Abstract
Autism spectrum disorders are a heterogeneous group of neurodevelopmental conditions characterised by impairments in reciprocal social interaction, communication and stereotyped behaviours. As increased DNA damage events have been observed in a range of other neurological disorders, it was hypothesised that they would be elevated in lymphoblastoid cell lines (LCLs) obtained from children with autism compared with their non-autistic siblings. Six case–sibling pairs of LCLs from children with autistic disorder and their non-autistic siblings were obtained from the Autism Genetic Resource Exchange (AGRE) and cultured in standard RPMI-1640 tissue culture medium. Cells were exposed to medium containing either 0, 25, 50, 100 and 200 µM hydrogen peroxide (an oxidative stressor) or 0, 5, 10, 20 and 40 µM s-nitroprusside (a nitric oxide producer) for 1h. Following exposure, the cells were microscopically scored for DNA damage, cytostasis and cytotoxicity biomarkers as measured using the cytokinesis-block micronucleus cytome assay. Necrosis was significantly increased in cases relative to controls when exposed to oxidative and nitrosative stress (P = 0.001 and 0.01, respectively). Nuclear division index was significantly lower in LCLs from children with autistic disorder than their non-autistic siblings when exposed to hydrogen peroxide (P = 0.016), but there was no difference in apoptosis, micronucleus frequency, nucleoplasmic bridges or nuclear buds. Exposure to s-nitroprusside significantly increased the number of micronuclei in non-autistic siblings compared with cases (P = 0.003); however, other DNA damage biomarkers, apoptosis and nuclear division did not differ significantly between groups. The findings of this study show (i) that LCLs from children with autism are more sensitive to necrosis under conditions of oxidative and nitrosative stress than their non-autistic siblings and (ii) refutes the hypothesis that children with autistic disorder are abnormally susceptible to DNA damage.
Introduction
Autism spectrum disorders are a heterogeneous group of neurodevelopmental conditions that are characterised by impairments in reciprocal social interaction, communication and stereotypical behaviours (1). The number of diagnosed cases has increased over the last 30 years from a prevalence of 0.4–0.5/1000 (2) to a median of 1.7/1000 for autistic disorder and 6.2/1000 for autism spectrum disorders (3). While broadening diagnostic criteria, earlier age of diagnosis and improved case ascertainment account for much of the increase (4), the fundamental underlying aetiology relating to these disorders remains unknown. Factors that are currently considered to contribute to the aetiology of autistic disorder include genes (5–8), epigenetic changes (9,10), imprinting (11), immune system dysfunction (12), hormone imbalance (13,14), environmental toxins (15–17), gastrointestinal factors (18,19), season of birth (20) and age of paternity (21,22).
Numerous studies have suggested that increased oxidative and nitrosative stress may play a key role in the pathogenesis of autism (23–32). Oxidative and nitrosative stress is the result of a homeostatic imbalance between elevated reactive oxygen/nitrogen species (ROS/RNS) and a decrease in either the efficiency of the endogenous antioxidant defence mechanisms or the cells’ ability to efficiently scavenge free radicals. Although small fluctuations may play a role in intracellular signalling (33), uncontrolled increased levels of oxidative and nitrosative stress can result in damage of cellular macromolecules including proteins (34,35), lipid membranes (36) and DNA (37).
Increased DNA damage in lymphocytes has been observed in a range of common neurobiological conditions including Down syndrome (38), ataxia-telangiectasia (39) and Bloom syndrome (40) as previously measured by the cytokinesis-block micronucleus cytome (CBMN-cyt) assay in lymphocytes (41). The CBMN-cyt assay is a comprehensive and well-validated method to measure DNA damage events in once-divided cytokinesis-blocked binucleated (BN) cells. DNA damage events include (i) micronuclei (MNi), which are biomarkers of chromosome breakage and/or whole chromosome loss; (ii) nucleoplasmic bridges (NPB), biomarkers of DNA misrepair and/or telomere end-fusions; (iii) nuclear buds (NBUDs), biomarkers for the elimination of amplified DNA and/or DNA repair complexes. Cytostatic effects are measured via the proportion of mono-, bi- and multinucleated cells represented by the nuclear division index (NDI) and cytotoxicity events are determined by the number of necrotic and/or apoptotic cells (41).
In vitro modelling using lymphoblastoid cell lines (LCLs) has shown that DNA damage can be induced under conditions of high oxidative or nitrosative stress such as cellular exposure to hydrogen peroxide or nitric oxide (NO) (42,43). To date, however, only one study has been published that examines the effect of ROS/RNS in LCLs obtained from children diagnosed with autistic disorder compared with unaffected controls (44). This study showed that baseline glutathione redox capacity was lower in whole-cell extracts and mitochondria in LCLs derived from children with autistic disorder compared with non-autistic controls. Furthermore, a greater decrease in the glutathione redox ratio together with an increase in free radical generation was observed under conditions of thimerosal-induced oxidative stress within these cell lines (44). In this study, acute exposure to physiological levels of NO decreased mitochondrial membrane potential to a greater extent in LCLs derived from autistic children although the glutathione redox and ATP levels were similarly decreased in both controls and autistic-derived cell lines (44). These results suggest that children with autism may have a reduced capacity to counteract the damaging effects of oxidative or nitrosative stress possibly because the glutathione redox system does not function optimally (44).
It was, therefore, hypothesised that LCLs obtained from children with autistic disorder may be more sensitive to the DNA damaging effects of ROS/RNS than their non-autistic siblings. This LCL model was chosen as numerous studies have previously shown that genomic instability is readily measured in LCLs using the CBMN-cyt assay (42,45,46), which is a robust, reproducible, well-validated assay for measuring DNA damage, cystostasis and cytotoxicity (41). LCLs from children with autism and their non-autistic siblings were selected from the Autism Genetic Resource Exchange repository, which also includes diagnostic and other phenotypic information (47). Unaffected siblings are an ideal control group because they enable a more accurate assessment as to whether any observed differences are due to the autism phenotype because they control for shared genes and potentially a common environment assuming that epigenetic markers induced early in life are retained in in vitro culture (10,48). Physiological concentrations of hydrogen peroxide were chosen as an oxidative stressor as it has been validated previously to produce dose-related changes in DNA damage biomarkers in peripheral blood lymphocytes (42) and LCLs (45). It was originally planned to use S-nitroso-N-acetylpenicillamine (SNAP) for the NO donor as per the study by James et al.(2009) (44); however, use of SNAP requires serum-free culture conditions and cellular growth proved to be insufficient to conduct the CBMN-cyt assay. Consequently, s-nitroprusside (SNP) was chosen as a NO donor as it is widely used in clinical practice (49) and LCLs can grow adequately in its presence to accumulate sufficient cytokinesis-blocked BN cells to perform the CBMN-cyt assay (50).
Materials and methods
Lymphoblastoid cell lines
Six case–sibling pairs of LCLs from children with autistic disorder and their non-autistic siblings were obtained from the Autism Genetic Resource Exchange (AGRE; Los Angeles, CA, USA). Case–sibling cell lines were derived from non-Hispanic Caucasian multiplex families in which (i) children shared the same parents, (ii) the case was diagnosed with autistic disorder using both the Autism Diagnostic Interview (ADI) and Autism Diagnostic Observation Schedule (ADOS) and (iii) both children were male. (AGRE ID numbers for cases were as follows: AU008404, AU038804, AU1165302, AU1267302, AU1344302, AU1348303; mean age 9.85±2.39 year. AGRE ID numbers for sibling controls were as follows: AU008405, AU038805, AU1165303, AU1267303, AU1344303, AU1348302; mean age 9.02±3.30 year.) The six case LCLs were the same ones used in the study by James et al. (2009) (44); however, our study differed because we used sibling controls rather than unrelated adult controls.
Cell culture
Cell lines were cultured and then cryopreserved at −80°C at a concentration of 5×106 in 1ml 90% foetal bovine serum (FBS) (Trace Biosciences, Australia) and 10% dimethyl sulphoxide (Sigma) before being cultured in RPMI-1640 (Sigma, Australia) supplemented with 10% FBS, 1% penicillin/streptomycin (5000 IU penicillin/5mg streptomycin) (Sigma, Australia), 1% sodium pyruvate (Sigma, Australia), 1% glutamine (Sigma, Australia) and 1% interleukin-2 (Roche Diagnostics, Australia) and incubated in a humidified atmosphere with 5% CO2 at 37 C (Sanyo MCO-17 AIC, Japan).
LCL tissue culture
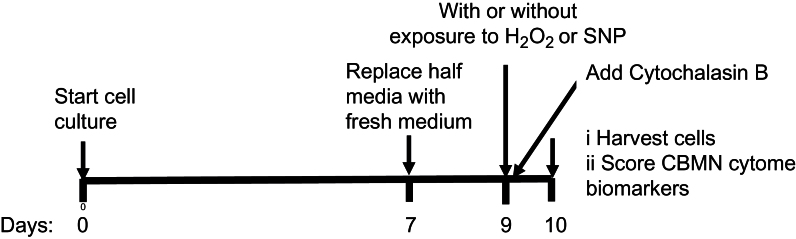
Cells were seeded at a density of 0.15×106 viable cells per millilitre in TV-25 flasks (Nunc, Australia) 9 days prior to exposure to hydrogen peroxide or SNP and grown in duplicate as outlined below (Figure 1). The seeding concentration of LCLs was determined in preliminary experiments aimed at optimising the number of viable cells at Day 9 required to complete the CBMN-cyt assay. On Day 7, half the culture medium was carefully replaced with fresh medium without disturbing the cells that were then incubated for a further 2 days.
Fig. 1.
Schematic diagram outlining the long-term culture and CBMN-cyt assay to test the cytotoxic and genotoxic effects of hydrogen peroxide (H2O2) and s-nitro-prusside (SNP) concentration on LCLs derived from children with autistic disorder compared with their non-autistic siblings.
Exposure of LCLs to hydrogen peroxide or SNP and CBMN-cyt assay
On the day of the assay (Day 9 of culture), cells were washed twice in Hanks’ balanced saline solution (HBSS) (Thermo-Fisher) before being exposed to supplemented RPMI-1640 cell culture medium containing a final concentration of either 0, 25, 50, 100 and 200 µM hydrogen peroxide (Chem Supply, Australia) or 0, 5, 10, 20 and 40 µM SNP (Sigma), in a 96-well plate (NUNC flat bottom, sterile with lid 200 μl/well) for 1h. SNP is extremely light sensitive, and it is therefore important that it be kept in a dark place until time of exposure. Following exposure, the cells were washed again in HBSS and resuspended in supplemented RPMI-1640 cell culture medium warmed to 37°C before being incubated for 24h with cytochalasin B (Sigma, Australia) at a final concentration of 4.5 μg/ml to inhibit cytokinesis. Cells were harvested in duplicate by pipetting 120 μl cell suspension in a cyto-centrifuge cup attached to a cyto-centrifuge filter card and slide and spun for 5min at 600rpm using a Shandon Scientific cyto-centrifuge (Shandon Products, UK), so that two spots were obtained for each concentration being tested. Slides were air dried for 30min before being fixed and stained using a commercial kit (Diff Quick, Lab-Aids, Australia), then air dried overnight before being cover-slipped with DePex (BDH, UK).
Slides were coded prior to scoring by P.T. Scoring was carried out by one person (P.M.), who did not have access to the code, using a bright field microscope (Leica 20EB) at ×1000 magnification under oil immersion. Established criteria were used for scoring DNA damage, cytostasis and cytotoxicity biomarkers as measured by the CBMN-cyt assay (41). Briefly, 250 cells were scored from each spot to determine mononucleated, BN, multinucleated, apoptotic and necrotic cell ratios and the NDI, a measure of cellular proliferation, was calculated using the formula NDI = (M1 + 2 M2 + 4 M>2)/total number of viable cells scored where M1, M2 and M>2 are the number of cells with one nucleus, two nuclei and >2 nuclei, respectively. This slightly modified method of measuring NDI was used because the standard method of measuring NDI (41) does not include cells with >4 nuclei, but in this study, cells with >4 nuclei were common in cultures treated with SNP. This modified form of the NDI is almost identical to the cytokinesis-block proliferation index described in the OECD Guideline Number 487 for Testing of Chemicals using the in vitro mammalian cell micronucleus test (51). A comparison of NDIs obtained using the two methods showed a high degree of correlation (R 2 = 0.9906, P < 0.0001) between scores across 120 slides from this study. Additionally, 1000 BN cells were scored from each spot for the following DNA damage biomarkers: BN cells with MNi (MNed cells), number of MNi, BN cells with NPB and BN cells with NBUDs. It was not possible to determine the frequency of DNA damage markers for cell line AU1165302 (case) when exposed to the highest concentration of hydrogen peroxide or SNP as insufficient numbers of BN were available to obtain statistically reliable data.
Statistical analysis
The data are presented as medians with interquartile ranges. Data were analysed using conditional logistic regression to determine the significance of differences in biomarkers between case–sibling pairs as well as the significance of within group differences with increasing dose. A P < 0.05 value was considered to be significant. The statistical analysis was performed using the clogit command in the Stata statistical package version 11 (52). The Mann–Whitney test was applied where a significant dose–response relationship was not observed although it was evident that the CBMN-cyt was increased at the highest dose compared with baseline.
Results
Comparison of control cultures
There were no significant differences in the NDI or any biomarkers of cytotoxicity or DNA damage between the untreated control cultures of LCLs from children with autism and their non-autistic siblings in both the hydrogen peroxide and SNP experiments.
Oxidative stress
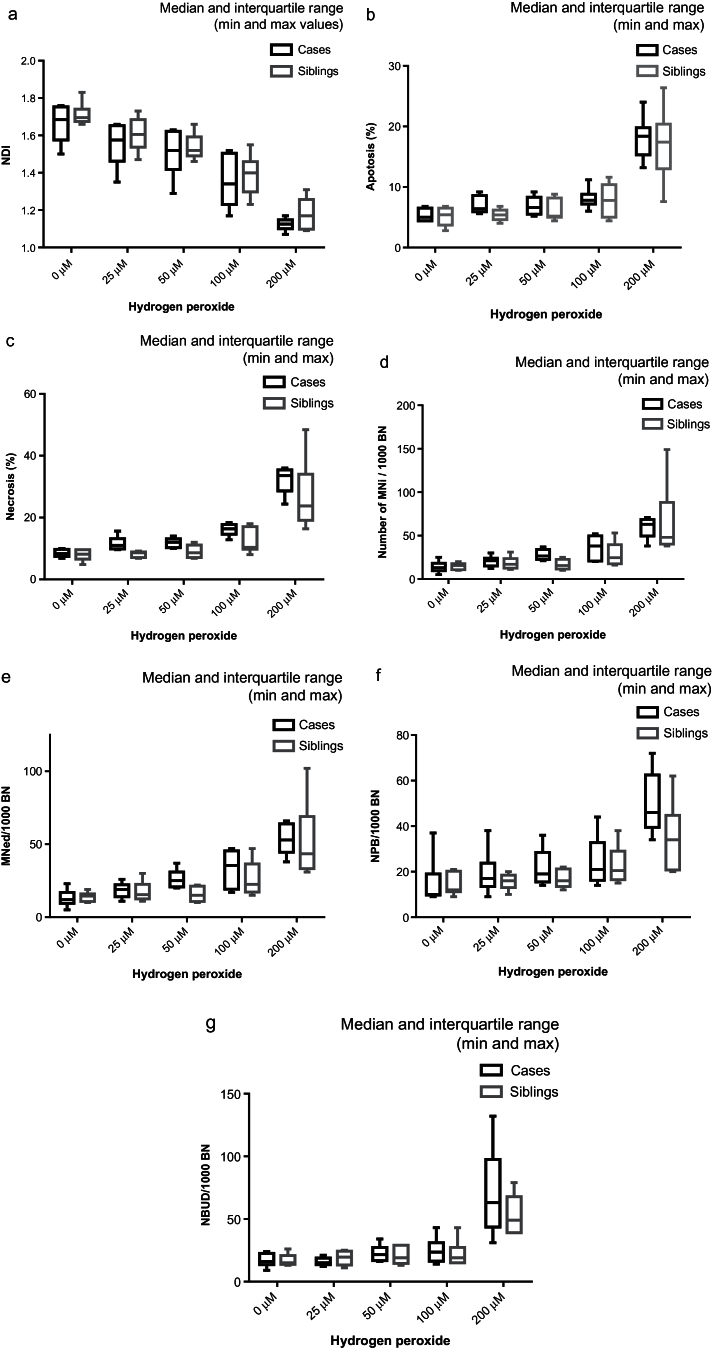
A significant decrease in the NDI, which is a measure of cellular proliferation, was observed as the concentration of hydrogen peroxide increased (P = 0.022) for both sets of LCLs (Table I and Figure 2a). A statistically significant dose–response relationship was not observed for any biomarker of DNA damage or cytotoxicity as the concentration of hydrogen peroxide increased from 0 to 200 μM (Tables I and II, Figure 2b–g). It was, however, evident that apoptosis (P = 0.0001), necrosis (P = 0.0001), MNi (P = 0.0001), NPBs (P = 0.0003) and NBUDs (P = 0.0001) were increased at 200 µM hydrogen peroxide relative to untreated controls.
Table I.
NDI and frequency of apoptotic and necrotic cells in LCLs obtained from children with autistic disorder compared with their non-autistic siblings exposed to increasing doses of hydrogen peroxide (median and interquartile range)
| Concentration (µM) | 0 | 25 | 50 | 100 | 200 | P value (dose) | P value (biomarker)* | P value (interaction) | |
|---|---|---|---|---|---|---|---|---|---|
| n (case–sibling pairs) | n = 6 | n = 6 | n = 6 | n = 6 | n = 5 | ||||
| NDI | Case | 1.69 (1.60–1.75) | 1.58 (1.50–1.65) | 1.52 (1.46–1.62) | 1.34 (1.25–1.50) | 1.13 (1.11–1.14) | 0.022 | 0.016 | |
| Sibling | 1.70 (1.68–1.71) | 1.61 (1.56–1.67) | 1.52 (1.5–1.57) | 1.40 (1.32–1.43) | 1.17 (1.10–1.24) | ||||
| Apoptotic (%) | Case | 5.0 (4.4–6.4) | 6.4 (6.0–8.4) | 6.6 (5.6–8.0) | 7.8 (7.6–8.0) | 18.4 (16.0–18.4) | 0.331 | 0.267 | |
| Sibling | 5.4 (4.0–6.4) | 5.4 (4.8–6.0) | 5.2 (5.2–8.0) | 7.8 (5.2–10.0) | 17.4 (14.8–18.4) | ||||
| Necrotic (%) | Case | 8.4 (7.6–9.6) | 11.0 (10.0–12.4) | 12.0 (10.4–12.8) | 16.4 (15.2–17.6) | 33.6 (30.0–35.2) | 0.798 | 0.001 | 0.008 |
| Sibling | 8.2 (7.2–9.6) | 8.6 (7.2–8.8) | 8.6 (7.2–10.8) | 10.4 (10.4–16.8) | 23.8 (20.0–29.2) | ||||
*The biomarker can distinguish between cases and siblings at this P value.
Fig. 2.
The effect of exposure to increasing doses of hydrogen peroxide on LCLs derived from children with autistic disorder compared with their non-autistic siblings (medians and interquartile ranges). (a) Nuclear division index, (b) percentage apoptosis, (c) percentage necrosis, (d) number of MNi/1000 BN cells, (e) BN cells with MNi/1000 BN cells, (f) BN cells with NPB/1000 BN cells and (g) BN cells with NBUDs/1000 BN cells.
Table II.
Frequency of DNA damage biomarkers per 1000 BN cells from LCLs obtained from children with autistic disorder compared with their non-autistic siblings exposed to increasing doses of hydrogen peroxide (median and interquartile range)
| Concentration (µM) | 0 | 25 | 50 | 100 | 200 | P value (dose) | P value (biomarker)* | |
|---|---|---|---|---|---|---|---|---|
| n (case–sibling pairs) | n = 6 | n = 6 | n = 6 | n = 6 | n = 5 | |||
| MNi | Case | 13 (11–16) | 21 (16–22) | 26.5 (23–33) | 38 (21–49) | 63 (61–66) | 0.358 | 0.351 |
| Sibling | 15.5 (11–17) | 17 (13–21) | 15.5 (12–22) | 24.5 (18–35) | 48 (41–68) | |||
| MNed | Case | 12 (11–15) | 19 (15–21) | 25 (21–29) | 35.5 (20–45) | 53 (51–62) | 0.125 | 0.107 |
| Sibling | 14.5 (11–15) | 15.5 (13–20) | 15 (11–21) | 22.5 (18–33) | 43.5 (34–58) | |||
| NPB | Case | 10 (10–13) | 17 (15–19) | 19 (16–26) | 21 (17–29) | 46 (45–53) | 0.107 | 0.054 |
| Sibling | 12 (12–20) | 16 (14–18) | 16 (14–21) | 20.5 (17–26) | 34 (21–39) | |||
| NBUD | Case | 16 (15–22) | 15 (14–18) | 21.5 (17–25) | 23.5 (17–27) | 63 (56–63) | 0.398 | 0.388 |
| Sibling | 15 (14–19) | 19.5 (14–24) | 19 (15–29) | 19 (15–22) | 49 (39–64) | |||
Mned, number of BN with micronuclei/1000 BN cells; Mni, number of micronuclei/1000 BN cells; NPB, number of BN with NPB/1000 BN cells, NBUD, number of BN with nuclear buds/1000 BN cells.
*The biomarker can distinguish between cases and siblings at this P value.
LCLs from children with autistic disorder had a significantly lower cellular proliferation (NDI) (P = 0.016) and increased necrosis (P = 0.001) compared with their non-autistic siblings (Table I and Figure 2a and c). There was significant interaction between dose and response by necrosis (P = 0.008) suggesting that the sensitivity of cases to necrosis increased with hydrogen peroxide dose. The median of MNi, MNed, NPB and NBUD was higher in cases relative to siblings at hydrogen peroxide concentrations of 50 µM or greater, but the overall differences did not achieve statistical significance.
Nitrosative stress
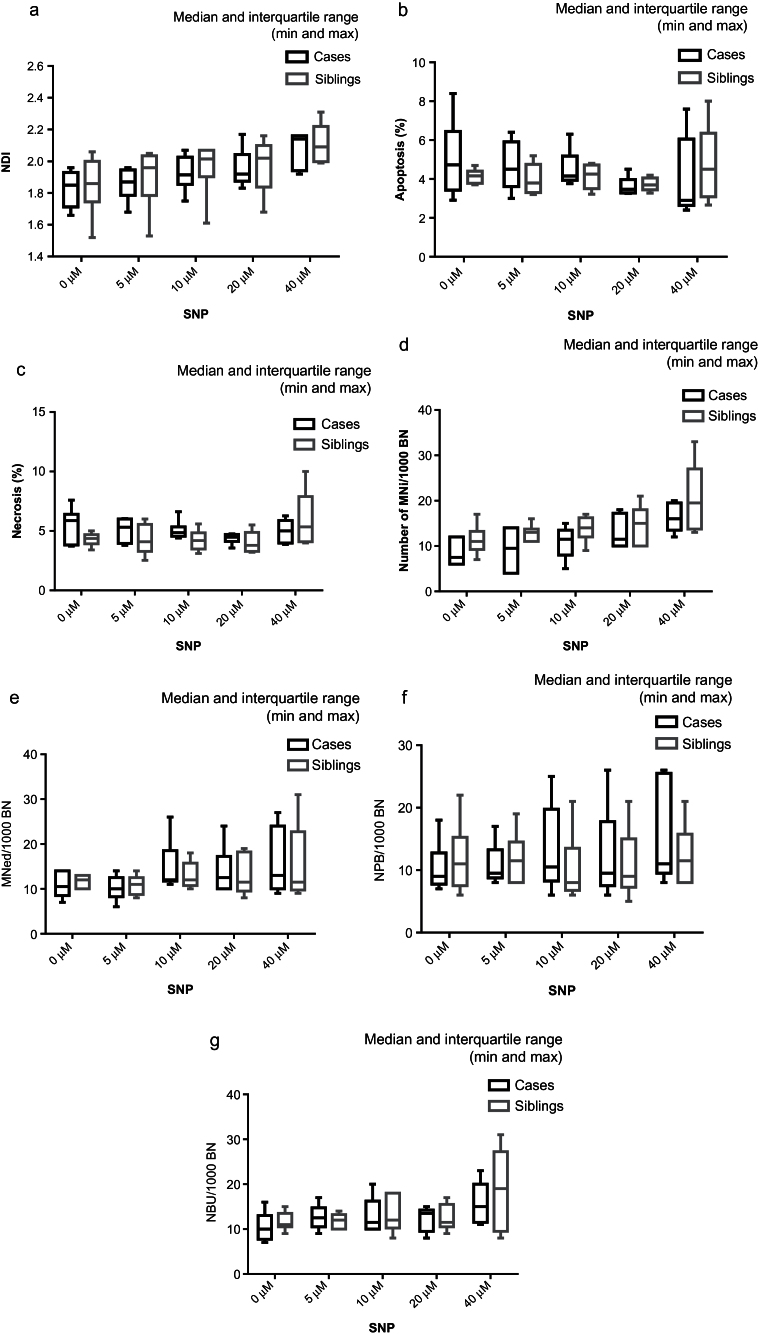
A significant dose–response was observed for the percentage of necrotic cells (P = 0.028) as well as an increase in the number of MNi per 1000 BN (P = 0.04) in both LCLs (Tables III andIV and Figure 3c and d). Significant dose–responses were not observed for NDI, apoptosis (Tables III andIV, Figure 3a and b), number of MNed cells, NPBs or NBUDs for either set of LCLs (Table IV and Figure 3e–g). Although a significant dose–response was not observed, the NDI increased at 40 µM SNP relative to untreated controls (Figure 3a).
Table III.
NDI and the frequency of apoptotic and necrotic cells in LCLs obtained from children with autistic disorder compared with their non-autistic siblings exposed to increasing doses of SNP (median and interquartile range)
| Concentration (μM) | 0 | 5 | 10 | 20 | 40 | P value (dose) | P value (biomarker)* | P value (interaction) | |
|---|---|---|---|---|---|---|---|---|---|
| n (case–sibling pairs) | n = 6 | n = 6 | n = 6 | n = 6 | n = 5 | ||||
| NDI | Case | 1.85 (1.73–1.92) | 1.87 (1.82–1.94) | 1.92 (1.89–2.01) | 1.92 (1.89–2.0) | 2.14 (1.96–2.16) | 0.150 | 0.415 | |
| Sibling | 1.86 (1.82–1.98) | 1.96 (1.87–2.03) | 2.02 (2.0–2.07) | 2.02 (1.89–2.08) | 2.09 (2.0–2.19) | ||||
| Apoptotic (%) | Case | 4.73 (3.6–5.8) | 4.5 (3.82–5.75) | 4.16 (4.0–4.8) | 3.48 (3.3–3.8) | 2.9 (2.89–4.5) | 0.835 | 0.357 | |
| Sibling | 4.17 (3.8–4.3) | 3.8 (3.34–4.6) | 4.25 (3.6–4.7) | 3.7 (3.5–4.0) | 4.5 (3.23–5.8) | ||||
| Necrotic (%) | Case | 5.87 (3.84–6.0) | 5.32 (4.0–6.0) | 4.85 (4.6–4.92) | 4.49 (4.3–4.7) | 5.0 (4.1–5.5) | 0.028 | 0.01 | 0.019 |
| Sibling | 4.37 (4.1–4.6) | 4.1 (3.53–5.4) | 4.2 (3.6–4.6) | 3.79 (3.3–4.67) | 5.35 (4.12–7.2) | ||||
*The biomarker can distinguish between cases and siblings at this P value.
Table IV.
Frequency of DNA damage biomarkers per 1000 BN cells from LCLs obtained from children with autistic disorder compared with their non-autistic siblings exposed to increasing doses of SNP (median and interquartile range)
| Concentration (µM) | 0 | 5 | 10 | 20 | 40 | P value (dose) | P value (biomarker)* | |
|---|---|---|---|---|---|---|---|---|
| n (case–sibling pairs) | n = 6 | n = 6 | n = 6 | n = 6 | n = 5 | |||
| MNi | Case | 7.5 (6–12) | 9.5 (4–14) | 11.5 (9–13) | 11.5 (10–17) | 16 (15–19) | 0.040 | 0.003 |
| Sibling | 11 (10–12) | 13 (11–13) | 14 (13–16) | 15 (10–17) | 19.5 (14–25) | |||
| MNed | Case | 10.5 (9–14) | 10 (9–12) | 12 (12–16) | 12.5 (10–15) | 13 (11–21) | 0.710 | 0.710 |
| Sibling | 12 (10–13) | 11 (9–12) | 12 (11–15) | 11.5 (10–18) | 11.5 (10–20) | |||
| NPB | Case | 9 (8–11) | 9.5 (9–12) | 10.5 (9–18) | 9.5 (8–15) | 11 (11–25) | 0.685 | 0.414 |
| Sibling | 11 (8–13) | 11.5 (8–13) | 8 (7–11) | 9 (8–13) | 11.5 (8–14) | |||
| NBUD | Case | 10 (8–12) | 12.5 (11–14) | 11.5 (10–15) | 13.5 (10–14) | 15 (12–17) | 0.729 | 0.769 |
| Sibling | 11 (10–13) | 12 (10–13) | 11 (9–13) | 11 (10–12) | 14.5 (10–21) | |||
Mned, number of BN with micronuclei/1000 BN cells; Mni, number of MNi/1000 BN cells; NPB, number of BN with NPB/1000 BN cells; NBUD,number of BN with NBUDs/1000 BN cells.
*The biomarker can distinguish between cases and siblings at this P value.
Fig. 3.
The effect of exposure to increasing doses of SNP on LCLs derived from children with autistic disorder compared with their non-autistic siblings (medians and interquartile ranges). (a) Nuclear division index, (b) percentage apoptosis, (c) percentage necrosis, (d) number of MNi/1000 BN cells, (e) BN cells with MNi/1000 BN cells, (f) BN cells with NPB/1000 BN cells and (g) BN cells with NBUDs/1000 BN cells.
Necrosis in LCLs from children with autistic disorder was significantly greater than in their non-autistic siblings (P = 0.01) and a significant interaction was observed between dose and necrosis effect (P = 0.019) (Table III). In addition, LCLs from children with autistic disorder had significantly less MNi (P = 0.003) than LCLs from their non-autistic siblings although there was no significant difference in the number of BN cells with MNi (Table IV). There was no significant difference between cases and controls in NDI, the frequency of apoptotic cells (Table III and Figure 3a and b) or the frequency of BN cells with MNi, NPBs or NBUDs (Table IV and Figure 3e–g).
Discussion
The most significant finding of this study was that LCLs from children with autistic disorder are more likely to become necrotic compared with LCLs from their non-autistic siblings under conditions of oxidative and nitrosative stress. In recent years, the conventional view that necrosis is a passive process caused by external physical injury has been overturned by research that has shown its initiation and progression to be tightly controlled by several signalling pathways (53–55). Central to this process is an imbalance in production and detoxification of ROS generated by the mitochondria and cytosolic glycolysis and RNS generated by nitric oxide synthetase (NOS). The balance between oxidised and reduced glutathione provides an important indicator of oxidative stress in the body. The same LCLs from children with autistic disorder were previously shown to have significantly lower free glutathione and glutathione redox capacity compared with LCLs from adults with no known psychiatric or neurological disorder when exposed to thimerosal, a mercury containing sulphhydryl pro-oxidant previously used as a preservative in vaccines (44).
Several indirect lines of evidence suggest that oxidative stress may be a significant factor in the aetiology of autism. Firstly, in vivo studies suggest that children with autism may generate higher levels of hydrogen peroxide than unaffected children as increased activity of superoxide dismutase (SOD) in erythrocytes (25,27,31,56), plasma (27) and platelets (56) has been reported in children with autism. SOD is a major producer of endogenous hydrogen peroxide in the body as a result of superoxide conversion during reactions involving the electron transport chain (57). One study, however, reported lower levels of SOD in erythrocytes of autistic children (58) while another found no significant difference in plasma SOD between autistic children and controls (23). Furthermore, erythrocyte xanthine oxidase activity has also been shown to be increased in autism (25). Xanthine oxidase, the rate limiting enzyme in purine catabolism, is an important producer of the superoxide radical (O2 −) used by SOD. Both hydrogen peroxide and superoxide have been shown to be efficient inducers of MNi and NPB, which are biomarkers of genome instability, in the WIL2-NS human B LCL (45).
Secondly, the physiological mechanisms required to remove hydrogen peroxide are impaired in many children with autism. Endogenously formed hydrogen peroxide is removed either enzymatically by catalase and peroxidases or non-enzymatically via the Fenton reaction by free metal iron ions. Children with autism show decreased catalase activity in erythrocytes (23,31) but not plasma (27). Furthermore, glutathione peroxidase activity was found to be lower (56,58), or not significantly different (31,59) in erythrocytes from children with autistic disorder than controls, and erythrocyte selenium, a mineral required for the optimal function of glutathione peroxidase was shown to be lower in children with autism in one study (60). In plasma, however, the results are conflicting having been reported as higher in two studies (23,27) and lower in a further two (32,58). Children with autism are also more likely to be low in ferritin, the main form of iron ion storage protein, compared with unaffected children (61) possibly indicating susceptibility to the presence of pro-oxidant free iron in body fluids, which is supported by the fact that plasma-free iron is significantly higher in children with autism (31). Children with autistic disorder have also been shown to have low serum transferrin and ceruloplasmin (62,63) both of which are important to prevent free iron and copper ions that drive the Fenton reaction, which in turn generates genotoxic hydroxyl radicals from hydrogen peroxide.
The third line of evidence is that children with autism have been shown to have significantly lower levels of antioxidant proteins and vitamins than unaffected children. Glutathione status is an accurate indicator of cell functionality and viability (64–66). Glutathione levels and glutathione redox ratios are reduced in about 75% of children with autistic disorder (67). Plasma antioxidants, vitamin C and E have been shown to be lower in children with autism (27). The expression of metallothionein (MT), an antioxidant protein required for metal homeostasis, metal detoxification and scavenging of ROS (68), is more complex. While a recent study showed that mRNA for MT is increased in children with autism compared with controls (31), a previous study found no significant difference (69). While a recent study reported that serum zinc is significantly higher in children with autism than controls (31), two previous studies showed that it was low but not significantly so (70,71) and a further study reported serum and erythrocyte zinc to be significantly lower (72). There was no difference in the up-regulation of MT genes in LCLs from children with autism compared with their non-autistic siblings following challenge using zinc chloride although the numbers were small (73). Transcription of MT genes is enhanced by metal-regulatory transcription factor (MTF-1), which requires zinc for its formation (74). A family association study revealed an association between a polymorphism of MTF-1 (rs 3790625) and autism (75).
Additionally, ROS react with membrane lipoproteins and polyunsaturated fatty acids leading to lipid peroxidation, which can be measured by thiobarbituric acid reactive substances (TBARS), such as malondialdehyde (MDA), in blood or by urinary 8-isoprostane-F2α (8-iso-PGF2α) levels. Erythrocyte and plasma TBARS, including MDA, are higher in children with autism (25,27,28), as is urinary 8-iso-prostane (76) supporting the hypothesis of elevated ROS in autistic disorder.
Our data indicate that under normal culture conditions, the oxidative stress level is insufficient to reveal increased susceptibility to DNA damage in LCLs from children with autistic disorder relative to controls. In contrast, cultured lymphocytes from patients with ataxia-telangiectasia, for example, had three times as many MNi as controls (39). Increasing the oxidative stress did not lead to a dose–response in autism LCLs, nor was there a significant difference between LCLs from cases or siblings. NDI was lower in LCLs from children with autistic disorder than LCLs from their non-autistic siblings when exposed to hydrogen peroxide, suggesting that autism is associated with decreased cellular proliferation under conditions of oxidative stress.
As discussed above, the current study showed there was a significant dose–response for necrosis levels that differed between LCLs from cases and their non-autistic siblings. RNS has been shown to be involved in the initiation and execution of necrosis although their role is not fully elucidated (53). NO has a dual role as a protective or toxic molecule depending on factors such as concentration, type of cell that it is synthesised in and the isoform of NOS (77). Physiological concentrations of NO decreased mitochondrial membrane potential in the same case LCLs compared with adult controls in the study by James et al. (2009) (44). This suggests that children with autism may have a reduced cytosol and mitochondrial capacity for glutathione redox (44) and may be more prone to apoptosis as nuclear features of apoptosis are thought to be preceded by alterations in mitochondrial structure and transmembrane potential (78–80); however, apoptosis was not affected by SNP in our experiment.
The observation that MNi are significantly increased by SNP, but not the NPB or NBUD frequency, suggests mechanisms other than misrepair of strand breaks that would lead to dicentric chromosome and NPB formation may result in breakage-fusion bridge cycles and NBUD formation (81). Alternative mechanisms could include malsegregation of chromosomes or lack of repair of DNA stand breaks. Mechanistic studies using centromere and telomere probes and markers of unrepaired strand breaks such as γH2AX may shed more light on the genomic defects caused by RNS in autistic disorder.
The lack of reduction in NDI with increasing concentration of SNP in the current study was unexpected as whole blood lymphocyte cultures exposed to the same concentrations of SNP and g12 cell lines exposed to 0–8mM SNP for 1h showed decreased cellular proliferation and increased numbers of MNi (50,82). Although the same concentrations of SNP were used as Andreassi et al. (2001) (50), the duration of SNP exposure in the latter study was for 48h compared with 1h exposure time in this study. Other differences in methodology that may have influenced the outcome was that our study used transformed cell lines that comprise only B lymphocytes rather than whole blood lymphocyte cultures or g12 cells and our LCLs were grown in RPMI-1640 rather than Ham’s F12 cell culture medium that differ in the concentration of micronutrients required for DNA synthesis and repair (e.g. RPMI-1640 has 1.32mg/l folic acid, 0.005mg/l vitamin B12 and 1mg/l pyridoxine hydrochloride and in comparison, Ham’s F12 has 1mg/l folic acid, 1.36mg/l vitamin B12 and 0.062mg/l pyridoxine hydrochloride) (83).
NO is the main RNS in the body, which has a central role in signalling (57). Plasma and erythrocyte NO are also elevated in children with autism compared with controls (23–25,84,85). Additionally, there is a weak but significant association between two genetic polymorphisms of inducible nitric oxide synthase (iNOS) (rs 8068149 and rs 1060826) and failure to use non-verbal language to regulate social interactions in children with autism spectrum disorders (86). NO has been shown to deplete intracellular glutathione (87) increasing the sensitivity of cells towards the toxic effects of NO (88–90).
The median baseline MN frequency for both sets of LCLs was at the higher end of studies reporting paediatric MN frequency based on the CBMN-cyt method using cultured lymphocytes (91). Possible reasons for the difference include (i) unlike peripheral blood lymphocytes that are mainly T lymphocytes, the cells used in our study are B lymphocytes that were transformed using the Epstein–Barr virus; (ii) B lymphocytes have different sensitivity to genotoxins compared with T lymphocytes (92–95); (iii) the process of immortalisation using Epstein–Barr virus may increase chromosomal instability and thus increase the number of MNi (96,97); and, (iv) the level of chromosome damage in cell lines may vary depending on passage number and duration in culture (98).
Although the results of this in vitro study do not provide evidence of genotoxic susceptibility in LCLs from autistic children, they may not reflect the effect in vivo for the following reasons: (i) the supraphysiological concentrations of certain micronutrients in RPMI-1640 medium required for DNA metabolism (e.g. 2000nM folic acid) (99) may have corrected differences in antioxidant or DNA repair capacity due to nutrient deficiencies that may occur in vivo as a result of poor dietary intake or as a result of genetic defects in uptake and metabolism of micronutrients; (ii) although NO donor compounds are relatively easy to use in vitro, they may not provide a steady rate of NO release that might occur in vivo and the parent compounds and/or their decomposition products may contribute additional undefined non-physiological affects that may confound the outcomes of the experiment.
Conclusion
The findings of this study suggest that children with autism are more sensitive to necrosis caused by oxidative and nitrosative stress than their non-autistic siblings. A number of lines of evidence have shown oxidative and nitrosative stress to be increased in many children with autism. LCLs grown in tissue culture medium designed for optimal cellular growth may not be a sensitive enough model for studies investigating oxidative and nitrosative stress in children with autistic disorder, if subtle defects in antioxidant or DNA damage responses may only become evident under normal physiological conditions. The strengths of our model are the use of well-matched LCLs, linked to validated diagnostic data and the use of a comprehensive and well-validated DNA damage and cytotoxicity assay, i.e. the CBMN-cyt assay.
Further studies are needed to determine: (i) the effect of ROS/RNS on other biomarkers of DNA damage, including telomere length and integrity, (ii) the impact of genotype on the various DNA repair enzymes of genomic stability in autism, (iii) the optimal nutrient concentration for DNA damage prevention and (iv) the interactive effects of folate, glutathione and xenobiotics in DNA metabolism pathways. The relationship of these findings to neural cells and lymphocytes in vivo also needs to be determined.
Funding
This research was funded by Commonwealth Scientific and Industrial Research Organisation Animal Food and Health Science . The Autism Genetic Resource Exchange is a program of Autism Speaks and is supported, in part, by grant 1U24MH081810 from the National Institute of Mental Health to Clara M. Lajonchere (PI).
Acknowledgements
We gratefully acknowledge the technical assistance provided by Ms Carolyn Salisbury of the Commonwealth Scientific and Industrial Research Organisation as well as the resources provided by the Autism Genetic Resource Exchange (AGRE) Consortium and the participating AGRE families.
References
- 1. American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders: text revision (DSM-IV-R), Washington D.C. [Google Scholar]
- 2. Wing L., Gould J. (1979). Severe impairments of social interaction and associated abnormalities in children: epidemiology and classification. J. Autism Dev. Disord., 9, 11–29 [DOI] [PubMed] [Google Scholar]
- 3. Elsabbagh M., Divan G., Koh Y. J., et al. (2012). Global prevalence of autism and other pervasive developmental disorders. Autism Res., 5, 160–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wazana A., Bresnahan M., Kline J. (2007). The autism epidemic: fact or artifact? J. Am. Acad. Child Adolesc. Psychiatry, 46, 721–730 [DOI] [PubMed] [Google Scholar]
- 5. Folstein S. E., Rosen-Sheidley B. (2001). Genetics of autism: complex aetiology for a heterogeneous disorder. Nat. Rev. Genet., 2, 943–955 [DOI] [PubMed] [Google Scholar]
- 6. Geschwind D. H., Levitt P. (2007). Autism spectrum disorders: developmental disconnection syndromes. Curr. Opin. Neurobiol., 17, 103–111 [DOI] [PubMed] [Google Scholar]
- 7. Pinto D., Pagnamenta A. T., Klei L., et al. (2010). Functional impact of global rare copy number variation in autism spectrum disorders. Nature, 466, 368–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. State M. W., Šestan N. (2012). The emerging biology of autism spectrum disorders. Science, 337, 1301–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams E. L., Casanova M. F. (2011). Above genetics: lessons from cerebral development in autism. Transl. Neurosci., 2, 106–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shanen N. C. (2006). Epigenetics of autism spectrum disorders. Hum. Mol. Genet., 15, R138–R150 [DOI] [PubMed] [Google Scholar]
- 11. Badcock C. (2011). The imprinted brain: how genes set the balance between autism and psychosis. Epigenomics, 3, 345–359 [DOI] [PubMed] [Google Scholar]
- 12. Krause I., He X., Gershwin M., Shoenfeld Y. (2002). Immune factors in autism: a critical review. J. Autism Dev. Disord., 32, 337–345 [DOI] [PubMed] [Google Scholar]
- 13. Baron-Cohen S. (2004). Autism: research into causes and intervention. Pediatr. Rehabil., 7, 73–78 [DOI] [PubMed] [Google Scholar]
- 14. Pfaff D. W., Rapin I., Goldman S. (2011). Male predominance in autism: neuroendocrine influences on arousal and social anxiety. Autism Res., 4, 163–176 [DOI] [PubMed] [Google Scholar]
- 15. Grandjean P., Landrigan P. J. (2006). Developmental neurotoxicity of industrial chemicals. Lancet, 368, 2167–2178 [DOI] [PubMed] [Google Scholar]
- 16. Kern J. K., Jones A. M. (2006). Evidence of toxicity, oxidative stress, and neuronal insult in autism. J. Toxicol. Environ. Health. B. Crit. Rev., 9, 485–499 [DOI] [PubMed] [Google Scholar]
- 17. Slotkin T. A., Levin E. D., Seidler F. J. (2006). Comparative developmental neurotoxicity of organophosphate insecticides: effects on brain development are separable from systemic toxicity. Environ. Health Perspect., 114, 746–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horvath K., Perman J. A. (2002). Autistic disorder and gastrointestinal disease. Curr. Opin. Pediatr., 14, 583–587 [DOI] [PubMed] [Google Scholar]
- 19. White J. F. (2003). Intestinal pathophysiology in autism. Exp. Biol. Med. (Maywood)., 228, 639–649 [DOI] [PubMed] [Google Scholar]
- 20. Lee L. C., Newschaffer C. J., Lessler J. T., Lee B. K., Shah R., Zimmerman A. W. (2008). Variation in season of birth in singleton and multiple births concordant for autism spectrum disorders. Paediatr. Perinat. Epidemiol., 22, 172–179 [DOI] [PubMed] [Google Scholar]
- 21. Hultman C. M., Sandin S., Levine S. Z., Lichtenstein P., Reichenberg A. (2011). Advancing paternal age and risk of autism: new evidence from a population-based study and a meta-analysis of epidemiological studies. Mol. Psychiatry, 16, 1203–1212 [DOI] [PubMed] [Google Scholar]
- 22. Puleo C. M., Schmeider J., Kolevzon A., Soorya L., Silverman J. M. (2012). Advanced paternal age predicts simplex autism. Autism, 16, 367–380 [DOI] [PubMed] [Google Scholar]
- 23. Söğüt S., Zoroğlu S. S., Ozyurt H., et al. (2003). Changes in nitric oxide levels and antioxidant enzyme activities may have a role in the pathophysiological mechanisms involved in autism. Clin. Chim. Acta., 331, 111–117 [DOI] [PubMed] [Google Scholar]
- 24. Sweeten T. L., Posey D. J., Shankar S., McDougle C. J. (2004). High nitric oxide production in autistic disorder: a possible role for interferon-gamma. Biol. Psychiatry, 55, 434–437 [DOI] [PubMed] [Google Scholar]
- 25. Zoroglu S. S., Armutcu F., Ozen S., Gurel A., Sivasli E., Yetkin O., Meram I. (2004). Increased oxidative stress and altered activities of erythrocyte free radical scavenging enzymes in autism. Eur. Arch. Psychiatry Clin. Neurosci., 254, 143–147 [DOI] [PubMed] [Google Scholar]
- 26. Kern J. K., Grannemann B. D., Trivedi M. H., Adams J. B. (2006). Sulfhydryl-reactive metals in autism. J. Toxicol. Environ. Health. A, 70, 715–721 [DOI] [PubMed] [Google Scholar]
- 27. Al-Gadani Y., El-Ansary A., Attas O., Al-Ayadhi L. (2009). Metabolic biomarkers related to oxidative stress and antioxidant status in Saudi autistic children. Clin. Biochem., 42, 1032–1040 [DOI] [PubMed] [Google Scholar]
- 28. Chauhan A., Chauhan V. (2006). Oxidative stress in autism. Pathophysiology, 13, 171–181 [DOI] [PubMed] [Google Scholar]
- 29. James S. J., Cutler P., Melnyk S., Jernigan S., Janak L., Gaylor D. W., Neubrander J. A. (2004). Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am. J. Clin. Nutr., 80, 1611–1617 [DOI] [PubMed] [Google Scholar]
- 30. James S. J., Melnyk S., Jernigan S., et al. (2006). Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am. J. Med. Genet. B. Neuropsychiatr. Genet., 141B, 947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vergani L., Lanza C., Rivaro P., et al. (2011). Metals, metallothioneins and oxidative stress in blood of autistic children. Research in Autism Spectrum Disorders, 5, 286–293 [Google Scholar]
- 32. Mostafa G. A., El-Hadidi E. S., Hewedi D. H., Abdou M. M. (2010). Oxidative stress in Egyptian children with autism: relation to autoimmunity. J. Neuroimmunol., 219, 114–118 [DOI] [PubMed] [Google Scholar]
- 33. Dröge W. (2002). Free radicals in the physiological control of cell function. Physiol. Rev., 82, 47–95 [DOI] [PubMed] [Google Scholar]
- 34. Levine R. L., Stadtman E. R. (2001). Oxidative modification of proteins during aging. Exp. Gerontol., 36, 1495–1502 [DOI] [PubMed] [Google Scholar]
- 35. Davis K. J. (1987). Protein damage and degradation by oxygen radicals. I. general aspects. J. Biol. Chem., 262, 9895–9901 [PubMed] [Google Scholar]
- 36. Negre-Salvayre A., Coatrieux C., Ingueneau C., Salvayre R. (2008). Advanced lipid peroxidation end products in oxidative damage to proteins: potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol., 153, 6–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beckman K. B., Ames B. N. (1997). Oxidative decay of DNA. J. Biol. Chem., 272, 19633–19636 [DOI] [PubMed] [Google Scholar]
- 38. Maluf S. W., Erdtmann B. (2001). Genomic instability in Down syndrome and Fanconi anemia assessed by micronucleus analysis and single-cell gel electrophoresis. Cancer Genet. Cytogenet., 124, 71–75 [DOI] [PubMed] [Google Scholar]
- 39. Tomanin R., Sarto F., Mazzotti D., Giacomelli L., Raimondi F., Trevisan C. (1990). Louis-Bar syndrome: spontaneous and induced chromosomal aberrations in lymphocytes and micronuclei in lymphocytes, oral mucosa and hair root cells. Hum. Genet., 85, 31–38 [DOI] [PubMed] [Google Scholar]
- 40. Honma M., Tadokoro S., Sakamoto H., et al. (2002). Chromosomal instability in B-lymphoblasotoid cell lines from Werner and Bloom syndrome patients. Mutat. Res., 520, 15–24 [DOI] [PubMed] [Google Scholar]
- 41. Fenech M. (2007). Cytokinesis-block micronucleus cytome assay. Nat. Protoc., 2, 1084–1104 [DOI] [PubMed] [Google Scholar]
- 42. Fenech M., Crott J., Turner J., Brown S. (1999). Necrosis, apoptosis, cytostasis and DNA damage in human lymphocytes measured simultaneously within the cytokinesis-block micronucleus assay: description of the method and results for hydrogen peroxide. Mutagenesis, 14, 605–612 [DOI] [PubMed] [Google Scholar]
- 43. Wang C., Trudel L. J., Wogan G. N., Deen W. M. (2003). Thresholds of nitric oxide-mediated toxicity in human lymphoblastoid cells. Chem. Res. Toxicol., 16, 1004–1013 [DOI] [PubMed] [Google Scholar]
- 44. James S. J., Rose S., Melnyk S., Jernigan S., Blossom S., Pavliv O., Gaylor D. W. (2009). Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J., 23, 2374–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Umegaki K., Fenech M. (2000). Cytokinesis-block micronucleus assay in WIL2-NS cells: a sensitive system to detect chromosomal damage induced by reactive oxygen species and activated human neutrophils. Mutagenesis, 15, 261–269 [DOI] [PubMed] [Google Scholar]
- 46. Crott J. W., Mashiyama S. T., Ames B. N., Fenech M. (2001). The effect of folic acid deficiency and MTHFR C677T polymorphism on chromosome damage in human lymphocytes in vitro. Cancer Epidemiol. Biomarkers Prev., 10, 1089–1096 [PubMed] [Google Scholar]
- 47.Autism Genetic Resource Exchange. www.agre.org.au. www.agre.org.au (accessed April 25, 2013).
- 48. Nguyen A., Rauch T. A., Pfeifer G. P., Hu V. W. (2010). Global methylation profiling of lymphoblastoid cell lines reveals epigenetic contributions to autism spectrum disorders and a novel autism candidate gene, RORA, whose protein product is reduced in autistic brain. FASEB J., 24, 3036–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Friederich J. A., Butterworth J. F., 4th. (1995). Sodium nitroprusside: twenty years and counting. Anesth. Analg., 81, 152–162 [DOI] [PubMed] [Google Scholar]
- 50. Andreassi M. G., Picano E., Del Ry S., Botto N., Colombo M. G., Giannessi D., Lubrano V., Vassalle C., Biagini A. (2001). Chronic long-term nitrate therapy: possible cytogenetic effect in humans? Mutagenesis, 16, 517–521 [DOI] [PubMed] [Google Scholar]
- 51. OECD (2010). Section 4. OECD Guideline for the testing of chemicals. Test No 487: In Vitro Mammalian Cell Micronucleus Test. Vol. TG-487, OECD Publishing, e-book, pp. 1–23 [Google Scholar]
- 52. StataCorp (2009). Stata Statistical Software: Release 11. StataCorp LP, College Station, TX [Google Scholar]
- 53. Festjens N., Vanden Berghe T., Vandenabeele P. (2006). Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim. Biophys. Acta, 1757, 1371–1387 [DOI] [PubMed] [Google Scholar]
- 54. Edinger A. L., Thompson C. B. (2004). Death by design: apoptosis, necrosis and autophagy. Curr. Opin. Cell Biol., 16, 663–669 [DOI] [PubMed] [Google Scholar]
- 55. Golstein P., Kroemer G. (2007). Cell death by necrosis: towards a molecular definition. Trends Biochem. Sci., 32, 37–43 [DOI] [PubMed] [Google Scholar]
- 56. Golse B., Durosay D.-R. P., Puget K., Michelson A. M. (1978). Perturbation de deux enzymes: la superoxyde-dismutase 1 et la glutathion-peroxydase dans la psychose infantile de developpement (autisme infantile). (Alterations in two enzymes: superoxide dismutase and glutathione peroxidase in infantile autism). Revue Neurologique (Paris), 134, 699–705 [PubMed] [Google Scholar]
- 57. Hurd T. R., Murphy M. P. (2009). Biological systems relevant for redox signaling and control. In Jacob C., Winyard P. G. (eds), Redox Signaling and Regulation in Biology and Medicine. Wiley-VCH, Weinheim, Germany, pp. 13–43 [Google Scholar]
- 58. Yorbik O., Sayal A., Akay C., Akbiyik D. I., Sohmen T. (2002). Investigation of antioxidant enzymes in children with autistic disorder. Prostaglandins. Leukot. Essent. Fatty Acids, 67, 341–343 [DOI] [PubMed] [Google Scholar]
- 59. Paşca S. P., Nemeş B., Vlase L., Gagyi C. E., Dronca E., Miu A. C., Dronca M. (2006). High levels of homocysteine and low serum paraoxonase 1 arylesterase activity in children with autism. Life Sci., 78, 2244–2248 [DOI] [PubMed] [Google Scholar]
- 60. Jory J., McGinnis W. R. (2007). Red-cell trace minerals in children with autism. Am. J. Biochem. Biotechnol., 4, 101–104 [Google Scholar]
- 61. Latif A., Heinz P., Cook R. (2002). Iron deficiency in autism and Asperger syndrome. Autism, 6, 103–114 [DOI] [PubMed] [Google Scholar]
- 62. Chauhan A., Chauhan V., Brown W. T., Cohen I. (2004). Oxidative stress in autism: increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin–the antioxidant proteins. Life Sci., 75, 2539–2549 [DOI] [PubMed] [Google Scholar]
- 63. Essa M. M., Guillemin G. J., Waly M. I., Al-Sharbati M. M., Al-Farsi Y. M., Hakkim F. L., Al-Sharfaee M. S. (2012). Reduced levels of antioxidant proteins in children with autism in Oman. Int. J. Nutr., Pharmacol. Neurolog. Dis., 2, 53–56 [Google Scholar]
- 64. Jones D. P. (2006). Redefining oxidative stress. Antioxid. Redox Signal., 8, 1865–1879 [DOI] [PubMed] [Google Scholar]
- 65. Kemp M., Go Y. M., Jones D. P. (2008). Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic. Biol. Med., 44, 921–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schafer F. Q., Buettner G. R. (2001). Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med., 30, 1191–1212 [DOI] [PubMed] [Google Scholar]
- 67. James S. J., Melnyk S., Fuchs G., Reid T., Jernigan S., Pavliv O., Hubanks A., Gaylor D. W. (2009). Efficacy of methylcobalamin and folinic acid treatment on glutathione redox status in children with autism. Am. J. Clin. Nutr., 89, 425–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Coyle P., Philcox J. C., Carey L. C., Rofe A. M. (2002). Metallothionein: the multipurpose protein. Cell. Mol. Life Sci., 59, 627–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Singh V. K., Hanson J. (2006). Assessment of metallothionein and antibodies to metallothionein in normal and autistic children having exposure to vaccine-derived thimerosal. Pediatr. Allergy Immunol., 17, 291–296 [DOI] [PubMed] [Google Scholar]
- 70. Faber S., Zinn G. M., Kern J. C., Kingston H. M. S. (2009). The plasma zinc/serum copper ratio as a biomarker in children with autism spectrum disorders. Biomarkers, 14, 171–180 [DOI] [PubMed] [Google Scholar]
- 71. Russo A. J., Bazin A. P., Bigega R., et al. (2012). Plasma copper and zinc concentration in individuals with autism correlated with selected symptom severity. Nutrition and Metabolic Insights, 5, 41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yorbik O., Akay C., Sayal A., Cansever A., Sohmen T., Cavdar A. O. (2004). Zinc status in autistic children. J. Trace Elem. Exp. Med., 17, 101–107 [Google Scholar]
- 73. Walker S. J., Segal J., Aschner M. (2006). Cultured lymphocytes from autistic children and non-autistic siblings up-regulate heat shock protein RNA in response to thimerosal challenge. Neurotoxicology, 27, 685–692 [DOI] [PubMed] [Google Scholar]
- 74. Wang Y., Wimmer U., Lichtlen P., Inderbitzin D., Stieger B., Meier P. J., Schaffner W. (2004). Metal-responsive transcription factor-1 (MTF-1) is essential for embryonic liver development and heavy metal detoxification in the adult liver. FASEB J., 18, 1071–1079 [DOI] [PubMed] [Google Scholar]
- 75. Serajee F. J., Nabi R., Zhong H., Huq M. (2004). Polymorphisms in xenobiotic metabolism genes and autism. J. Child Neurol., 19, 413–417 [DOI] [PubMed] [Google Scholar]
- 76. Ming X., Stein T. P., Brimacombe M., Johnson W. G., Lambert G. H., Wagner G. C. (2005). Increased excretion of a lipid peroxidation biomarker in autism. Prostaglandins. Leukot. Essent. Fatty Acids, 73, 379–384 [DOI] [PubMed] [Google Scholar]
- 77. Thippeswamy T., McKay J. S., Quinn J. P., Morris R. (2006). Nitric oxide, a biological double-faced janus–is this good or bad? Histol. Histopathol., 21, 445–458 [DOI] [PubMed] [Google Scholar]
- 78. Newmeyer D. D., Farschon D. M., Reed J. C. (1994). Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell, 79, 353–364 [DOI] [PubMed] [Google Scholar]
- 79. Petit P. X., Lecoeur H., Zorn E., Dauguet C., Mignotte B., Gougeon M. L. (1995). Alterations in mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J. Cell Biol., 130, 157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zamzami N., Marchetti P., Castedo M., Zanin C., Vayssière J. L., Petit P. X., Kroemer G. (1995). Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J. Exp. Med., 181, 1661–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fenech M., Kirsch-Volders M., Natarajan A. T., et al. (2011). Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis, 26, 125–132 [DOI] [PubMed] [Google Scholar]
- 82. Lin W., Wei X., Xue H., Kelimu M., Tao R., Song Y., Zhou Z. (1998). Genotoxicity of nitric oxide produced from sodium nitroprusside. Mut. Res., 413, 121–127 [DOI] [PubMed] [Google Scholar]
- 83.Sigma Aldrich. www.sigmaaldrich.com/australia.html. www.sigmaaldrich.com/australia.html (accessed April 25, 2013).
- 84. Tostes M. H. F. S., Teixeira H. C., Gattaz W. F., Brandao M. A. F., Raposo N. R. B. (2012). Altered neurotropin, neuropeptide, cytokines and nitric oxide levels in autism. Pharmacopsychiatry, 45, 241–243 [DOI] [PubMed] [Google Scholar]
- 85. Essa M. M., Guillemin G. J., Waly M. I., Al-Sharbati M. M., Al-Farsi Y. M., Hakkim F. L., Ali A., Al-Shafaee M. S. (2012). Increased markers of oxidative stress in autistic children of the Sultanate of Oman. Biol. Trace Elem. Res., 147, 25–27 [DOI] [PubMed] [Google Scholar]
- 86. Kim H.-W., Cho S.-C., Kim J.-W., Cho I. H., Kim S. A., Park M., Cho E. J., Yoo H.-J. (2008). Family based association study between NOS-I and -IIA polymorphisms and autism spectrum disorders in Korean trios. Am J Med Genet Part B, 150B, 300–306 [DOI] [PubMed] [Google Scholar]
- 87. Berendji D., Kolb-Bachofen V., Meyer K. L., Kröncke K. D. (1999). Influence of nitric oxide on the intracellular reduced glutathione pool: different cellular capacities and strategies to encounter nitric oxide-mediated stress. Free Radic. Biol. Med., 27, 773–780 [DOI] [PubMed] [Google Scholar]
- 88. Walker M. W., Kinter M. T., Roberts R. J., Spitz D. R. (1994). Nitric oxide-induced cytotoxicity: involvement of cellular resistance to oxidative stress and the role of glutathione in protection. Pediatr. Res, 37, 41–49 [DOI] [PubMed] [Google Scholar]
- 89. Wink D. A., Nims R. W., Darbyshire J. F., et al. (1994). Reaction kinetics for nitrosation of cysteine and glutathione in aerobic nitric oxide solutions at neutral pH. Insights into the fate and physiological effects of intermediates generated in the NO/O2 reaction. Chem. Res. Toxicol., 7, 519–525 [DOI] [PubMed] [Google Scholar]
- 90. Luperchio S., Tamir S., Tannenbaum S. R. (1996). NO-induced oxidative stress and glutathione metabolism in rodent and human cells. Free Radic. Biol. Med., 21, 513–519 [DOI] [PubMed] [Google Scholar]
- 91. Neri M., Ceppi M., Knudsen L. E., Merlo D. F., Barale R., Puntoni R., Bonassi S. (2005). Baseline micronuclei frequency in children: estimates from meta- and pooled analyses. Environ. Health Perspect., 113, 1226–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wuttke K., Streffer C., Müller W. U. (1993). Radiation induced micronuclei in subpopulations of human lymphocytes. Mutat. Res., 286, 181–188 [DOI] [PubMed] [Google Scholar]
- 93. Holmén A., Karlsson A., Bratt I., Högstedt B. (1994). Micronuclei and mitotic index in B-, T4- and T8-cells treated with mitomycin C and gamma-irradiation. Mutat. Res., 309, 93–99 [DOI] [PubMed] [Google Scholar]
- 94. Högstedt B., Karlsson A., Bratt I., Holmén A. (1993). Micronucleus induction in human B and T lymphocytes separated by an immunomagnetic technique. Hereditas, 119, 99–103 [DOI] [PubMed] [Google Scholar]
- 95. Högstedt B., Bratt I., Holmén A., Hagmar L., Skerfving S. (1988). Frequency and size distribution of micronuclei in lymphocytes stimulated with phytohemagglutinin and pokeweed mitogen in workers exposed to piperazine. Hereditas, 109, 139–142 [DOI] [PubMed] [Google Scholar]
- 96. Gruhne B., Sompallae R., Marescotti D., Kamranvar S. A., Gastaldello S., Masucci M. G. (2009). The Epstein-Barr virus nuclear antigen-1 promotes genomic instability via induction of reactive oxygen species. Proc. Natl. Acad. Sci. U. S. A., 106, 2313–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wu C. C., Liu M. T., Chang Y. T., et al. (2010). Epstein-Barr virus DNase (BGLF5) induces genomic instability in human epithelial cells. Nucleic Acids Res., 38, 1932–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Oh J. H., Kim Y. J., Moon S., Nam H. Y., Jeon J. P., Lee J. H., Lee J. Y., Cho Y. S. (2013). Genotype instability during long-term subculture of lymphoblastoid cell lines. J. Hum. Genet., 58, 16–20 [DOI] [PubMed] [Google Scholar]
- 99. Fenech M. F. (2010). Nutriomes and nutrient arrays - the key to personalised nutrition for DNA damage prevention and cancer growth control. Genome Integr., 1, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]