Abstract
Background
Olfactory disorders are common complaints in ENT clinics. We investigated causes and relevant features of olfactory disorders and the need for gustatory testing in patients with olfactory dysfunction.
Material/Methods
A total of 140 patients seeking medical consultations were enrolled. All patients were asked about their olfactory disorders in a structured interview of medical history and underwent thorough otolaryngologic examinations and imaging of the head.
Results
Causes of olfactory disorders were classified as: upper respiratory tract infection (URTI), sinonasal diseases (NSD), head trauma, idiopathic, endoscopic sinus surgery, congenital anosmia, and other causes. Each of the various causes of olfactory dysfunction had its own distinct clinical features. Nineteen of 54 patients whose gustation was assessed had gustatory disorders.
Conclusions
The leading causes of olfactory dysfunction were URTI, NSD, head trauma, and idiopathic causes. Gustatory disorders were fairly common in patients with olfactory dysfunction. High priority should be given to complaints of olfactory disorders.
Keywords: olfactory dysfunction, diagnosis, etiology, chemosensory assessment
Background
Olfaction is an important sensory modality for human being and plays an important role in daily life. It helps to detect hazards in the surrounding environment and to taste food. When the sense of smell is impaired, it may influence nutritional intake and affects interpersonal relations [1,2]. Compared with visual loss and hearing loss, olfactory loss is generally overlooked by people due to lack of awareness. It is well documented that more than 200 diseases can contribute to olfactory dysfunction [3]. Olfactory dysfunction is present in 7% of the general population of the USA [4] and a prevalence up to 24.5% has been reported in elderly adults [5]. Although olfactory disorders are common [6,7], there has been no previous epidemiological investigation or relevant studies of olfactory dysfunction reported in China. Furthermore, some patients with olfactory dysfunction complain of gustatory disorders.
The aim of the present study was to investigate the causes and relevant features of olfactory disorders, as well as the need for gustatory testing in patients with olfactory dysfunction.
Material and Methods
Patients
The study group was composed of 140 consecutive patients (72 men and 68 women, aged 16 to 75 years) with olfactory disorders who presented to the outpatient clinic of the Department of Otolaryngology-Head and Neck Surgery, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China. All patients were asked about their olfactory disorders in a structured interview of medical history and underwent thorough otolaryngologic examinations, including nasal endoscopy and chemosensory assessment. Imaging of the head by use of computed tomography (CT) or magnetic resonance imaging (MRI) was performed according to history-taking and nasal endoscopy. In the structured interview, information was collected about the kind of olfactory disorders the patients experienced and the duration of the problem. In addition, the interview included information about rhinological symptoms, occupational exposure, systematic diseases, medical conditions, and smoking. Rhinological symptoms included nasal obstruction, rhinorrhea, epistaxis, sneezing, itching, and facial pain. According to history-taking and olfactory testing, the olfactory disorders were classified into 4 categories: hyposmia, anosmia, parosmia, and phantosmia. Three patients with sensitivity complaints had additional complaints of parosmia and/or phantosmia. Nasal endoscopy was performed with a 0-degree rigid endoscope (Karl Storz, Tuttlingen, Germany) to evaluate the condition of the nasal mucosa (inflammation, edema, polyps, and secretions) and anatomical findings (septal deviation and patency of the olfactory cleft). Rigid endoscopes 2.7 mm and 4mm in diameter were used. The study complied with the principles of the Declaration of Helsinki on Biomedical Research Involving Human Subjects and was approved by the Institutional Review Board of Beijing Chaoyang Hospital, Capital Medical University.
Chemosensory assessment
Olfactory testing
Olfactory function was assessed with the extended version of the Sniffin’ Sticks test (Burghart GmbH, Wedel, Germany), which was performed according to methods published previously [8,9]. It involved 3 subtests for odor thresholds, odor discrimination, and odor identification. Olfactory evaluation required approximately 30 minutes.
Odorants were presented in felt-tipped pens. For odor presentation, the pen cap was removed by the examiner for approximately 3 seconds and the pen tip was placed approximately 2 cm in front of the patients’ nostrils.
Odor thresholds for n-butanol was assessed using a single-staircase, triple-forced choice procedure. Sixteen dilutions were prepared in a geometric series starting from a 4% n-butanol (16 stages of 1:2 dilutions). Three pens were presented in a randomized order, with 2 containing the solvent and the other the odorant at a certain dilution. The patient had to determine the odor-containing pen. Reversal of the staircase was triggered when the odor was determined correctly in 2 successive trials. Odor thresholds were defined as the mean of the last 4 of 7 staircase reversal points.
In the odor discrimination task, 16 triplets of pens were presented. The 3 pens were presented in a randomized order, with 2 containing the same odorant and the other a different odorant. Using a triple-forced choice procedure, patients had to identify which of 3 odor-containing pens smelled different from the other 2. When measuring odor thresholds and discrimination, patients were blindfolded to prevent visual identification of the target pens.
Odor identification was assessed for 16 common odors. Using a multiple-forced choice procedure, patients were asked to indicate odors by selecting the best label from a list of 4 descriptors. Results of the 3 subtests were presented as a composite “TDI score” [10], which was derived from the sum of the results obtained for odor thresholds, discrimination, and identification measures. Hyposmia was indicated by a TDI score less than 31, with a TDI score below 16 considered as functional anosmia [11].
Gustatory testing
Gustatory function was assessed with “three-drop test”, which was performed according to methods published previously [12]. Sucrose, sodium chloride, citric acid, and quinine hydrochloride (J & K Chemical Reagent Company, Beijing, China) were prepared n solutions of different concentrations. For liquid presentation, liquid was placed onto the middle of the anterior third of patients’ extended tongues by the examiner. Three drops of liquid were placed in a randomized order, with 2 containing distilled water and the other a tastant at a certain concentration. After closing the mouth, the patients had to identify the taste as “sweet”, “sour”, “salty”, or “bitter”, and then rinsed their mouths with tap water before the next trial. If the subject chose a wrong answer, then a greater concentration of solution was applied. The sequence of presentation of the 4 tastants was randomized across patients tested. The score for each tastant ranged between 0 and 4 and the total score for the entire test ranged between 0 and 16. Hypogeusia was indicated by a score below 9.
Statistical analysis
Results were analyzed using SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL). Descriptive statistics are presented as means ±SD. T tests for independent samples were used for comparisons between groups. χ2 tests were used to calculate analyses of frequencies. The alpha level was set at 0.05.
Results and Analyses
Characteristics of patients with olfactory disorders
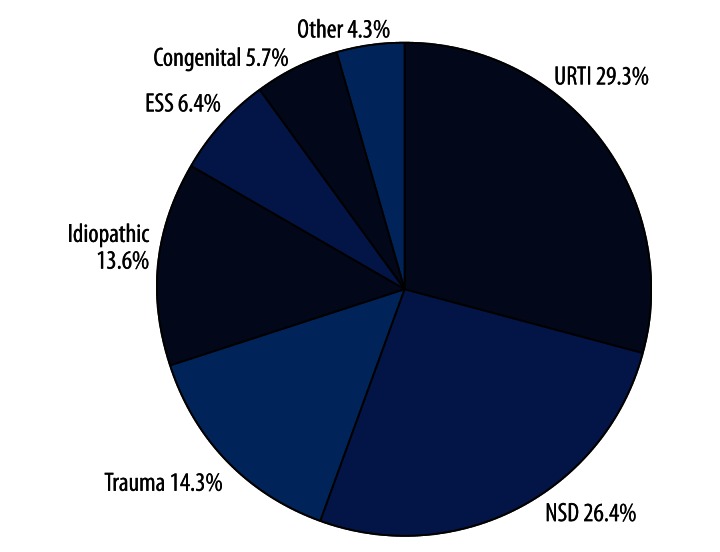
A total of 140 consecutive patients were included (72 men and 68 women). All of them had olfactory disorders as established by means of the Sniffin’ Sticks test. Forty-three were hyposmic and 97 were functionally anosmic. According to the chief complaints, 3 of 97 functionally anosmic patients were also diagnosed as parosmia and/or phantosmia. The proportion of different causes of the olfactory disorders is shown in Figure 1. Major causes of olfactory disorders were: upper respiratory tract infection (URTI) (n=41; 29.3%), sinonasal disease (SND) (n=37; 26.4%), head trauma (n=20; 14.3%), endoscopic sinus surgery (ESS) (n=9; 6.4%), congenital anosmia (n=8; 5.7%), and other causes including intoxication and brain surgery (n=6; 4.3%). In 19 patients (13.6%), it was impossible to clinically identify the relevant causes of olfactory disorders; in 14 of these patients aged 60 years or older, aging may be responsible for olfactory loss [13]; in SND patients, 30 had chronic rhinosinusitis (CRS) with/without nasal polyps, 4 had allergic rhinitis, and 3 had olfactory cleft disease.
Figure 1.
Proportion of patients with olfactory disorders with different causes.
Relations between olfactory disorders and causes
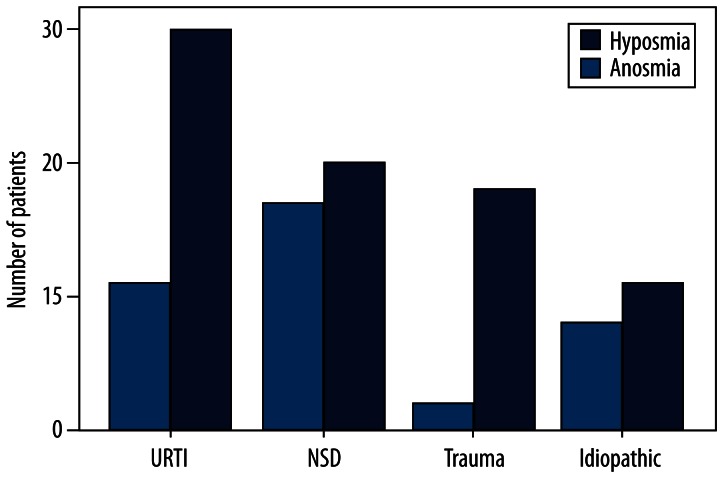
The prevalence of patients with hyposmia and anosmia as the major causes is shown in Figure 2. More patients were found to be anosmic than hyposmic across these causes. Statistical differences were found for SND (17 hyposmic vs. 20 anosmic; χ2=4.184, p=0.041) and head trauma (2 hyposmic vs. 18 anosmic; χ2=5.470, p=0.019). These differences were not statistically significant for URTI (11 hyposmic vs. 30 anosmic) and idiopathic causes (8 hyposmic vs. 11 anosmic).
Figure 2.
Prevalence of hyposmia and anosmia of major causes.
Relations between olfactory disorders and gender
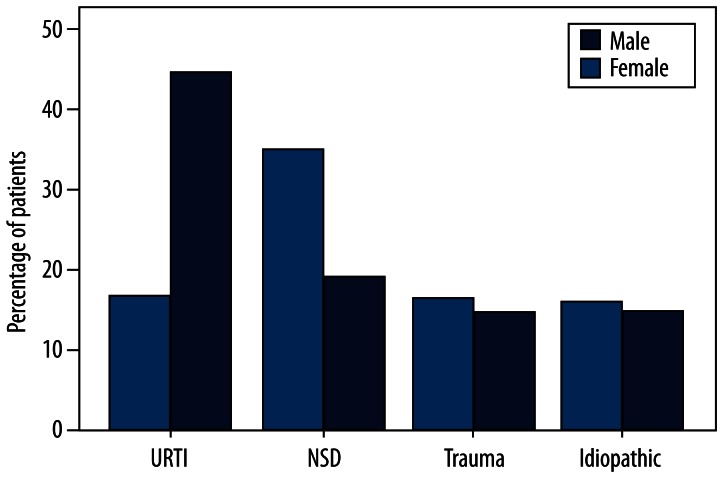
The proportion of the major causes in males and females is shown in Figure 3. Sex-related differences were statistically significant for URTI (16.7% male vs. 42.6% female; χ2=11.398, p=0.001) and SND (34.7% male vs. 17.6% female; χ2=5.244, p=0.041). These sex-related differences were not significant for head trauma (14.5% male vs. 15% female), idiopathic causes (15.3% male vs. 13.2% female). Sex-related difference was not statistically significant for patients with hyposmia and anosmia (males, 20 with hyposmia vs. 52 with anosmia; females, 23 with hyposmia vs. 45 with anosmia).
Figure 3.
Proportion of olfactory disorders with major causes in different genders.
Relations between olfactory disorders and age
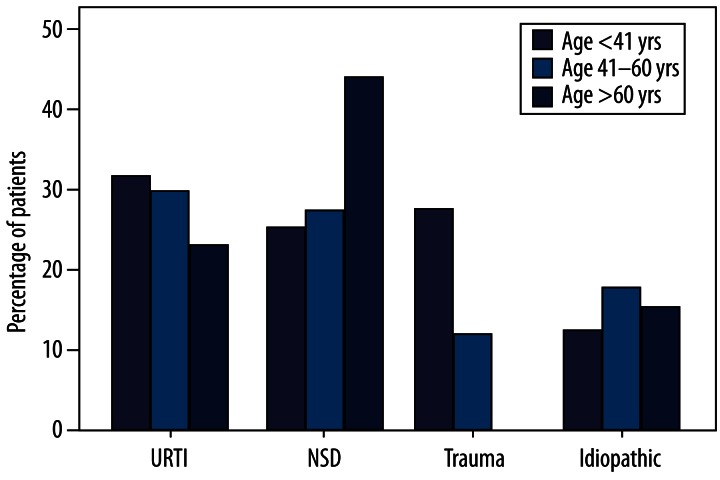
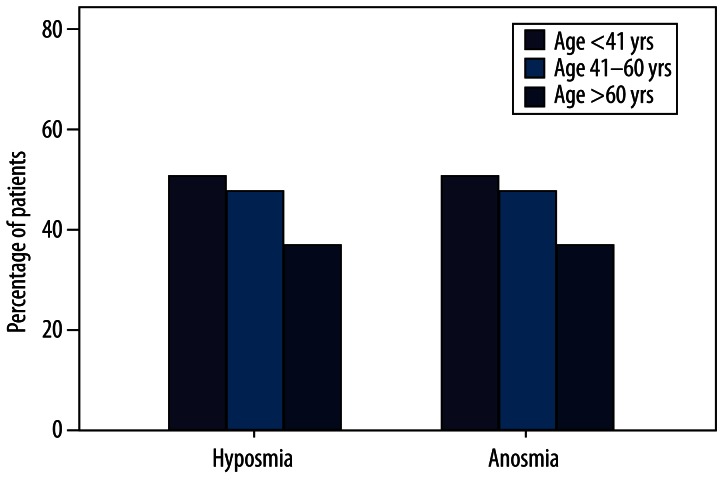
Patients were classified into 3 age groups: group A, younger than 41 years (n=51); group B, 41–60 years (n=75); and group C, older than 60 years (n=14). Different causes were present at different percentages in these 3 age groups. Percentages for the major causes are shown in Figure 4: URTI (group A, 31.4%; group B, 29.3%; group C, 21.4%); SND (group A, 23.5%; group B, 25.3%; group C, 42.9%); head trauma (group A, 25.5%; group B, 9.3%; group C, 0%); and idiopathic causes (group A, 9.8%; group B, 16.0%; group C, 14.3%). Statistical differences were significant for head trauma in group A (χ2=8.225, p=0.004). No such differences were found in patients with head trauma in groups B and C and other causes in different age groups. Percentages for patients with hyposmia and anosmia in different age groups are shown in Figure 5: hyposmia (group A, 41.2%; group B, 25.3%; group C, 21.4%) and anosmia (group A, 58.8%; group B, 74.7%; group C, 78.6%). Age-related difference was significant for patients with hyposmia (n=21) and anosmia (n=30) in group A (χ2=4.126, p=0.042). No such differences were found in the other 2 groups (group B, 19 hyposmic vs. 56 anosmic; group C, 3 hyposmic vs. 11 anosmic).
Figure 4.
Proportion of olfactory disorders with major causes in different age groups.
Figure 5.
Proportion of hyposmia and anosmia in different age groups.
Gustatory disorders in patients
Gustatory function was evaluated in 54 patients (27 males and 27 females) across various etiologies. Nineteen (8 males and 11 females, 35.2%) had gustatory disorders established by means of the 3-drop test and the other 35 had normal gustatory function. Patients’ gustatory disorders were due to various causes: URTI (n=7), NSD (n=4), head trauma (n=3), congenital anosmia (n=2), idiopathic (n=1), ESS (n=1), and other causes (n=1). Patients with gustatory disorders were from various age groups: group A (n=4), group B (n=14), and group C (n=1). Sex-related difference was not statistically significant for patients with gustatory disorders. Mean taste score for patients with hyposmia was 9.63±3.20 and 10.57±3.19 for those with anosmia. There was no statistically significant difference in taste scores between hyposmic and anosmic groups. Mean TDI score for patients with normogeusia was 5.59±9.05 and 6.67±9.20 for those with hypogeusia. The statistical difference in TDI scores was not significant between normogeusic and hypogeusic groups.
Discussion
The principal purpose of this study was to investigate causes and relevant features of olfactory disorders, and the need for gustatory testing in patients with olfactory dysfunction. Olfactory disorders are common complaints in otolaryngology. Clinically, olfaction can fail in 3 ways: decreased olfactory sensitivity (hyposmia and anosmia), distorted quality of an odorant stimulation (parosmia), and perceived odor when no odorant is present (phantosmia) [14]. The first is called quantitative olfactory dysfunction, and the latter 2 are qualitative olfactory dysfunctions. Our aim was to make quantitative and/or qualitative diagnosis of olfactory disorders and, if possible, identify their exact causes based on a structured interview of medical history, a thorough otolaryngologic examination, assessment of olfactory function, and CT or MRI performed according to history taking and nasal endoscopy. Because the existing olfactory test batteries – the Sniffin’ Sticks [15] used in the current study, the University of Pennsylvania Smell Identification Test [16], and the T & T Olfactometer [17] – all aim at quantifying olfactory dysfunction, testing aiming at qualitative olfactory dysfunction has been lacking. The diagnosis of parosmia and/or phantosimia was obtained from patients’ chief complaints in the structured interviews.
According to our results, complaints of quantitative olfactory dysfunction are more common than qualitative olfactory dysfunction, which is in accordance with findings of previous reports [1,18,19]. Despite our problem with identifying the exact etiology of olfactory dysfunction in 13.6% of patients seeking medical consultation, URTI was the most common cause for olfactory dysfunction among various causes, followed by NDS and head trauma. Thus, the present findings agree well with those of previous reports [20,21].
Severity-related data on olfactory dysfunction shows that the proportion of anosmic patients is higher than hyposmic patients. This finding is consistent with that of a previous report [20]. The relatively high proportion of anomic patients seeking medical consultation may partly be due to their lack of awareness of their gradual olfactory loss. This speculation is supported by cases of patients with congenital anosmia in the current study. They were not aware of their olfactory dysfunction until they were older than 10 years, and all of them have been sensing no smell for more than 30 years. Even so, some of them were still not willing to seek medical consultation until their family members urged them.
Among patients with URTI olfactory dysfunction, possible mechanisms of olfactory disorders encompass damage to peripheral olfactory neuroepithelium and degeneration of central olfactory pathways after viral invasion [22]. Female patients were more common than male [23,24]. This is likely due to the higher occurrence of the common cold in women [22], which provides more opportunities to develop olfactory dysfunction following viral infections.
In SND patients, CRS was the leading causative factor in olfactory dysfunction. As a common complaint, olfactory dysfunction affects approximately 65–80% of patients with CRS [25–27]. The underlying pathogeneses of CRS-induced olfactory dysfunction may be mechanical obstruction of the olfactory cleft and damage to olfactory neuroepithelium from chronic inflammation [28]. When compared with patients with other causes, hyposmia is more commonly seen among patients with SND. These data show that SND-induced olfactory loss diminishes gradually. In general, CRS with nasal polyps is often responsible for olfactory dysfunction and is particularly associated with anosmia [29], thus there is the possibility that the degree of olfactory dysfunction is associated with the severity of CRS [25]. When compared with female patients, more male patients had olfactory dysfunction. This could be the result of more male patients with CRS seeking medical help.
In posttraumatic patients, a number of possible mechanisms may contribute to olfactory dysfunction, including damage to the olfactory neuroepithelium or nasal cavities, injury to olfactory filaments, and contusions and hemorrhage lesions in the olfactory region of the brain [30]. The degree of olfactory dysfunction is associated with the severity of head trauma [31–33]. A higher proportion of posttraumatic patients were anosmic, which may be explained by the speculation that severe head trauma is responsible for serious damage to the central olfactory pathway. On the other hand, posttraumatic olfactory dysfunction mainly presented in patients under 41 years of age, which may be due to the higher incidence of head trauma in younger people.
A number of patients complained of olfactory loss following ESS. By means of nasal endoscopy, no mechanical obstruction of olfactory cleft was found in these patients. It has been reported that relevant factors accounting for the postoperative changes of olfactory function may contribute to changes in intranasal airflow [34]. Moreover, a number of studies indicate that certain intranasal volumes are significantly associated with olfactory function [35–37]. Thus, changes in intranasal airflow pattern may be responsible for olfactory loss in patients following ESS.
Olfactory dysfunction with idiopathic causes appeared in 19 patients (13.6%). This could be the result of degeneration of peripheral olfactory neuroepithelium and the central olfactory pathway; however, the exact causes cannot be identified clinically. Some causative factors, such as viral insults, nasal pathologies, and aging, were neglected by patients due to their lack of awareness. A relatively low proportion of elderly patients sought medical consultation. The low number of elderly patients can be explained by the following factors. Firstly, aging may contribute to olfactory loss [13]. Age-related olfactory dysfunction develops over time, so people may be unaware of the gradual change of smell. Secondly, elderly people may pay less attention to olfactory loss when compared with younger people. The relatively high proportion of hyposmia in younger patients in the present data supports this speculation.
In our study, 35.2% of patients whose gustatory function was evaluated had various degrees of gustatory disorders. However, there was no gustation-related difference between patients with hyposmia and those with anosmia [38]. Although olfactory function was investigated, no statistical difference was found between patients with dysgeusia and those with normogeusia. Thus, these data showed that gustation is not correlated with olfaction in patients with olfactory dysfunction, although gustatory dysfunction is fairly common among patients with olfactory disorders. The small sample size of patients whose taste function was tested may not contribute to this finding, since Yang et al. did not find the association between smell and taste function when a larger sample size was investigated [38]. When compared with healthy subjects, patients with olfactory dysfunction presented decreased gustatory function [38,39]. However, the underlying mechanisms on these clinical phenomena are still unknown. Given the relatively high proportion of gustatory impairments in patients with olfactory dysfunction, gustatory testing is still recommended in patients with olfactory disorders.
Although impacts of olfactory dysfunction on quality of life were not taken into account in the present study, olfactory dysfunction in the longer term could have negative psychosocial consequences for patients [2]. In the present study, 5 patients complained that olfactory disorders had seriously affected their daily lives. Three of them had qualitative and quantitative olfactory dysfunction concurrently and complained that parosmia and/or phantosmia, not simply olfactory loss, were responsible for the decline of their quality of life. These examples suggest that qualitative olfactory dysfunction is more distressing to patients’ quality of life than quantitative olfactory dysfunction [14]. Therefore, clinicians, especially otolaryngologists, should pay more attention to patients with olfactory complaints.
Further investigations are needed for a thorough understanding of how olfactory dysfunction affects quality of life in Chinese patients. In the near future we will use relevant questionnaires with patients seeking medical consultation.
Conclusions
Complaints of quantitative olfactory dysfunction were more common than complaints of qualitative olfactory dysfunction. URTI, NSD, head trauma, and idiopathic causes were the leading etiologies of olfactory disorders. Gustatory disorders were fairly common in patients with olfactory disorders. High priority should be given to complaints of olfactory disorders and to further investigations in this field.
Footnotes
Source of support: China Natural Scientific Foundation under project No. 30973284
Reference
- 1.Bramerson A, Nordin S, Bende M. Clinical experience with patients with olfactory complaints, and their quality of life. Acta Otolaryngol. 2007;127:167–74. doi: 10.1080/00016480600801357. [DOI] [PubMed] [Google Scholar]
- 2.Hummel T, Nordin S. Olfactory disorders and their consequences for quality of life. Acta Otolaryngol. 2005;125:116–21. doi: 10.1080/00016480410022787. [DOI] [PubMed] [Google Scholar]
- 3.Murphy C, Doty RL, Duncan HJ. Clinical Disorders of Olfaction. In: Doty RL, editor. Handbook of Olfaction and Gustation. New York: Marcel Dekker; 2003. pp. 461–78. [Google Scholar]
- 4.Wysocki CJ, Gilbert AN National Geographic Smell Survey. Effects of age are heterogenous. Ann N Y Acad Sci. 1989;561:12–28. doi: 10.1111/j.1749-6632.1989.tb20966.x. [DOI] [PubMed] [Google Scholar]
- 5.Murphy C, Schubert CR, Cruickshanks KJ, et al. Prevalence of olfactory impairment in older adults. JAMA. 2002;288:2307–12. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- 6.Wysocki CJ, Pelchat ML. The effects of aging on the human sense of smell and its relationship to food choice. Crit Rev Food Sci Nutr. 1993;33:63–82. doi: 10.1080/10408399309527613. [DOI] [PubMed] [Google Scholar]
- 7.Nordin S, Bramerson A, Bende M. Prevalence of self-reported poor odor detection sensitivity: the Skovde population-based study. Acta Otolaryngol. 2004;124:1171–73. doi: 10.1080/00016480410017468. [DOI] [PubMed] [Google Scholar]
- 8.Hummel T, Sekinger B, Wolf SR, et al. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22:39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- 9.Hummel T, Kobal G, Gudziol H, Mackay-Sim A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007;264:237–43. doi: 10.1007/s00405-006-0173-0. [DOI] [PubMed] [Google Scholar]
- 10.Wolfensberger M, Schnieper I, Welge-Lussen A. Sniffin’Sticks: a new olfactory test battery. Acta Otolaryngol. 2000;120:303–6. doi: 10.1080/000164800750001134. [DOI] [PubMed] [Google Scholar]
- 11.Kobal G, Klimek L, Wolfensberger M, et al. Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur Arch Otorhinolaryngol. 2000;257:205–11. doi: 10.1007/s004050050223. [DOI] [PubMed] [Google Scholar]
- 12.Mueller C, Kallert S, Renner B, et al. Quantitative assessment of gustatory function in a clinical context using impregnated “taste strips”. Rhinology. 2003;41:2–6. [PubMed] [Google Scholar]
- 13.Bramerson A, Johansson L, Ek L, et al. Prevalence of olfactory dysfunction: the skovde population-based study. Laryngoscope. 2004;114:733–37. doi: 10.1097/00005537-200404000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Leopold D. Distortion of olfactory perception: diagnosis and treatment. Chem Senses. 2002;27:611–15. doi: 10.1093/chemse/27.7.611. [DOI] [PubMed] [Google Scholar]
- 15.Kobal G, Hummel T, Sekinger B, et al. “Sniffin’ sticks”: screening of olfactory performance”. Rhinology. 1996;34:222–26. [PubMed] [Google Scholar]
- 16.Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94:176–78. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Zusho H. Olfactometry in Japan. Rhinology. 1983;21:281–85. [PubMed] [Google Scholar]
- 18.Landis BN, Konnerth CG, Hummel T. A study on the frequency of olfactory dysfunction. Laryngoscope. 2004;114:1764–69. doi: 10.1097/00005537-200410000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Nordin S, Murphy C, Davidson TM, et al. Prevalence and assessment of qualitative olfactory dysfunction in different age groups. Laryngoscope. 1996;106:739–44. doi: 10.1097/00005537-199606000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Temmel AF, Quint C, Schickinger-Fischer B, et al. Characteristics of olfactory disorders in relation to major causes of olfactory loss. Arch Otolaryngol Head Neck Surg. 2002;128:635–41. doi: 10.1001/archotol.128.6.635. [DOI] [PubMed] [Google Scholar]
- 21.Nordin S, Bramerson A. Complaints of olfactory disorders: epidemiology, assessment and clinical implications. Curr Opin Allergy Clin Immunol. 2008;8:10–15. doi: 10.1097/ACI.0b013e3282f3f473. [DOI] [PubMed] [Google Scholar]
- 22.Seiden AM. Postviral olfactory loss. Otolaryngol Clin North Am. 2004;37:1159–66. doi: 10.1016/j.otc.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Mott AE, Leopold DA. Disorders in taste and smell. Med Clin North Am. 1991;75:1321–53. doi: 10.1016/s0025-7125(16)30391-1. [DOI] [PubMed] [Google Scholar]
- 24.Sugiura M, Aiba T, Mori J, Nakai Y. An epidemiological study of postviral olfactory disorder. Acta Otolaryngol Suppl. 1998;538:191–96. doi: 10.1080/00016489850182918. [DOI] [PubMed] [Google Scholar]
- 25.Litvack JR, Mace JC, Smith TL. Olfactory function and disease severity in chronic rhinosinusitis. Am J Rhinol Allergy. 2009;23:139–44. doi: 10.2500/ajra.2009.23.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soler ZM, Mace J, Smith TL. Symptom-based presentation of chronic rhinosinusitis and symptom-specific outcomes after endoscopic sinus surgery. Am J Rhinol. 2008;22:297–301. doi: 10.2500/ajr.2008.22.3172. [DOI] [PubMed] [Google Scholar]
- 27.Jiang RS, Lu FJ, Liang KL, et al. Olfactory function in patients with chronic rhinosinusitis before and after functional endoscopic sinus surgery. Am J Rhinol. 2008;22:445–48. doi: 10.2500/ajr.2008.22.3195. [DOI] [PubMed] [Google Scholar]
- 28.Rudmik L, Smith TL. Olfactory improvement after endoscopic sinus surgery. Curr Opin Otolaryngol Head Neck Surg. 2012;20:29–32. doi: 10.1097/MOO.0b013e32834dfb3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raviv JR, Kern RC. Chronic rhinosinusitis and olfactory dysfunction. Adv Otorhinolaryngol. 2006;63:108–24. doi: 10.1159/000093757. [DOI] [PubMed] [Google Scholar]
- 30.Costanzo RM, Miwa T. Posttraumatic olfactory loss. Adv Otorhinolaryngol. 2006;63:99–107. doi: 10.1159/000093753. [DOI] [PubMed] [Google Scholar]
- 31.Doty RL, Yousem DM, Pham LT, et al. Olfactory dysfunction in patients with head trauma. Arch Neurol. 1997;54:1131–40. doi: 10.1001/archneur.1997.00550210061014. [DOI] [PubMed] [Google Scholar]
- 32.Green P, Iverson GL. Effects of injury severity and cognitive exaggeration on olfactory deficits in head injury compensation claims. NeuroRehabilitation. 2001;16:237–43. [PubMed] [Google Scholar]
- 33.Green P, Rohling ML, Iverson GL, Gervais RO. Relationships between olfactory discrimination and head injury severity. Brain Inj. 2003;17:479–96. doi: 10.1080/0269905031000070242. [DOI] [PubMed] [Google Scholar]
- 34.Pade J, Hummel T. Olfactory function following nasal surgery. Laryngoscope. 2008;118:1260–64. doi: 10.1097/MLG.0b013e318170b5cb. [DOI] [PubMed] [Google Scholar]
- 35.Hornung DE, Leopold DA. Relationship between uninasal anatomy and uninasal olfactory ability. Arch Otolaryngol Head Neck Surg. 1999;125:53–58. doi: 10.1001/archotol.125.1.53. [DOI] [PubMed] [Google Scholar]
- 36.Damm M, Vent J, Schmidt M, et al. Intranasal volume and olfactory function. Chem Senses. 2002;27:831–39. doi: 10.1093/chemse/27.9.831. [DOI] [PubMed] [Google Scholar]
- 37.Jun BC, Song SW, Kim BG, et al. A comparative analysis of intranasal volume and olfactory function using a three-dimensional reconstruction of paranasal sinus computed tomography, with a focus on the airway around the turbinates. Eur Arch Otorhinolaryngol. 2010;267:1389–95. doi: 10.1007/s00405-010-1217-z. [DOI] [PubMed] [Google Scholar]
- 38.Yang L, Wei Y, Zhang W, et al. Examination of chemosensory functions in patients with dysosmia. Med Sci Monit. 2012;18(3):CR154–59. doi: 10.12659/MSM.882520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landis BN, Scheibe M, Weber C, et al. Chemosensory interaction: acquired olfactory impairment is associated with decreased taste function. J Neurol. 2010;257:1303–8. doi: 10.1007/s00415-010-5513-8. [DOI] [PubMed] [Google Scholar]