal standards were stored in 0.02 N HCl at −20°C and diluted to the appropriate concentrations in DMEM before use.
Aliquots of treated DMEM and standards in DMEM were made basic by the addition of 0.5ml of saturated sodium bicarbonate and extracted with 5ml of a mixture of pentane, dichloromethane and ethyl acetate (45:45:10, vol:vol:vol). The organic layer was centrifuged and transferred by freeze pouring into tubes containing 100 µl of 0.1 N HCl in methanol. The tubes were dried by centrifugal vacuum evaporation and derivatised with 200 µl of 3mg/ml PFPH in acetonitrile at 60°C for 30min. Afterwards, the tubes were made basic with 0.5ml of saturated sodium bicarbonate and extracted as above. The organic layer was transferred to tubes containing 0.5ml of 1M H2SO4, vortexed, centrifuged and the organic layer discarded. The 1M H2SO4 was made basic by the addition of 0.3ml of 50% wt/vol K2CO3 and the supernatant transferred to new tubes as described above. The solvent was then dried under nitrogen at 55°C after which the residue was reconstituted in 200 µl of 20% methanol in 0.1M ammonium formate for LC–MS/MS analysis.
LC–MS/MS: The samples were analysed on a Thermo Scientific Vantage LC–MS/MS with an Accela UPLC system using a 3- × 150-mm 2.6µ Phenomenex Kinetex PFP column. An ammonium formate methanol solvent system was held isocratic at 15% methanol for 2min and then increased to 85% methanol over 7min. The flow rate was 0.67ml/min and the column temperature was 50°C. The listed ion transitions (see Table I for selected reaction monitoring analysis) were monitored using the Xcalibur ‘Easy Method’ software using 1-min retention time windows and with a cycle time of 0.3 s.
Table I.
THS analytes and LC–MS/MS parameters
| Parent | Product | CE | RT | Name | LLOQ | ULOQ | R 2 |
|---|---|---|---|---|---|---|---|
| 177.123 | 80.077 | 29 | 4.2 | d0-COT | 0.82 | 67 | 0.998 |
| 186.165 | 84.105 | 28 | 4.2 | d9-COT | – | – | – |
| 177.124 | 159.090 | 15 | 4.3 | N-formyl-nornicotine | 0.46 | 111 | 0.999 |
| 178.094 | 148.15 | 8 | 4.4 | d0-NNN | 0.015 | 33 | 0.999 |
| 182.115 | 152.186 | 8 | 4.4 | d4-NNN | – | – | – |
| 208.121 | 122.040 | 11 | 5.1 | d0-NNK | 0.015 | 33 | 0.999 |
| 212.125 | 126.119 | 11 | 5.1 | d4-NNK | – | – | – |
| 157.099 | 130.040 | 21 | 6.0 | d0-Bipyridine | 0.274 | 67 | 0.999 |
| 161.113 | 133.100 | 21 | 6.0 | d4-Bipyridine | – | – | – |
| 234.113 | 207.150 | 25 | 7.2 | d0-Nicotelline | 0.030 | 67 | 0.999 |
| 242.114 | 214.240 | 25 | 7.2 | d8-Nicotelline | – | – | – |
| 388.111 | 161.095 | 10 | 8.4 | d0-NNA–PFPH | 0.030 | 22 | 0.998 |
| 391.136 | 164.115 | 10 | 8.4 | d3-NNA–PFPH | – | – | – |
| 359.092 | 134.059 | 14 | 9.4 | d0-PON–PFPH | 0.091 | 67 | 0.993 |
| 363.107 | 138.082 | 14 | 9.4 | d4-PON–PFPH | – | – | – |
CE = collision energy (volts); RT = retention time (minutes); LLOQ = lower limit of quantification; ULOQ = upper limit of quantification; R 2 = fit.
Gas chromatography–ion trap–tandem mass spectrometry (GC–IT–MS/MS) analysis of cellulose substrates: In addition to the chemicals present in the DMEM medium, we also determined nicotine and TSNAs in the cellulose substrates by extraction with methanol and analysis by GC–IT–MS/MS, as previously described (4,11). In summary, each substrate exposed to THS (or THS + HONO) was weighed before extraction with 10ml of methanol spiked with quinoline (internal standard) in a 40-ml amber flask. The flasks were stirred for 25min and slurries centrifuged for 20min at 10 000rpm to separate the supernatant from suspended cellulose matrix. Recoveries of nicotine, NNN, NNA and NNK ranged from 90 to 115%. Extracts were then analysed using a Varian 3800 gas chromatograph (Varian Chromatography Systems) equipped with a CP8400 autosampler and an ion trap mass detector Varian 2000. Methanol extracts were injected directly into the GC operating in splitless mode at 200°C. Nicotine and TSNAs were separated on a 30-m VF-5 MS, low bleed column. The mass spectrometer was operated in the electron ionisation mode at 70eV in the mass range 50–350 m∕z. For MS/MS, precursor ions of NNN (m∕z 147), NNA (m∕z 148) and NNK (m∕z 177) were used, whereas fragment ions selected for quantification were 105, 130 and 132 for NNN; 148 for NNA; and 146, 149 and 159 for NNK.
Comet assay
Human hepatocellular carcinoma (HepG2) cells [American Type Culture Collection (ATCC) Manassas, VA, USA] were grown in DMEM supplemented with 10% fetal bovine serum (FBS) and subcultured twice a week. Cells were plated at 2×105 cells per well in 6-well plates with the growth medium, which was replaced with serum-free DMEM for 6h before treatment with THS/DMEM extracts with or without FBS. The HepG2 cultures were also exposed to varying concentrations (0.01, 0.1, 1.0, 10, 100 µM) of NNA or NNK, the stock solutions of which were dissolved in the phosphate buffer, pH 7.4. Treatment with THS or NNA/NNK was conducted in DMEM suspension at 37°C for up to 24h.
After treatment, cells were trypsinised and processed with the Trevigen CometAssay single gel electrophoresis kit (Trevigen, Gaithersburg, MD, USA) per the manufacturer’s instructions. Briefly, cells were washed and suspended in 0.5ml of phosphate-buffered saline after trypsinisation. Cells were counted and adjusted to 1×105 cells/ml and then mixed at a ratio of 1:10 with molten LMAgarose that was maintained at 37°C. Fifty microlitre of the mixture was pipetted onto a CometSlide immediately. After solidification at 4°C for 10min, the slides were incubated in prechilled lysis solution at 4°C overnight. The slides were immersed in freshly prepared alkaline solution (200mM NaOH and 1mM EDTA) for 1h, followed by electrophoresis in the alkaline solution at 21V for 30min. After electrophoresis, excess solution was gently drained and the slides were immersed twice in H2O and then in 70% ethanol for 5min. The slides were finally dried at 45°C and stained with SYBR Green I. The slides were viewed under an epifluorescence microscope and analysed using CometScore software (TriTek Corporation, Sumerduck, VA, USA).
The extent of DNA damage (strand breaks) was quantified by percentage (%) of DNA in tails. For each data point, 90 random cells from three separate fields of view were counted. The differences between the mean values obtained from the Comet assay were analysed for statistical significance (P < 0.05 and P < 0.01) using Student’s t-test. Statistical analysis of the dose response was calculated using one-way analysis of variance.
LA–QPCR assay
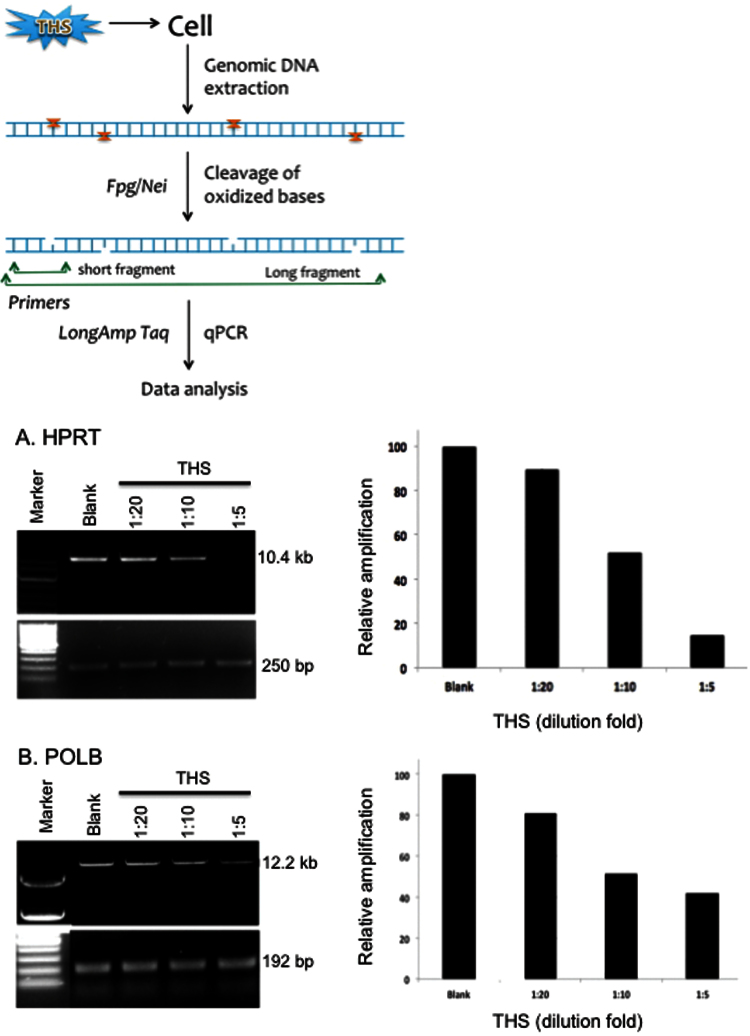
The normal human lung epithelial BEAS-2B cell line (ATCC) was maintained in DMEM with 10% FBS before treatment. After exposure to chronic THS-treated medium (×5, ×10 and ×20 dilutions) as described above, the cells were harvested for genomic DNA extraction using the QIAGEN Genomic-tip 20/G kit (Qiagen, Valencia, CA, USA) per the manufacturer’s instructions (33). This kit minimises DNA oxidation during the isolation step and has been used previously for LA–QPCR assays (32,35). After quantitation by Pico Green (Molecular Probes) in a 96-well plate, equal amounts of genomic DNA were digested with two Escherichia coli base excision repair (BER) enzymes, formamidopyrimidine DNA glycosylase (Fpg) and endonuclease VIII (Nei), which are able to remove a variety of oxidised DNA bases and induce strand breaks by cleaving the phosphodiester bond with their associated apurinic/apyrimidinic (AP) lyase activity (36).
To examine the formation of oxidative DNA damage in two human genes, hypoxanthine phosphoribosyltransferase 1 (HPRT) and polymerase β (POLB), LA–QPCR assay was performed in THS-exposed BEAS-2B cells as described previously (32). LongAmp Taq DNA polymerase (New England Biolabs, Beverly, MA, USA) was used to amplify a 10.4kb region of HPRT gene in human genomic DNA using the following primers: 5′-TGG GAT TAC ACG TGT GAA CCA ACC-3′ and 5′-GCT CTA CCC TCT CCT CTA CCG TCC-3′; and to amplify a 12.2kb region for POLB gene with primers 5′-CAT GTC ACC ACT GGA CTC TGA AC-3′ and 5′-CCT GGA GCA ACA AAA TTG CT-3′. Preliminary assays were carried out to ensure the linearity of PCR amplification with respect to the number of cycles and DNA concentration. Because amplification of a small region would be relatively independent of oxidative DNA damage (low probability), a small DNA fragment for each gene (250bp for HPRT and 192bp for POLB) was also amplified for normalisation of the data obtained with the large fragments using the following primers: 5′-TGC TCG AGATGT GAT GAA GG-3′ and 5′-CTG CAT TGT TTT GCC AGT GT-3′ for HPRT; and 5′-AGT GGG CTG GAT GTA ACCTG-3′ and 5′-CCA GTA GAT GTG CTG CCA GA-3′ for POLB. All of the amplified products were resolved and visualised using agarose gel electrophoresis and quantitated with ImageQuant (Molecular Dynamics). The data were plotted as columns with relative amplification as Y-axis, which was calculated comparing the values of exposed samples with the control.
Results
Generation of THS and chemical analysis
Laboratory chamber experiments were performed to generate both acute and chronic THS samples using chromatography paper as a model surface. Although organic solvents can extract a range of compounds from these exposed cellulose substrates, water-based extraction (DMEM) was used to determine compounds that would be available to cells under more realistic (life-like) conditions. To analyse the chemical components in the SHS and THS extracts, LC–MS/MS procedure was used. Table II summarises the concentrations of compounds detected and quantified in the DMEM (ng/ml) and methunol extracts of THS-, SHS-laden substrates (ng/g). Concentrations of all analytes were below limits of detection in DMEM and DMEM extracts of unexposed blank substrates.
Table II.
Concentrations of THS constituents in DMEM media (ng/ml) and in cellulose substrates (ng/g)
| Sample name | COT | N-Formylnornicotine | NNN | NNK | NNA | Nicotelline | 2,3′-Bipyridine | PON | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Acute THS | ||||||||||
| DMEM only | BLOQ | BLOQ | BLOQ | BLOQ | BLOQ | BLOQ | BLOQ | BLOQ | ||
| Blank | BLOQ | BLOQ | BLOQ | BLOQ | BLOQ | BLOQ | BLOQ | BLOQ | ||
| THS | DMEM | 27.0 | 50.9 | 0.264 | 0.903 | 0.43 | 0.752 | 189 | 228 | |
| 3MM paper | n.a. | n.a. | BLOQa | 15.9a | 1.6a | n.a. | n.a. | n.a. | ||
| THS + HONO | DMEM | 14.9 | 27.0 | 0.145 | 0.517 | 1.82 | 0.312 | 107 | 163 | |
| 3MM paper | n.a. | n.a. | BLOQa | 18.1a | 5.4a | n.a. | n.a | n.a. | ||
| SHS | 11.3 | 23.6 | 0.259 | 0.149 | BLOQ | 0.404 | 90.2 | 134 | ||
| Chronic THS | ||||||||||
| DMEM only | BLOQ | BLOQ | BLOQ | BLOQ | BLOQ | BLOQ | BLOQ | BLOQ | ||
| Blank | BLOQ | BLOQ | BLOQ | BLOQ | BLOQ | BLOQ | BLOQ | BLOQ | ||
| THS | DMEM | 133 | 585 | 1.57 | 7.20 | 0.39 | 8.04 | 147 | 13.0 | |
| 3MM paper | n.a. | n.a. | 19.5a | 78.6a | 1.4a | n.a. | n.a. | n.a. | ||
BLOQ = below the limit of quantitation.
aTSNA concentrations were obtained using GC–IT–MS/MS method and are expressed in nanogram per gram of 3MM paper (ng/g).
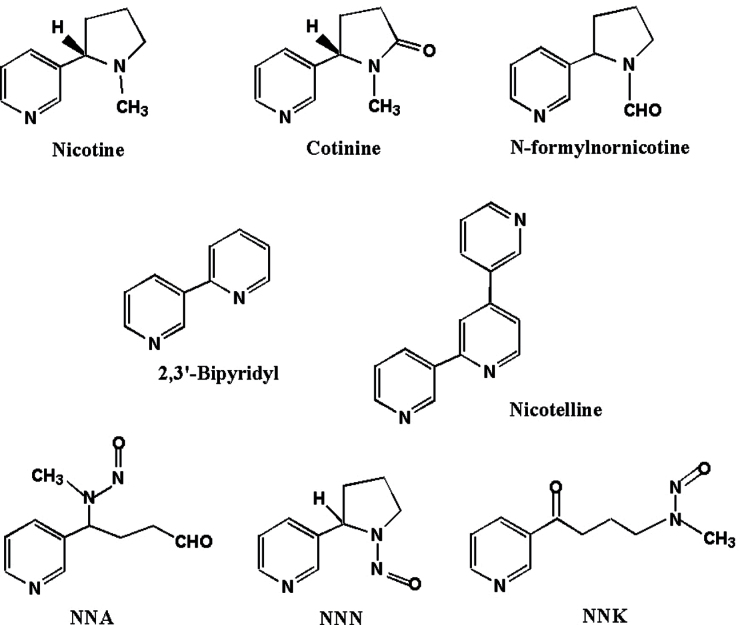
The compounds detected in all SHS and THS samples included COT, N-formylnornicotine, NNA, NNK, NNN, bipyridine and nicotelline. Some of them have been previously reported, including COT, NNA, NNK and NNN (4). The chemical structures of these compounds are shown in Figure 2. All the analytes tested in the DMEM only and blank paper groups were below detection levels (below the limit of quantitation).
Fig. 2.
Chemical structures of the compounds identified in THS.
Because NNA can degrade during the LC–MS/MS procedure, it was measured by a newly developed quantitative analytical method based on NNA–PFPH derivatisation that had a 30 pg limit of quantitation per sample. Similarly, pseudooxynicotine (PON) can be measured by the PON–PFPH assay (Table II). NNA, NNN and NNK were detected in the acute THS sample (before treatment with HONO) at sub-nanogram per millilitre. As expected, the HONO-exposed acute THS sample contained at least four times the NNA as that without HONO treatment (see Table II). This finding was also confirmed using GC–MS/MS, as shown in Table II, and is consistent with the results of Sleiman et al. (4). Although the LC–MS data indicate a decrease in NNK concentration after exposure of THS to HONO, the GC–MS results reveal a substantial increase. This discrepancy could be due to a difference in extraction efficiency between DMEM used for the LC–MS analysis and methanol used for GC–MS measurement. Another possibility, supported by the observation of similar lower concentration ratios (exposed to unexposed ratio of 0.4:0.5) for all compounds except NNA, is that the group of strip(s) exposed to HONO could have received less initial deposition of THS and/or experienced loss of part of THS on the substrates during the exposure to HONO.
NNA was also detected in the chronic THS samples at a level similar to that in acute THS, but its concentration was several fold lower than that of NNN or NNK. It is noteworthy that the levels of a number of fairly stable compounds in the chronic exposure sample (196 days with 258h of smoke) were considerably higher than those in the acute (overnight) condition, consistent with accumulation from frequent addition of THS to the chamber. However, the NNA concentration was similar in acute (without added HONO exposure) and chronic THS samples (0.43 vs. 0.39ng/ml), which could be due to its instability and reactivity during an extended exposure period, especially if O3 was present in the environment (4).
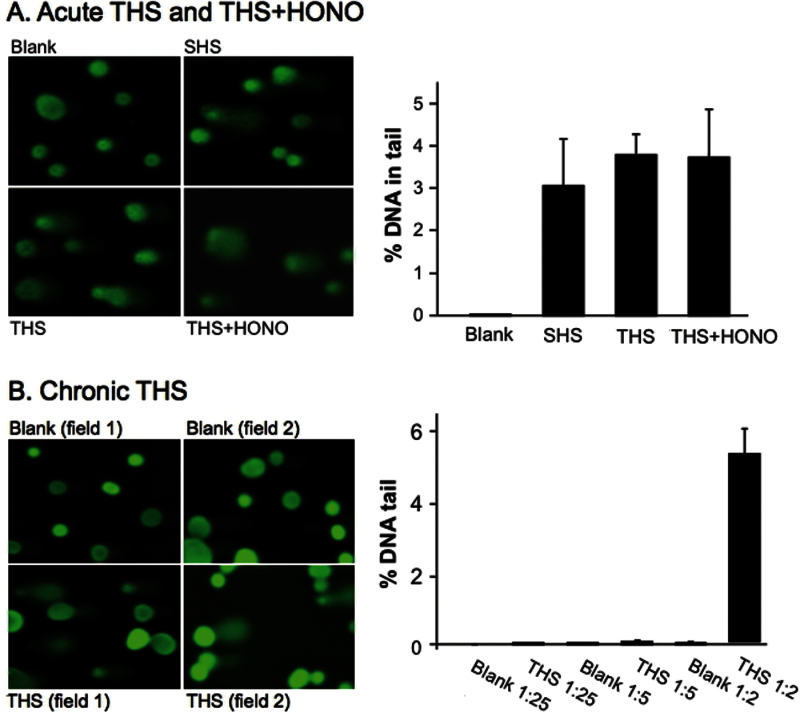
THS and NNA induce DNA strand breaks in the Comet assay
Both acute and chronic THS, as well as SHS (right after smoking), were extracted from the paper substrates using DMEM medium as described in Materials and methods. For acute THS and SHS, undiluted DMEM samples were used to treat metabolically competent human HepG2 cells in culture for 24h. No cytotoxicity was observed at this dose, and representative comet assay images are shown in the left panel of Figure 3A. The comet DNA in the tail measured in acute THS-exposed cells had a mean value close to 4%. Based on this value, the exposure resulted in significant DNA damage compared with the control (P < 0.01). Also, SHS-treated cells showed a slightly higher than 3% increase in % DNA in the tail compared with the control, which was also statistically significant. The SHS sample was used mainly as a positive control for THS testing in this experiment as SHS itself is known to be genotoxic from previously published Comet assays (27,37). Except for NNN, the levels of TSNA in the extracts of SHS were lower than for extracts of acute THS, even before exposure to HONO. The concentrations of the other compounds were in roughly the same ratios (SHS/unexposed acute THS) as observed for exposed acute THS/unexposed acute THS. This difference would be due to variability in surface coverage among the substrates. Therefore, the results for THS and SHS are not statistically different.
Fig. 3.
(A) Genotoxic effect of acute THS on human genomic DNA as measured by the Comet assay. HepG2 cells were exposed to undiluted THS or SHS samples at 37°C for 24h. The extent of DNA damage was analysed by measuring the % DNA in tail to total DNA from 90 randomly selected cells. The histogram depicts the average median % of DNA in tail ± SEM of three independent experiments. (B) HepG2 cells were exposed to chronic THS at varying dilutions under identical conditions as above.
For exposure to chronic THS, cells were first treated with a series of diluted chronic THS–DMEM extracts for 24h and tested for cytotoxicity. The finding that undiluted samples (as shown in Table II) were cytotoxic can be attributed to the higher concentrations of chemical compounds deposited on the substrates over the extended exposure compared with deposition over the short period of exposure to acute THS. Therefore, diluted samples (×2, ×5, ×25, ×100) and corresponding blank samples were used to treat BEAS-2B cells to establish a dose–response relationship. The data showed that significant positive Comet assay results only occurred at 2-fold dilution. Figure 3B shows that exposure to chronic THS for 24h at this dose caused a significantly higher level of DNA damage than the control (~5% DNA in tail, P < 0.01). Therefore, chronic THS exposure (~5%, ×2 dilution) resulted in more DNA damage than did the acute exposure (~4%, undiluted), which could be partially explained by the higher levels of the same chemicals accumulated in chronic THS (Table II).
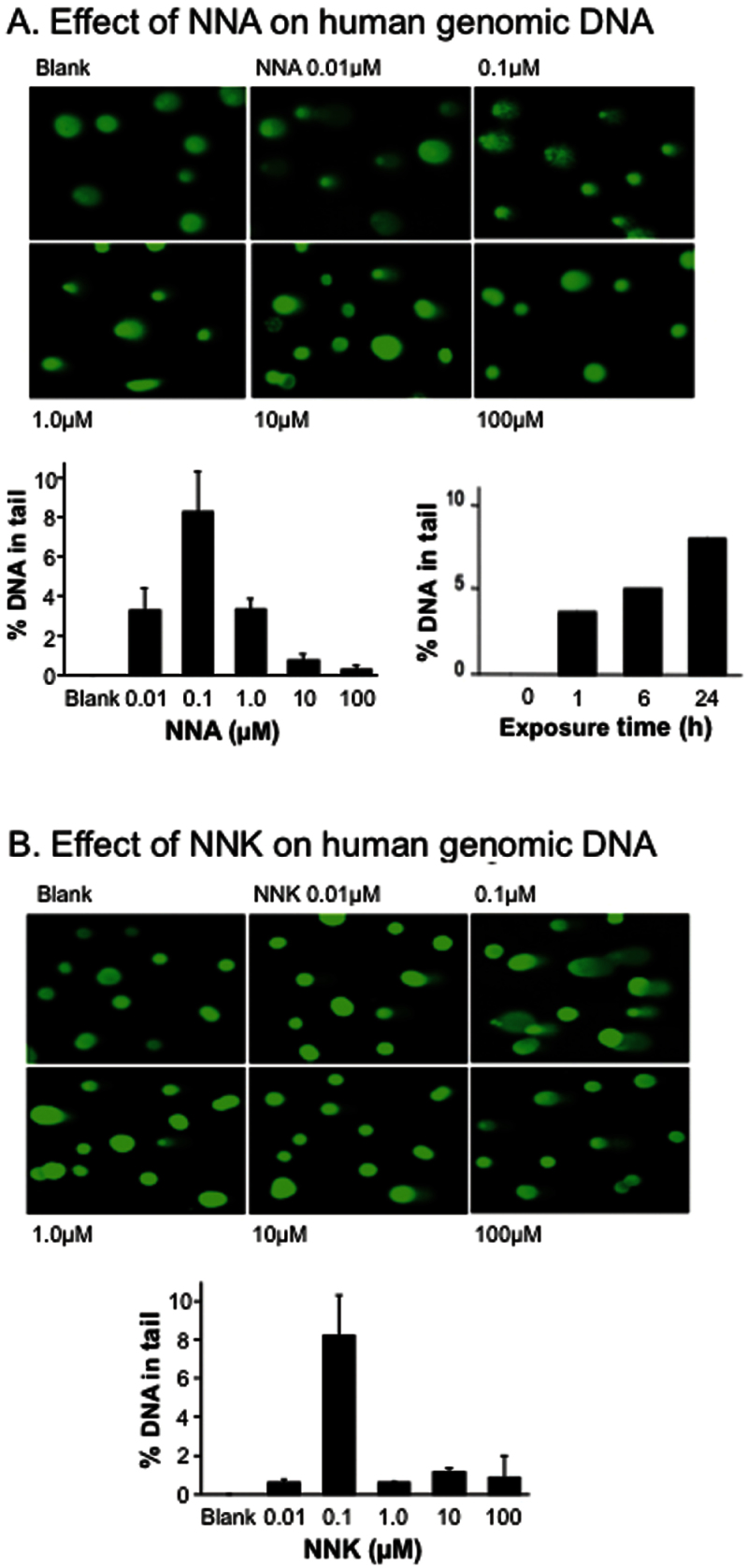
The ability of NNA to cause DNA damage is poorly understood. To gain insight, we used the Comet assay to measure the formation of DNA strand breaks in human HepG2 cells treated with NNA (Figure 4A). For positive control, similar cell cultures were exposed to its isomer NNK under the same doses, as shown in Figure 4B. Previous studies showed that the Comet assay is sensitive and specific enough to measure NNK-induced DNA damage, including strand breaks and alkali-labile sites (40,41). As shown in Figure 4A, NNA was able to cause dose-dependent DNA strand breaks in HepG2 cultures. These cells were exposed to NNA at non-cytotoxic concentrations ranging from 0.01 to 100 µM for 24h. Interestingly, more DNA damage was observed at the lower levels of NNA exposure (from 0.01 to 1 µM). To examine the time-dependent effect, a 0.1 µM dose, the optimal dose for DNA strand breaks within our dose range, was chosen to carry out the Comet assay. As shown in Figure 4A, NNA caused DNA damage after as little as 1h of exposure and led to increasing levels of strand breaks within the 24h reaction period.
Fig. 4.
(A) Genotoxic effect of NNA in human HepG2 cells as measured by the Comet assay. Cultured cells were exposed to increasing doses of NNA dissolved in DMEM with or without 10% FBS for 24h, before being processed by the Comet assay. The histogram on the lower left depicts the mean % of DNA in tail ± SEM of three independent experiments. The histogram on the lower right depicts the time-dependent change in % DNA in tail in HepG2 cells at 0.1 µM concentration of NNA. (B) Genotoxic effect of NNK in HepG2 cells measured under identical assay conditions. The histogram depicts the mean % of DNA tail ± SEM of three independent experiments.
THS leads to accumulation of oxidative DNA damage in the LA–QPCR assay
To examine whether THS exposure results in accumulation of oxidative base damage, we exposed BEAS-2B cells to chronic THS samples. The levels of base damage in the HPRT and POLB genes of THS-exposed versus control cells were compared using LA–QPCR. We chose these two genes as Van Houten’s group had already standardised the conditions for LA–QPCR amplifying a long region (10–12kb) with the proper set of primers (32). In the protocol, isolated cellular DNA was treated with two E. coli DNA glycosylases, Fpg and Nei, to excise oxidised DNA bases, which generate single DNA strand breaks, thus preventing PCR amplification of the long region. A decrease in the PCR product reflects a higher amount of oxidative DNA damage.
Figure 5 shows that there was a high level of oxidative DNA damage in both genes when exposed to chronic THS extracts (acute THS was not tested). This was reflected by the decreasing levels of the top long PCR band with increasing THS doses. However, amplification of a smaller fragment for each gene was similar in extent between the samples because the probability of damage in a small fragment is much lower than in a much longer fragment. The relative level of PCR amplification after normalisation of the small fragment for both genes was found to be significantly lower in BEAS-2B cells upon exposure.
Fig. 5.
Induction of oxidative DNA damage in human lung epithelial cells after 24h exposure to chronic THS. Cultured BEAS-2B cells were treated with three concentrations of THS extracts for 24h followed by LA–QPCR analysis. Representative gels show PCR amplified fragments of HPRT and POLB genes. Amplification of the large fragment was normalised to the amplification product of a small fragment of the corresponding gene. Quantitation of the amplified products is represented in the histograms with relative amplification arbitrarily set as 100. Each experiment was repeated three times.
The data presented in this study indicate that both THS and NNA cause elevated levels of DNA strand breaks in exposed human cells and that one key class of DNA damage induced by THS is the oxidation of DNA bases. We conclude that THS is genotoxic to human cells in vitro, and suggest that exposure to THS could lead to DNA damage-related adverse effects in the human body.
Discussion
This study is the first to explore the genotoxicity of THS and, thus, its findings are highly relevant to addressing the gap in knowledge about the health impacts of THS because genotoxicity is associated with development of diseases, and a critical event of the early steps in tobacco carcinogenesis is the formation of mutagenic DNA damage by the genotoxic compounds. To address this issue, we generated both acute and chronic THS samples from two different laboratory settings and studied their potential effects on genomic stability in cultured human cells.
Several conclusions can be drawn from the LC–MS/MS analysis of THS extract samples. First, key nicotine metabolites and nicotine-derived compounds can be quantified at low levels (Table II), thus establishing them as tracers and possible biomarkers of exposure. Such analysis is greatly aided by the development of novel or ultra-sensitive analytical chemistry methods (Jacob et al., manuscript in preparation), such as the NNA–PFPE and PON–PFPE assays. Of the compounds detected in THS (Table II), COT is the major proximate metabolite of nicotine and also one of the most prevalent nicotine oxidation by-products found in the environment (2). It has been widely used as a biomarker for cigarette smoke exposure (40). NNA, NNK and NNN, as important TSNAs in THS, were all detected but at relatively low levels compared with other compounds in the samples. However, toxicity is not always proportional to quantity. For instance, NNK is a potent carcinogen even at low concentrations (41). Nevertheless, THS is expected to be a complex and heterogeneous mixture containing many toxic SVOCs and VOCs. Second, comparing the levels of the compounds identified in acute versus chronic THS samples indicates that THS is cumulative as most of the compounds in chronic THS samples exhibited higher levels compared with acute THS, which was also reflected in the Comet assay showing a greater percentage of DNA in tail (×2 dilution, 5% vs. undiluted, 4%). Third, chemical transformation for certain chemicals did occur during the long exposure/aging period: NNA levels in the chronic samples were lower than NNA levels in the acute samples. Fourth, some comparisons may be made between the amounts of representative constituents such as TSNAs in THS samples and the amounts of TSNAs that may be detected in indoor environment. In the previous work described by Sleiman et al. (4), the levels of TSNAs formed on indoor surfaces (under typical HONO levels) were estimated to be NNA: 2.2–3500ng/m2; NNK: 0.31–500ng/m2, depending on the indoor matrix used for testing, whereas in our cell culture exposure, the amounts of NNA added to the cells were at concentrations of 0.39–1.82ng/ml, and for NNK, 0.51–7.2ng/ml (Table II). These values calculated as per millilitre are comparable to the total amounts of NNA and NNK deposited per square metre of the surface area, and even at the low end of the estimated range for indoor surface concentrations.
The alkali Comet assay used to assess the genotoxicity of THS allowed for the detection of a broad spectrum of DNA damage. It is well known that published Comet results are difficult to directly compare because of variations among assay conditions (25). To address this, a commonly used commercial Comet assay was selected for this work. The HepG2 cells used contain P450 enzymes that are able to carry out normal biotransformations and are frequently used as a model for testing cytotoxicity and genotoxicity of chemicals that may require metabolic activation in vivo (42,43). This assay allows detection of the following DNA lesions: (i) single-strand breaks, such as those resulting from direct attack to the sugar phosphate moieties of DNA, unstable DNA adducts and incomplete excision repair mechanisms. The latter involves enzymatic removal of stable DNA adducts such as oxidised and bulky adducts leading to an leading to an AP site (7); (ii) double-strand breaks that are cytotoxic; and (iii) DNA cross-links. This method is particularly useful for testing the effects of very small quantities of chemicals in the sample (44). In fact, we were able to detect a significant increase of DNA damage at low nanomolar concentrations of NNA and NNK (as low as 10nM where P < 0.05). It is also notable that the extent of increase in % DNA in tail in acute or chronic THS-exposed cells was at the low end when compared with those reported in many other published works, which is expected given the relatively low levels of chemicals in THS samples. Also, the % DNA in tail was lower in the short-term THS, THS + HONO and SHS samples than in the chronic THS samples, reflecting the level of compounds in these paper strips. It should be noted that a direct comparison of the results obtained from acute and chronic THS would be impossible as their chemical composition may change with the smoking–aging–smoking process, and even for the same compounds, their concentrations can vary with their stability and reactivity. As in the case of the THS + HONO sample, although a higher level of NNA was found in it than in the THS only sample, it did not exhibit a higher induction of strand breaks in the Comet assay. It can be expected that there are many other chemical compounds in THS that have genotoxic potential and contribute to the overall genotoxic potency of THS. NNA, though it appears to be unique to THS, may not be the primary driver of the genotoxic effects of THS. Overall, these results show that THS-mediated DNA strand breaks are highly persistent after 24h exposure, which may lead to increased mutations in cells upon exposure to THS and ultimately higher cancer risk.
Although NNN and NNK are already known to be present in cigarette smoke, NNA is a new and important chemical found in THS. NNA levels can be increased dramatically in the presence of HONO, a common indoor air pollutant (4). In this work, we used the Comet assay to examine the genotoxicity of NNA, which is poorly understood. NNK has been shown to be genotoxic in this assay in several studies (38,39). The only work that can be found in the literature for NNA involved the use of 6-thioguanine-based mutagenicity assay in human lymphoblastoid cells to suggest that NNA is mutagenic but less than NNK in the cells expressing P450 CYP2D6 cDNA (19). Our data based on the Comet assay clearly showed that these TSNAs exhibited similar patterns in dose response and in the highest level of strand breaks induced (Figure 4). Therefore, this study confirms that NNA is also genotoxic as the carcinogen NNK at nanomolar level exposures. Also, it is interesting to note that the most effective dose range for NNA and NNK was between 0.01 and 1 µM. Given that the NNA concentrations in acute and chronic THS exposure are around 2nM, and that [NNK]s are 4.3nM (acute) and 34nM (chronic), respectively, concentrations of NNA and NNK in THS samples are close to or within the dose ranges of the pure compounds that exhibited increased levels of DNA strand breaks. Also, it can be seen that decreased levels of strand breaks occurred at higher genotoxin concentrations, and several reasons might account for, including dimerisation of NNA in aqueous solution (unpublished data), DNA damage response and repair at different chemical concentrations and cellular toxicity of NNA/NNK.
Because of the importance of oxidative stress-induced DNA lesions in environmental mutagenesis and carcinogenesis, we next examined the formation of oxidative DNA damage in specific human genes induced by THS in BEAS-2B cells, which are suitable for the study of cellular response to oxidative stress-induced DNA lesions since bronchial epithelial cells/tissues are directly exposed to oxidants during cigarette smoking. Our data provide the first evidence that THS causes increased levels of oxidative DNA damage in exposed cells. The cause for THS-induced oxidation of DNA bases remains to be determined. Both NNK (45,46) and NNA (unpublished data) cause the formation of 8-oxo-dG, and free radicals or other ROS may be a plausible source for THS-induced oxidative DNA damage. Church and Pryor (47) reported that mainstream cigarette smoke contains two different populations of free radicals: one in the tar such as the quinone/hydroquinone (Q/QH2) redox system and one in the gas phase including small oxygen- and carbon-centred radicals. In an unpublished work carried out at LBNL, ROS and carbon-centred radicals (C•) were detected in THS after 22h of aging, suggesting the presence of persistent ROS and/or potential formation of ROS and free radicals during aging. Consistent with that data, our results support the observation that THS contains free radicals and/or generates ROS to attack DNA base or sugar moieties, which can also contribute to the strand breaks observed in the Comet assay.
Given that the LA–QPCR assay yielded significant DNA damage at a 1:20 dilution of chronic THS samples, it seems to be more sensitive than the Comet assay that began to show significant results at 1:2 dilution as shown in Figure 3. Although these two methods detect different types of DNA damage, they are expected to have some overlap. Oxidative DNA lesions may contribute to strand breaks, if the AP sites generated from enzymatic removal of these lesions are not repaired promptly. We have also observed that, as can be seen in Figure 5, the extent of increase in the levels of oxidised DNA damage at a 1:20 sample dilution was less than those at higher concentrations of THS (1:10 and 1:5). This may indicate that DNA repair more efficiently removed lower levels of DNA damage occurring during the 24h exposure time. Oxidative DNA base damage could be repaired by several mechanisms such as BER, nucleotide excision repair and nucleotide incision repair pathways (7,48), The levels of DNA damage measured are expected to reflect a dynamic equilibrium between DNA damage and DNA repair in the cell. However, cells can become saturated at higher chemical doses and repair may slow down (7). It should be noted that although the LA–QPCR is gene specific, it cannot identify the specific nature of the damaged base other than Fpg/Nei sensitive sites that could include a broad spectrum of oxidised base lesions (36). It is expected that the THS-derived oxidised bases are diverse, and many of the oxidised DNA lesions have been found to be highly mutagenic and/or toxic (30). Furthermore, mutations induced by oxidative stress such as the free radicals in smoke are an important prerequisite for a number of radical-mediated disorders, such as emphysema and cancer.
In conclusion, we used two different assays to evaluate the in vitro genotoxic nature of THS and its unique component NNA, both of which showed that THS causes significant levels of DNA damage in human cell lines. We also confirmed the genotoxic potential of NNA in the Comet assay system. Future efforts should investigate the effects of THS and novel compounds on genomic DNA in animals or cells of exposed human subjects such as blood samples. Understanding the mechanisms of DNA damage is critical for the analysis and management of tobacco-induced mutagenesis and carcinogenesis and should clearly include the THS route of smoke exposure. Ultimately, knowledge of the mechanisms by which THS exposure increases the chance of disease development in exposed individuals should lead to new strategies for prevention. Moreover, toxicological data are necessary to frame and enforce tobacco control policies to minimise health hazards and protect vulnerable subpopulations such as children.
Funding
This work was supported by the Grant 19XT-0070 (to B.H.) and Grant 20PT-0184 (California Thirdhand Smoke Consortium) from the University of California Tobacco-Related Disease Research Program (TRDRP), under U.S. Department of Energy (Contract no. DE-AC02-05CH11231). M.S. was supported by TRDRP New Investigator Grant 20KT-0051. Instrumentation and analytical chemistry at UCSF were supported by the National Institutes of Health (S10 RR026437 to P.J.) and (P30 DA012393 to Reese T. Jones, PI).
Acknowledgements
The authors thank Dr K. Asotra for helpful suggestions and M. Russell (LBNL) for experimental assistance.
References
- 1. Matt G. E., Quintana P. J., Destaillats H., et al. (2011). Thirdhand tobacco smoke: emerging evidence and arguments for a multidisciplinary research agenda. Environ. Health Perspect., 119, 1218–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petrick L. M., Sleiman M., Dubowski Y., Gundel L. A., Destaillats H. (2011). Tobacco smoke aging in the presence of ozone: a room-sized chamber study. Atmos. Environ., 45, 4959–4965 [Google Scholar]
- 3. Sleiman M., Destaillats H., Smith J. D., Liu C.-L., Ahmed M., Wilson K. R., Gundel L. A. (2010). Secondary organic aerosol formation from ozone-initiated reactions with nicotine and secondhand tobacco smoke. Atmos. Environ., 44, 4191–4198 [Google Scholar]
- 4. Sleiman M., Gundel L. A., Pankow J. F., Jacob P., 3rd, Singer B. C., Destaillats H. (2010). Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc. Natl Acad. Sci. U.S.A., 107, 6576–6581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Winickoff J. P., Friebely J., Tanski S. E., Sherrod C., Matt G. E., Hovell M. F., McMillen R. C. (2009). Beliefs about the health effects of “thirdhand” smoke and home smoking bans. Pediatrics, 123, e74–e79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hecht S. S. (2003). Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer, 3, 733–744 [DOI] [PubMed] [Google Scholar]
- 7. Hang B. (2010). Formation and repair of tobacco carcinogen-derived bulky DNA adducts. J. Nucleic Acids, 2010, 709521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith C. J., Perfetti T. A., Garg R., Hansch C. (2003). IARC carcinogens reported in cigarette mainstream smoke and their calculated log P values. Food Chem. Toxicol., 41, 807–817 [DOI] [PubMed] [Google Scholar]
- 9. CEPA (2005). Proposed Identification of Environmental Tobacco Smoke as a Toxic Air Contaminant. California Environmental Protection Agency, Sacramento, CA [Google Scholar]
- 10. Destaillats H., Singer B. C., Lee S. K., Gundel L. A. (2006). Effect of ozone on nicotine desorption from model surfaces: evidence for heterogeneous chemistry. Environ. Sci. Technol., 40, 1799–1805 [DOI] [PubMed] [Google Scholar]
- 11. Sleiman M., Maddalena R. L., Gundel L. A., Destaillats H. (2009). Rapid and sensitive gas chromatography-ion-trap tandem mass spectrometry method for the determination of tobacco-specific N-nitrosamines in secondhand smoke. J. Chromatogr. A, 1216, 7899–7905 [DOI] [PubMed] [Google Scholar]
- 12. Singer B. C., Hodgson A. T., Nazaroff W. M. (2003). Gas-phase organics in environmental tobacco smoke: 2. Exposure-relevant emission factors and indirect exposures from habitual smoking. Atmos. Environ., 37, 5551–5561 [Google Scholar]
- 13. Van Loy M. D., Riley W. J., Daisey J. M., Nazaroff W. W. (2001). Dynamic behavior of semivolatile organic compounds in indoor air. 2. Nicotine and phenanthrene with carpet and wallboard. Environ. Sci. Technol., 35, 560–567 [DOI] [PubMed] [Google Scholar]
- 14. Hoh E., Hunt R. N., Quintana P. J., Zakarian J. M., Chatfield D. A., Wittry B. C., Rodriguez E., Matt G. E. (2012). Environmental tobacco smoke as a source of polycyclic aromatic hydrocarbons in settled household dust. Environ. Sci. Technol., 46, 4174–4183 [DOI] [PubMed] [Google Scholar]
- 15. Fleming T., Anderson C., Amin S., Ashley J. (2012). Third-hand tobacco smoke: significant vector for PAH exposure or non-issue? Integr. Environ. Assess. Manag., 8, 763–764 [DOI] [PubMed] [Google Scholar]
- 16. Hoffmann D., Brunnemann K. D., Prokopczyk B., Djordjevic M. V. (1994). Tobacco-specific N-nitrosamines and Areca-derived N-nitrosamines: chemistry, biochemistry, carcinogenicity, and relevance to humans. J. Toxicol. Environ. Health, 41, 1–52 [DOI] [PubMed] [Google Scholar]
- 17. Schick S. F., Glantz S. (2007). Concentrations of the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in sidestream cigarette smoke increase after release into indoor air: results from unpublished tobacco industry research. Cancer Epidemiol. Biomarkers Prev., 16, 1547–1553 [DOI] [PubMed] [Google Scholar]
- 18. Hecht S. S. (2002). Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol., 3, 461–469 [DOI] [PubMed] [Google Scholar]
- 19. Crespi C. L., Penman B. W., Gelboin H. V., Gonzalez F. J. (1991). A tobacco smoke-derived nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, is activated by multiple human cytochrome P450s including the polymorphic human cytochrome P4502D6. Carcinogenesis, 12, 1197–1201 [DOI] [PubMed] [Google Scholar]
- 20. Hecht S. S., Chen C. B., Hirota N., Ornaf R. M., Tso T. C., Hoffmann D. (1978). Tobacco-specific nitrosamines: formation from nicotine in vitro and during tobacco curing and carcinogenicity in strain A mice. J. Natl Cancer Inst., 60, 819–824 [DOI] [PubMed] [Google Scholar]
- 21. Rehan V. K., Sakurai R., Torday J. S. (2011). Thirdhand smoke: a new dimension to the effects of cigarette smoke on the developing lung. Am. J. Physiol. Lung Cell Mol. Physiol., 301, L1–L8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh N. P., McCoy M. T., Tice R. R., Schneider E. L. (1988). A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res., 175, 184–191 [DOI] [PubMed] [Google Scholar]
- 23. Hartmann A., Agurell E., Beevers C., et al. (2003). Recommendations for conducting the in vivo alkaline Comet assay. 4th International Comet Assay Workshop. Mutagenesis, 18, 45–51 [DOI] [PubMed] [Google Scholar]
- 24. Olive P. L., Banáth J. P. (2006). The comet assay: a method to measure DNA damage in individual cells. Nat. Protoc., 1, 23–29 [DOI] [PubMed] [Google Scholar]
- 25. Hoffmann H., Högel J., Speit G. (2005). The effect of smoking on DNA effects in the comet assay: a meta-analysis. Mutagenesis, 20, 455–466 [DOI] [PubMed] [Google Scholar]
- 26. DeMarini D. M. (2004). Genotoxicity of tobacco smoke and tobacco smoke condensate: a review. Mutat. Res., 567, 447–474 [DOI] [PubMed] [Google Scholar]
- 27. Husgafvel-Pursiainen K., Sorsa M., Møller M., Benestad C. (1986). Genotoxicity and polynuclear aromatic hydrocarbon analysis of environmental tobacco smoke samples from restaurants. Mutagenesis, 1, 287–292 [DOI] [PubMed] [Google Scholar]
- 28. Leanderson P., Tagesson C. (1990). Cigarette smoke-induced DNA-damage: role of hydroquinone and catechol in the formation of the oxidative DNA-adduct, 8-hydroxydeoxyguanosine. Chem. Biol. Interact., 75, 71–81 [DOI] [PubMed] [Google Scholar]
- 29. Asami S., Manabe H., Miyake J., Tsurudome Y., Hirano T., Yamaguchi R., Itoh H., Kasai H. (1997). Cigarette smoking induces an increase in oxidative DNA damage, 8-hydroxydeoxyguanosine, in a central site of the human lung. Carcinogenesis, 18, 1763–1766 [DOI] [PubMed] [Google Scholar]
- 30. Cadet J., Berger M., Douki T., Ravanat J. L. (1997). Oxidative damage to DNA: formation, measurement, and biological significance. Rev. Physiol. Biochem. Pharmacol., 131, 1–87 [DOI] [PubMed] [Google Scholar]
- 31. Valavanidis A., Vlachogianni T., Fiotakis K. (2009). Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int. J. Environ. Res. Public Health, 6, 445–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santos J. H., Meyer J. N., Mandavilli B. S., Van Houten B. (2006). Quantitative PCR-based measurement of nuclear and mitochondrial DNA damage and repair in mammalian cells. Methods Mol. Biol., 314, 183–199 [DOI] [PubMed] [Google Scholar]
- 33. Dey S., Maiti A. K., Hegde M. L., et al. (2012). Increased risk of lung cancer associated with a functionally impaired polymorphic variant of the human DNA glycosylase NEIL2. DNA Repair (Amst.), 11, 570–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schick S. F., Farraro K. F., Fang J., et al. (2012). An apparatus for generating aged cigarette smoke for controlled human exposure studies. Aerosol Sci. Technol., 46, 1246–1255 [Google Scholar]
- 35. Jung D., Cho Y., Meyer J. N., Di Giulio R. T. (2009). The long amplicon quantitative PCR for DNA damage assay as a sensitive method of assessing DNA damage in the environmental model, Atlantic killifish (Fundulus heteroclitus). Comp. Biochem. Physiol. C Toxicol. Pharmacol., 149, 182–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hang B. (2006). Base Excision Repair. World Scientific Publishing Co. Pte. Ltd, Singapore [Google Scholar]
- 37. DeMarini D. M. (1983). Genotoxicity of tobacco smoke and tobacco smoke condensate. Mutat. Res., 114, 59–89 [DOI] [PubMed] [Google Scholar]
- 38. Wolz L., Krause G., Scherer G. (2002). The comet assay with MCL-5 cells as an indicator of genotoxic treatment with chemicals and cigarette smoke condensates. Altern. Lab. Anim., 30, 331–339 [DOI] [PubMed] [Google Scholar]
- 39. Lacoste S., Castonguay A., Drouin R. (2006). Formamidopyrimidine adducts are detected using the comet assay in human cells treated with reactive metabolites of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Mutat. Res., 600, 138–149 [DOI] [PubMed] [Google Scholar]
- 40. Benowitz N. L., Hukkanen J., Jacob P., 3rd (2009). Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol., 192, 29–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hecht S. S. (1999). DNA adduct formation from tobacco-specific N-nitrosamines. Mutat. Res., 424, 127–142 [DOI] [PubMed] [Google Scholar]
- 42. Zhuge J., Luo Y., Yu Y. N. (2003). Heterologous expression of human cytochrome P450 2E1 in HepG2 cell line. World J. Gastroenterol., 9, 2732–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dehn P. F., White C. M., Conners D. E., Shipkey G., Cumbo T. A. (2004). Characterization of the human hepatocellular carcinoma (hepg2) cell line as an in vitro model for cadmium toxicity studies. In Vitro Cell. Dev. Biol. Anim., 40, 172–182 [DOI] [PubMed] [Google Scholar]
- 44. Anderson D., Yu T. W., McGregor D. B. (1998). Comet assay responses as indicators of carcinogen exposure. Mutagenesis, 13, 539–555 [DOI] [PubMed] [Google Scholar]
- 45. Chung F. L., Xu Y. (1992). Increased 8-oxodeoxyguanosine levels in lung DNA of A/J mice and F344 rats treated with the tobacco-specific nitrosamine 4-(methylnitrosamine)-1-(3-pyridyl)-1-butanone. Carcinogenesis, 13, 1269–1272 [DOI] [PubMed] [Google Scholar]
- 46. Sipowicz M. A., Amin S., Desai D., Kasprzak K. S., Anderson L. M. (1997). Oxidative DNA damage in tissues of pregnant female mice and fetuses caused by the tobacco-specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Cancer Lett., 117, 87–91 [DOI] [PubMed] [Google Scholar]
- 47. Church D. F., Pryor W. A. (1985). Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect., 64, 111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Friedberg E. C., Walker G. C., Siede W., Wood R. D., Schultz R. A., Ellenberger T. (2006). DNA Repair and Mutagenesis. 2nd ed. ASM Press, Washington, DC [Google Scholar]