Abstract
Maternal weight loss during exclusive breastfeeding may influence the growth of exclusively breast-fed infants through impaired quality or quantity of breast milk. This study evaluated how maternal weight loss from 2 to 24 wk postpartum was related to infant weight and length gain in 1309 lactating HIV-infected mothers and their exclusively breast-fed infants. Malawian mother-infant pairs in the Breastfeeding, Antiretrovirals, and Nutrition Study were randomized with a 2 × 3 factorial design to a 2-arm nutritional intervention with a lipid-based nutrient supplement (LNS), meeting nutritional needs of lactation, or no LNS and a 3-arm antiretroviral (ARV) intervention (maternal, infant, or no ARV regimen). Linear regression models were used to relate maternal weight loss (weight loss vs. no weight loss) to infant weight and length gain from birth to 24 mo, stratifying by gender and controlling for maternal BMI at 2 wk (mean ± SD: 23.2 ± 3.0 kg/m2) and interacting maternal BMI with weight loss. In adjusted models, compared with daughters of women who did not lose weight, length and weight gain were lower in daughters whose mothers had a lower BMI at 2 wk postpartum coupled with the weight loss. For example, among mothers with an initial BMI of 18 kg/m2, daughters of those who lost weight gained less weight [β = −0.29 kg (95% CI: −0.53, −0.06)] and length [β = −0.88 cm (95% CI: −1.52, −0.23)] from birth to 24 wk than daughters of those who gained weight. Though effects were only observed in girls, suggesting possible gender differences in suckling and feeding behavior, these findings indicate that maternal weight loss with low energy reserves represents a risk factor for poor infant growth outcomes.
Introduction
During exclusive breastfeeding, the mother is the sole source of nutrition for her infant. Breastfeeding can deplete the energy and nutrient reserves of women with inadequate dietary intake and very poor maternal nutrition can impair breast milk quantity and nutrient quality (1–4). Increases in maternal weight during lactation have previously been linked to improved quantity and nutrient quality of breast milk (4), yet it is not well understood how these weight changes translate into breast milk output and infant growth due to the complexity of this relationship (1).
Maternal breast milk supply is regulated to match infant demand through frequency and intensity of infant suckling (1, 5). In the absence of adequate dietary intake to meet the energy costs of lactation and owing to the hormonal influences of lactation, maternal fat stores may be mobilized to meet the demand, with implications for maternal weight changes (1). Increased milk intake will subsequently influence infant growth and may lead to further increases in infant demand and burden on maternal nutritional status, as a rapidly growing infant will likely demand more milk (1). Given adequate maternal nutrient stores and dietary intake, most women may be able to meet infant breast milk demand; however, in cases where mothers are undernourished and losing weight, breast milk energy output may be limited if the mother exceeds her lactational capacity (the ability to produce milk on demand) (6), which could adversely affect infant growth.
In resource-poor settings with high HIV prevalence, 6 mo of exclusive breastfeeding by HIV-infected women is recommended to promote child survival if replacement feedings are not acceptable, feasible, affordable, sustainable, and safe (7). Because HIV infection and lactation increase metabolic demands (8), lactating HIV-infected women may be unable to simultaneously meet their own and their infant’s nutritional needs.
Few studies have reported how the overall pattern of maternal weight changes during breastfeeding influence infant growth; however, there is evidence of associations between maternal anthropometry and subsequent infant growth. In Bolivian mother-infant pairs, maternal weight and height at 3 mo were positively correlated with infant weight gain between 3 and 6 mo (9). In Indian women, maternal fat mass loss from baseline to 6 mo was associated with higher infant weight gain (P = 0.04) at 6 mo (10). In Kenyan HIV-infected and uninfected mothers and their infants, higher maternal weight was associated with higher length-for-age, weight-for-age, and weight-for-length Z-scores, while taller maternal stature was associated with higher length-for-age and weight-for-age Z-scores (11).
Our objective was to examine how maternal weight loss from 2 to 24 wk relates to infant growth during exclusive breastfeeding in mother-infant pairs participating in the Breastfeeding, Antiretrovirals, and Nutrition (BAN)4 Study. Mothers in the BAN study who received the lipid-based nutrient supplement (LNS) experienced less weight loss during exclusive breastfeeding than mothers who did not receive LNS, regardless of antiretroviral drug (ARV) assignment (12). We assume infant growth is a proxy indicator of lactation adequacy (13). We hypothesized that the effects of maternal weight loss on infant weight and length gain will depend on initial maternal energy stores, with more detrimental effects in women with limited stores.
Methods
Study population.
The BAN Study design has been described in detail elsewhere (12, 14–16). Briefly, BAN was a randomized controlled trial of 2369 mother-infant pairs conducted from April 2004 to January 2010 in Lilongwe, Malawi. HIV-1–positive pregnant women (n = 3572) were recruited from 4 antenatal clinics. The initial screening criteria included age ≥14 y, CD4 count ≥250 cells/mm3, and no prior antiretroviral medication use, and the secondary eligibility criteria included infant birth weight ≥2 kg and no conditions that would preclude the use of the study drugs (14).
Mother-infant pairs were randomized using a permuted-block method to 1 of 6 treatment arms according to a 2-arm nutritional and 3-arm antiretroviral factorial design. One-half of the mothers received daily maternal LNS providing the estimated added energy and protein requirements of lactation as well as the RDA of micronutrients, excluding vitamin A (12). Mother-infant pairs were further randomized to a maternal or infant ARV or no antiretroviral regimen (14). Randomized infants diagnosed with HIV-1 within 2 wk of delivery (n = 119) were withdrawn from the study and referred for care (14). To buffer the effects of seasonal food shortages and prevent sharing of maternal LNS, all participants were given 2 kg/wk of maize for family consumption. During a drought from February to August 2005, a monthly ration of corn/soy flour, similar to the quantity in the maize supplement, and 1 L of vitamin A-fortified corn oil was provided by the World Food Program in lieu of the maize package to an estimated 260 mothers (17).
The Malawi National Health Science Research Committee and the institutional review boards at University of North Carolina at Chapel Hill and the U.S. CDC approved the BAN protocol. All women provided written informed consent.
Anthropometrics and study procedures.
Study visits at birth and 2, 4, 6, 8, 12, 18, 21, and 24 wk postpartum were conducted at the BAN Study clinic at Bwaila Hospital in Lilongwe. All mothers were provided intensive counseling to exclusively breastfeed their infants for 24 wk and to rapidly wean by 28 wk (18). Only data up to 24 wk were included in this analysis, corresponding with the period of exclusive breastfeeding. At all visits, one measurement of maternal and infant weight was obtained to the nearest 0.1 kg unit using Tanita digital electronic scales, which were calibrated regularly with a standard 1-kg weight. Maternal height was measured with a wall-mounted stadiometer at 2 wk postpartum. At all visits, one measurement of infant recumbent length was obtained using a wooden length board made to UNICEF specifications (19). Nurses (n = 25) and nutrition assistants (n = 5) were trained in anthropometric measurements using recommended methods and standardization procedures (19).
Maternal reports of parity, marital status, and years of maternal education were obtained at the screening visit. Maternal reports of maternal and infant morbidity occurring prior to the visit was also obtained. In addition, if the mother reported current illness, a physician report was obtained as well. Infant exclusive breastfeeding status was obtained by maternal report.
Malawi has a subtropical climate with 4 seasons: cool (May to mid-August), hot (mid-August to November), rainy (November to April), and post-rainy (April to May). Availability of food and levels of malnutrition and morbidity due to infectious disease vary by season due to rainfall and agricultural production (20). The season of poor food security usually occurs from November/December to March/April, when crop stores are depleted.
Statistical analysis.
Statistical analyses were conducted with STATA 12.1. An α of 0.05 was used for all statistical tests. An a priori P value for significant interactions was set at 0.10 (21). A small number (<1%) of implausible infant weights and lengths and maternal weights at 24 wk were replaced by values interpolated from prior and subsequent measurements.
Mother-infant pairs were included in the analysis sample if they had data at 0 and 24 wk for the infant and 2 and 24 wk for the mother. Multiple births (n = 49), infants diagnosed with HIV after 2 wk postpartum (n = 58), and infants who were mixed-fed or weaned prior to 24 wk (n = 248) were excluded. It was necessary to limit the analysis sample to infants who were exclusively breastfed, because our main aim was to understand the relationship between maternal and infant nutritional status when mothers were the sole source of infant nutrition.
Owing to normal physiologic changes in the immediate postpartum period and other factors that could potentially influence the maternal weight measurement at delivery (edema, timing of measurement relative to delivery), we analyzed the pattern of maternal weight change from 2 to 24 wk. For simplicity of interpretation and to test the theory proposed by Brown and Dewey (22), which postulates that weight loss will result in suboptimal milk energy output for women with low energy reserves but not for women of normal weight, we utilized a dichotomous maternal weight change variable (weight loss vs. no weight loss). Alternate specifications of maternal weight change, as continuous and categorical variables (tertiles and quartiles of maternal weight change), were examined as potential primary exposure variables. Compared with the categorical weight loss (weight loss vs. no weight loss), continuous maternal weight change and quantile categories (tertiles and quartiles) were observed to have a similar model fit and predictors.
To understand factors influencing maternal weight loss from 2 to 24 wk, the primary exposure variable, logistic regression was used to evaluate predictors of maternal weight loss, including season, parity, treatment arm, maternal education, and maternal CD4 count. A season variable was created based on month of birth, which captures maternal exposure to food insecurity during pregnancy as well as food availability to the mother following delivery. October was selected as the referent birth month, as this represents the time of year when mothers were not exposed to periods of food insecurity during the latter part of pregnancy, but were exposed during breastfeeding. Given no observed differences in the likelihood of maternal weight loss between the months of September and November (P > 0.1), these months were combined as the reference month for season. A Wald test was utilized to test whether the group of birth month variables was significantly different from the referent months September to November.
The dependent variables for the primary analysis were infant weight or length gain from birth to 24 wk. In a separate analysis for each growth outcome, linear regression models evaluated the influence of maternal weight loss, controlling for infant initial anthropometric measurement, and maternal BMI at 2 wk. The interaction of maternal BMI and maternal weight loss tested whether the effects of maternal weight loss varied by initial maternal energy reserves. Adjusted models also included birth month, parity, BAN treatment arm, maternal education, maternal CD4 count, and infant morbidity. Due to occasional missed visits, infant morbidity was modeled as the proportion of visits from 0 to 24 wk when the child had specific morbidities (fever, vomiting, or diarrhea). Infant growth models were gender stratified given the significant gender differences observed in infant weight and length gain and the significant 3-way interactions among maternal weight loss, maternal BMI, and gender in the adjusted infant growth models. We used the same method in linear and logistic regression models to examine the overall significant pattern of seasonality effects. A sensitivity analysis including HIV-infected infants in the primary analysis was conducted to determine whether inclusion of these infants influenced the results.
Results
The mean weight loss of mothers from 2 to 24 wk was 0.93 ± 3.4 kg (Table 1). From 2 to 24 wk, 63.3% of mothers lost weight, whereas 36.7% gained weight or had no weight changes during this period. The proportion of mothers receiving the LNS did not differ between the weight loss and no weight loss groups (P = 0.85). Mean weight loss was 2.9 ± 2.1 kg in women who lost weight (n = 829) and the mean weight gain was 2.5 ± 2.3 kg in women who gained weight (n = 469). Initial maternal BMI did not differ by infant gender, but mothers of boys lost marginally more weight from 2 to 24 wk than mothers of girls (−1.1 ± 3.3 vs. −0.76 ± 3.5 kg; P = 0.07).
TABLE 1.
Characteristics of 1309 BAN mother-infant pairs in the primary analysis of the effects of maternal weight loss on infant growth1
| Characteristic | Value |
| Mothers | |
| Age, y | 26.7 ± 5.1 |
| Height, cm | 156.9 ± 5.5 |
| Weight, kg | 56.2 ± 8.8 |
| BMI, kg/m2 | 22.8 ± 3.2 |
| Primiparous, % | 9.6 |
| Married, % | 92.3 |
| Education beyond primary school, % | 36.1 |
| Hemoglobin,2 g/L | 109 ± 12 |
| CD4+ count,2 cells/mm3 | 475 ± 196 |
| Low CD4+ count,2,3 % | 29.9 |
| Received ARV intervention, % | 35.8 |
| Received LNS intervention, % | 51.8 |
| Maternal weight change from 2 to 24 wk, kg | −0.93 ± 3.4 |
| Infants | |
| Male gender, % | 51.5 |
| Birth weight, kg | 3.04 ± 0.40 |
| Low birth weight,4 % | 6.0 |
| Birth length, cm | 48.3 ± 1.9 |
| Hemoglobin, g/L | 174 ± 20.1 |
| Received ARV intervention, % | 38.4 |
| Weight gain from birth to 24 wk, kg | 4.13 ± 0.82 |
| Length gain from birth to 24 wk, cm | 15.7 ± 2.2 |
Values are means ± SDs or percent. ARV, antiretroviral drug; BAN, Breastfeeding, Antiretrovirals, and Nutrition; LNS, lipid-based nutrient supplement.
Measured during pregnancy.
Low CD4+ is <350 cells/mm3.
Low birth weight is 2–2.5 kg, as infants had to weigh ≥2.0 kg to be eligible for the BAN Study.
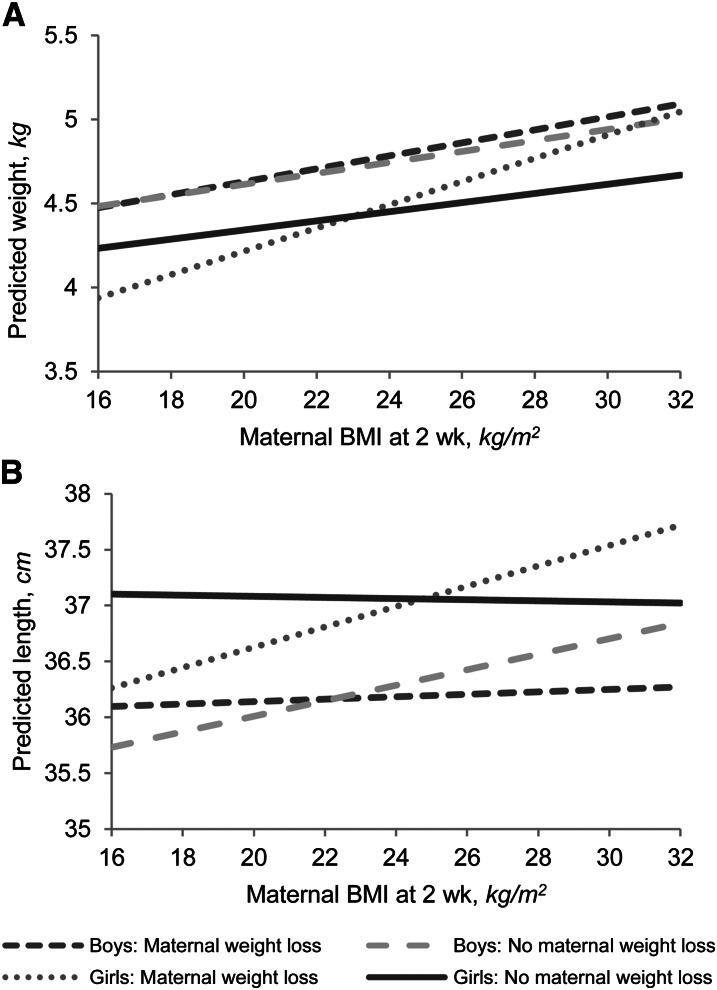
Maternal weight loss was associated with less length and weight gain in girls, particularly among mothers with lower BMI values at 2 wk, as evidenced by the significant interactions observed between weight loss and BMI for both weight gain and length gain (Table 2; Fig. 1). For example, based on confounder-adjusted model predictions among mothers with a BMI of 18 kg/m2, daughters of those who lose weight will gain an estimated 0.88 cm (P = 0.01) less length and 0.29 kg less weight (P = 0.01) than daughters of women who gain weight. Maternal weight loss from 2 to 24 wk did not affect weight or length gain in boys, even at varying BMI levels based on the nonsignificant interaction terms in adjusted and unadjusted models (Table 3; Fig. 1). In adjusted models, few factors significantly influenced infant weight and length gain from birth to 24 wk, consistent with previous findings in BAN infants (17). Higher birth length was associated with decreased 0–24 wk length gain in boys and girls. After adjustment for maternal factors, maternal ARV prophylaxis was associated with lower length gain in boys and girls, whereas infant ARV prophylaxis was associated with lower length gain only in girls. Maternal LNS was not associated with infant weight or length gain. Few seasonal effects on infant growth outcomes were observed compared with the referent months of September through November; July birth was associated with greater weight gain in boys and girls, while February was associated with increased weight gain, but only in girls. January births were associated with decreased length gain in boys, while August births were associated with increased length gain in girls. Sensitivity analyses showed that inclusion of HIV-infected infants in the analysis sample who had maternal and infant weight data and were exclusively breastfed at 24 wk (n = 28) did not influence the results (data not shown).
TABLE 2.
Linear regression models for girls showing the effects of maternal weight loss on infant weight and length gain from 0 to 24 wk in BAN mother-infant pairs included in the primary analysis of the effects of maternal weight loss on infant growth1
| Weight gain, kg |
Length gain, cm |
|||||||||||
| Crude, n = 633 |
Adjusted, n = 609 |
Crude, n = 632 |
Adjusted, n = 608 |
|||||||||
| Coefficient | 95% CI | P value | Coefficient | 95% CI | P value | Coefficient | 95% CI | P value | Coefficient | 95% CI | P value | |
| Birth weight or length | −0.26 | (−0.43, −0.10) | 0.002 | −0.30 | (−0.47, −0.13) | 0.001 | −0.46 | (−0.55, −0.37) | <0.001 | −0.46 | (−0.56, −0.36) | <0.001 |
| Maternal weight loss | −0.97 | (−1.86, −0.08) | 0.03 | −1.29 | (−2.20, −0.38) | 0.01 | −2.38 | (−4.83, 0.08) | 0.06 | −3.36 | (−5.88, −0.85) | 0.01 |
| Maternal BMI at 2 wk | 0.03 | (−0.001, 0.06) | 0.06 | 0.02 | (−0.01, 0.05) | 0.12 | −0.005 | (−0.08, 0.07) | 0.90 | −0.03 | (−0.11, 0.05) | 0.49 |
| Maternal weight loss × BMI | 0.04 | (0.004, 0.08) | 0.03 | 0.06 | (0.02, 0.09) | 0.01 | 0.1 | (−0.01, 0.20) | 0.07 | 0.14 | (0.03, 0.25) | 0.01 |
| CD4 cells < cells/mm3 | 0.03 | (−0.10, 0.16) | 0.70 | −0.19 | (−0.55, 0.17) | 0.31 | ||||||
| Fever | 0.01 | (−0.06, 0.07) | 0.84 | 0.11 | (−0.07, 0.28) | 0.23 | ||||||
| Vomiting | −0.0005 | (−0.13, 0.13) | 1.00 | −0.01 | (−0.38, 0.37) | 0.98 | ||||||
| Diarrhea | −0.03 | (−0.16, 0.10) | 0.63 | −0.003 | (−0.36, 0.35) | 0.99 | ||||||
| Primiparous | −0.13 | (−0.34, 0.07) | 0.20 | 0.21 | (−0.35, 0.76) | 0.46 | ||||||
| Birth month (ref: September, October, November) | ||||||||||||
| January | 0.04 | (−0.21, 0.28) | 0.78 | 0.04 | (−0.66, 0.73) | 0.92 | ||||||
| February | 0.32 | (0.05, 0.59) | 0.02 | 0.66 | (−0.09, 1.41) | 0.08 | ||||||
| March | −0.02 | (−0.28, 0.24) | 0.87 | −0.32 | (−1.05, 0.41) | 0.39 | ||||||
| April | 0.14 | (−0.11, 0.39) | 0.26 | −0.08 | (−0.78, 0.61) | 0.82 | ||||||
| May | 0.09 | (−0.14, 0.32) | 0.43 | 0.35 | (−0.28, 0.98) | 0.27 | ||||||
| June | 0.17 | (−0.06, 0.39) | 0.14 | 0.64 | (0.02, 1.26) | 0.04 | ||||||
| July | 0.42 | (0.17, 0.66) | 0.001 | 0.16 | (−0.52, 0.84) | 0.64 | ||||||
| August | 0.13 | (−0.09, 0.35) | 0.25 | 0.88 | (0.28, 1.49) | 0.004 | ||||||
| December | 0.23 | (−0.04, 0.49) | 0.09 | 0.21 | (−0.52, 0.94) | 0.57 | ||||||
| Married | −0.05 | (−0.29, 0.19) | 0.70 | 0.10 | (−0.56, 0.76) | 0.77 | ||||||
| Education | 0.05 | (−0.08, 0.18) | 0.42 | 0.16 | (−0.20, 0.52) | 0.38 | ||||||
| Maternal ARV intervention | −0.04 | (−0.20, 0.11) | 0.58 | −0.43 | (−0.87, 0.02) | 0.06 | ||||||
| Maternal LNS intervention | 0.08 | (−0.05, 0.20) | 0.22 | 0.21 | (−0.12, 0.55) | 0.21 | ||||||
| Infant ARV intervention | −0.11 | (−0.26, 0.05) | 0.18 | −0.58 | (−1.01, −0.15) | 0.01 | ||||||
| Intercept | 4.06 | (3.32, 4.80) | <0.001 | 4.22 | (3.42, 5.01) | <0.001 | 37.64 | (33.07, 42.21) | <0.001 | 38.03 | (33.21, 42.86) | <0.001 |
ARV, antiretroviral drug; BAN, Breastfeeding, Antiretrovirals, and Nutrition; LNS, lipid-based nutrient supplement.
FIGURE 1.
Predicted infant weight (A) and length (B) at 24 wk for varying maternal BMI levels in BAN mother-infant pairs included in the primary analysis of the effects of maternal weight loss on infant growth. Predicted curves from linear regression models containing infant initial anthropometric measurement, maternal weight loss from 2 to 24 wk, maternal BMI at 2 wk postpartum, and an interaction term between maternal weight loss and BMI. Weight gain: boys, n = 673; girls, n = 633. Length gain: boys, n = 667; girls, n = 632. BAN, Breastfeeding, Antiretrovirals, and Nutrition.
TABLE 3.
Linear regression models for boys showing the effects of maternal weight loss on infant weight and length gain from 0 to 24 wk in BAN mother-infant pairs included in the primary analysis of the effects of maternal weight loss on infant growth1
| Weight gain, kg |
Length gain, cm |
|||||||||||
| Crude, n = 673 |
Adjusted, n = 653 |
Crude, n = 667 |
Adjusted, n = 647 |
|||||||||
| Coefficient | 95% CI | P value | Coefficient | 95% CI | P value | Coefficient | 95% CI | P value | Coefficient | 95% CI | P value | |
| Birth weight or length | −0.20 | (−0.35, −0.04) | 0.01 | −0.15 | (−0.31, 0.01) | 0.06 | −0.42 | (−0.51, −0.34) | <0.001 | −0.43 | (−0.51, −0.35) | <0.001 |
| Maternal weight loss | −0.10 | (−1.12, 0.92) | 0.84 | −0.09 | (−1.14, 0.97) | 0.87 | 1.30 | (−1.30, 3.90) | 0.33 | 1.50 | (−1.15, 4.14) | 0.27 |
| Maternal BMI at 2 wk | 0.03 | (−0.003, 0.07) | 0.08 | 0.02 | (−0.02, 0.06) | 0.27 | 0.07 | (−0.02, 0.16) | 0.13 | 0.06 | (−0.03, 0.15) | 0.20 |
| Maternal weight loss × BMI | 0.01 | (−0.04, 0.05) | 0.79 | 0.01 | (−0.04, 0.05) | 0.82 | −0.06 | (−0.17, 0.05) | 0.30 | −0.07 | (−0.18, 0.05) | 0.26 |
| CD4 <350 cells/mm3 | 0.06 | (−0.08, 0.20) | 0.39 | 0.12 | (−0.24, 0.47) | 0.52 | ||||||
| Fever | −0.03 | (−0.10, 0.04) | 0.37 | 0.16 | (−0.004, 0.33) | 0.06 | ||||||
| Vomiting | −0.01 | (−0.15, 0.12) | 0.83 | −0.38 | (−0.72, −0.05) | 0.03 | ||||||
| Diarrhea | −0.05 | (−0.17, 0.06) | 0.38 | 0.15 | (−0.14, 0.45) | 0.31 | ||||||
| Primiparous | 0.01 | (−0.22, 0.24) | 0.91 | −0.25 | (−0.82, 0.32) | 0.39 | ||||||
| Birth month (ref: September, October, November) | ||||||||||||
| January | −0.12 | (−0.38, 0.14) | 0.36 | −0.76 | (−1.40, −0.11) | 0.02 | ||||||
| February | 0.05 | (−0.20, 0.30) | 0.71 | −0.22 | (−0.85, 0.41) | 0.49 | ||||||
| March | 0.22 | (−0.04, 0.48) | 0.10 | 0.17 | (−0.49, 0.82) | 0.61 | ||||||
| April | −0.03 | (−0.29, 0.23) | 0.80 | −0.21 | (−0.87, 0.45) | 0.54 | ||||||
| May | 0.01 | (−0.22, 0.25) | 0.92 | 0.17 | (−0.42, 0.77) | 0.57 | ||||||
| June | −0.11 | (−0.36, 0.14) | 0.40 | −0.26 | (−0.89, 0.37) | 0.42 | ||||||
| July | 0.30 | (0.06, 0.55) | 0.02 | 0.30 | (−0.33, 0.92) | 0.35 | ||||||
| August | 0.17 | (−0.09, 0.42) | 0.20 | 0.50 | (−0.15, 1.15) | 0.13 | ||||||
| December | 0.03 | (−0.22, 0.28) | 0.80 | −0.20 | (−0.82, 0.43) | 0.54 | ||||||
| Married | 0.17 | (−0.06, 0.39) | 0.15 | 0.12 | (−0.46, 0.69) | 0.69 | ||||||
| Education | 0.0005 | (−0.13, 0.13) | 0.99 | 0.31 | (−0.02, 0.64) | 0.07 | ||||||
| Maternal ARV intervention | −0.13 | (−0.29, 0.04) | 0.12 | −0.59 | (−1.00, −0.18) | 0.01 | ||||||
| Maternal LNS intervention | 0.07 | (−0.06, 0.19) | 0.29 | −0.03 | (−0.35, 0.29) | 0.88 | ||||||
| Infant ARV intervention | −0.07 | (−0.23, 0.09) | 0.42 | −0.25 | (−0.65, 0.15) | 0.22 | ||||||
| Intercept | 4.16 | (3.29, 5.02) | <0.001 | 4.16 | (3.23, 5.09) | <0.001 | 35.04 | (30.75, 39.34) | <0.001 | 35.48 | (31.04, 39.91) | <0.001 |
ARV, antiretroviral drug; BAN, Breastfeeding, Antiretrovirals, and Nutrition; LNS, lipid-based nutrient supplement.
Taller maternal height and the maternal ARV arm were associated with increased odds of maternal weight loss, whereas the LNS intervention, primiparity, low CD4 count, and maternal BMI at 2 wk were not associated with the likelihood of maternal weight loss (Table 4). Compared with September to November, all other birth months were associated with maternal weight loss [Wald test: χ2 (df: 9) = 81.3; P < 0.001].
TABLE 4.
Predictors of odds of maternal weight loss from 2 to 24 wk among BAN mothers included in the primary analysis of the effects of maternal weight loss on infant growth1
| OR | 95% CI | P value | |
| BMI at 2 wk postpartum | 1.02 | (0.98, 1.06) | 0.32 |
| Maternal height | 1.02 | (1.00, 1.05) | 0.04 |
| CD4 <350 cells/mm3 | 1.14 | (0.88, 1.49) | 0.33 |
| Primiparous | 1.01 | (0.66, 1.54) | 0.96 |
| Birth month (ref: September, October, November)2 | |||
| January | 0.35 | (0.22, 0.57) | <0.001 |
| February | 0.32 | (0.20, 0.53) | <0.001 |
| March | 0.19 | (0.12, 0.31) | <0.001 |
| April | 0.29 | (0.18, 0.46) | <0.001 |
| May | 0.35 | (0.22, 0.54) | <0.001 |
| June | 0.42 | (0.26, 0.66) | <0.001 |
| July | 1.05 | (0.62, 1.80) | 0.85 |
| August | 0.77 | (0.47, 1.26) | 0.30 |
| December | 0.62 | (0.37, 1.03) | 0.07 |
| Married | 1.49 | (0.95, 2.33) | 0.08 |
| Education | 1.22 | (0.94, 1.58) | 0.13 |
| Maternal ARV intervention | 1.74 | (1.34, 2.25) | <0.001 |
| Maternal LNS intervention | 0.98 | (0.77, 1.25) | 0.87 |
| Intercept | 0.03 | (0.0008, 1.12) | 0.06 |
Maternal weight loss from 2 to 24 wk compared with weight change ≥0. ARV, antiretroviral drug; BAN, Breastfeeding, Antiretrovirals, and Nutrition; LNS, lipid-based nutrient supplement.
There was a significant overall effect of birth months (December-August) compared with referent months based on a Wald test of whether the group of all birth month variables significantly differed from zero: Wald χ2 (9) 81.33; P < 0.001.
Discussion
To the authors’ knowledge, this is the first study to examine how maternal weight changes relate to infant weight and length gain in a population of HIV-infected women and their exclusively breast-fed, HIV-exposed, uninfected infants. We expected to observe adverse effects of low maternal BMI in both boys and girls; however, lower maternal BMI affected only the growth of girls, as the interaction between maternal BMI and weight loss was not significant in either of the boys’ growth models. Given our differential findings by gender, it appears that the milk production of thin mothers of girls could have been limited by substrates available for milk biosynthesis or their lactational capacity (1). Perhaps mothers of boys with lower BMIs were better able to mobilize existing nutrient stores or dietary intake into breast milk production than mothers of girls, yet we are unable to test this as we do not have the macronutrient content of breast milk. Gender preferences in feeding practices are unlikely to explain our findings, because there is little prior evidence of gender preferences in Malawi (23).
The observed gender-specific findings in maternal weight loss effects are in contrast to what was expected, because boys gain weight and length more rapidly than girls (24), which may enhance their susceptibility to environmental insults such as suboptimal breast milk quality and quantity. Boys are more vulnerable to undernutrition in utero, as they have faster growth rates and their placentas are thought to have less reserve capacity compared with girls (25). As a result of this fetal environment, boys may be programmed to be more adaptive to nutritional insults postpartum and may have an enhanced ability to demand more milk through suckling and feeding behavior despite suboptimal maternal milk output.
Prior studies in animals and humans have reported sex differences in milk content. In Kenyan mothers, more well-off mothers produced higher fat milk for sons compared with daughters; conversely, in poor mothers, the opposite association was observed, where poorer mothers produced richer milk for daughters than for sons (26). In Massachusetts mothers, mothers of sons produced higher energy milk than mothers of daughters (27). In rhesus macaques, mothers of sons, especially if primiparous, produced more energy-dense, high-fat milk, but produced less milk overall, compared with mothers of daughters (28). Though mothers produced similar milk energy amounts between boys and girls, it is unclear whether reduced quantity but higher energy content adversely affects growth (28). Because higher breast milk energy and protein consumption has been observed to positively influence infant growth (29), our gender-specific findings could possibly be attributable to sex differences in milk energy output.
Notably, few factors influenced infant weight and length gain in the adjusted models. Because BAN infants were exclusively breastfed, a requirement for inclusion in this analysis, this suggests that previously observed predictors of infant growth such as seasonality and parity are not as influential when the infant is exclusively breastfeeding. Exclusive breastfeeding appears to buffer the adverse effects of seasonality on infant weight and length gain. Infant breast milk intake was observed to decline during the rainy season in The Gambia, which is when mothers usually lose weight, and was initially attributed to maternal energy restriction (30). However, dietary supplementation of lactating women in a subsequent study in this population had no effects on maternal milk volume (30). Given these findings and additional studies reporting seasonal variations in breast milk intake in Kenya and the Democratic Republic of Congo, the authors concluded that the observed seasonal variations in breast milk output were attributed to increased infant morbidity and altered breastfeeding patterns due to strenuous maternal farm work (30). Because BAN infants were closely followed and treated for illnesses throughout the study, seasonal morbidity effects on breast milk intake could have been limited compared with other infants. Moreover, mothers were intensively counseled to exclusively breastfeed. As such, their infants were protected by the immunological components of milk and were not exposed to pathogens through contaminated foods and fluids. In adjusted models, most morbidities were not predictors of infant weight or length gain. In the length gain-adjusted models, vomiting was associated with diminished length gain only in the boys. Similar to previous findings in BAN infants (17), no consistent effects of the LNS were observed on infant growth, whereas adverse ARV effects were observed in both boys and girls.
Similar to other studies in HIV-infected (31) and non HIV-infected populations (32, 33), maternal weight change patterns were heterogeneous during exclusive breastfeeding, with some mothers losing and others gaining weight. Whereas taller stature was associated with maternal weight loss, the likelihood of weight loss was not related to initial BMI. Primiparity, low CD4 count, marital status, and education beyond primary school were not associated with maternal weight loss. Though maternal CD4 count has previously been associated with weight loss in a population of breastfeeding HIV-infected Zambian women (31), the inclusion requirements for BAN (CD4 >250 cells/mm3) may have reduced our ability to observe negative effects of low maternal CD4. The maternal ARV intervention was associated with weight loss from 2 to 24 wk. One of the drugs in the BAN regimen was lopinavir/ritonavir (Kaletra), which is frequently associated with diarrhea (34). As such, diarrhea may have contributed to the weight loss in this group. Maternal LNS had no association with maternal weight loss from 2 to 24 wk postpartum, which may reflect the concurrent provision of maize to mothers in both arms so that women were less likely to be in energy imbalance (12). Though the LNS was found to have a small protective effect on maternal weight loss from 0 to 28 wk in an intent-to-treat analysis of BAN mothers (n = 2369), the difference between treatment arms was reported to be of little clinical importance (12). Mothers who gave birth from January through June were protected from being in the maternal weight loss group. These women were exposed to food insecurity during pregnancy and in the postpartum period but were not as exposed to food insecurity for the duration of their postpartum weight changes compared with mothers of infants born between September and November. Given these seasonal patterns of effects on maternal weight change patterns, strategies to mitigate food shortages in Malawi should include policies to improve the food storage capacity during periods of food shortages as well as to enhance food production throughout the year. Though many factors were associated with the odds of maternal weight loss, few of these factors were associated with infant weight and length gain, which indicates that maternal breast milk output was sufficient to diminish the effects of these factors even though the mother was losing weight.
This research has some limitations. First, the outcome variables were change in weight/length from birth to 6 mo, and the primary exposure is maternal weight changes during a concurrent period; hence, this is a cross-sectional analysis and we are unable to discern the temporal relationship between maternal weight loss and infant growth outcomes. Moreover, we had available data only on maternal and infant weight and length and did not have detailed information on body composition, which would have allowed us to understand how changes in maternal fat stores related to infant growth and body composition. Effects of maternal weight change on infant growth are likely mediated through breast milk quantity and quality, which was not measured in this study. We excluded ∼45% of the randomized mother-infant pairs from our analysis who were lost to follow-up (n = 620), multiple births (n = 49), weaned early (n = 248), or had an HIV-infected infant in first 2 wk (n = 119) or from 2 to 24 wk (n = 58). Compared with randomized infants who were not included in the analysis sample (n = 1060), infants included in our analysis (n = 1309) had higher birth weight (P = 0.005), lower prevalence of low birth weight (P = 0.009), greater birth length (P = 0.002), and were more likely to receive the ARV intervention (P = 0.006); however, there were no differences between included and excluded infants in infant gender (P = 0.14) or hemoglobin at birth (P = 0.96). Because worse-off infants were lost-to-follow up or excluded, we may have underestimated the effects of maternal weight changes on infant growth.
Although low maternal BMI and exclusive breastfeeding are likely to occur in other populations following the WHO recommendations (35) as well as in other low-resource settings, we do not know if our findings are generalizable to other populations. We previously noted that BAN infants benefited from the intensive, exclusive breastfeeding counseling and provision of health care, which may have prevented more growth faltering (17). Moreover, mothers were provided with supplemental foods, which may have prevented further weight loss. As such, our findings may provide a conservative estimate of associations between maternal weight loss and infant growth outcomes, as maternal weight loss and poor infant growth would be more likely to occur in other populations.
This study also has many strengths. Applying a mother-infant dyad modeling framework is an innovative approach to examine these mother-infant anthropometry relationships. Supporting this analysis approach are the detailed exclusive breastfeeding data, which allowed us to exclude weaned and mixed-fed infants from our analyses, as the mother was no longer the sole source of nutrition for her growing infant. Moreover, our analysis focused on the period of the most rapid infant weight and length gain and highest maternal energy demands, enhancing our ability to observe the effects of maternal weight loss. Finally, understanding these relationships is especially important in the context of HIV to guide future interventions and programs to promote maternal and infant health.
In conclusion, our findings suggest that maternal weight loss coupled with low energy reserves may adversely affect infant growth. Further research to understand this relationship is indicated, especially to determine why the effects were seen only in girls.
Acknowledgments
The authors are grateful to the following: Yusuf Ahmed, Mounir Ait-Khaled, Sandra Albrecht, Shrikant Bangdiwala, Ronald Bayer, Margaret Bentley, Brian Bramson, Emily Bobrow, Nicola Boyle, Sal Butera, Charles Chasela, Charity Chavula, Joseph Chimerang’ambe, Maggie Chigwenembe, Maria Chikasema, Norah Chikhungu, David Chilongozi, Grace Chiudzu, Lenesi Chome, Anne Cole, Amanda Corbett, Amy Corneli, Anna Dow, Ann Duerr, Henry Eliya, Sascha Ellington, Joseph Eron, Sherry Farr, Yvonne Owens Ferguson, Susan Fiscus, Valerie Flax, Ali Fokar, Shannon Galvin, Laura Guay, Chad Heilig, Irving Hoffman, Elizabeth Hooten, Mina Hosseinipour, Michael Hudgens, Stacy Hurst, Lisa Hyde, Denise Jamieson, George Joaki (deceased), David Jones, Elizabeth Jordan-Bell, Zebrone Kacheche, Esmie Kamanga, Gift Kamanga, Coxcilly Kampani, Portia Kamthunzi, Deborah Kamwendo, Cecilia Kanyama, Angela Kashuba, Damson Kathyola, Dumbani Kayira, Peter Kazembe, Caroline C. King, Rodney Knight, Athena P. Kourtis, Robert Krysiak, Jacob Kumwenda, Hana Lee, Edde Loeliger, Dustin Long, Misheck Luhanga, Victor Madhlopa, Maganizo Majawa, Alice Maida, Cheryl Marcus, Francis Martinson, Navdeep Thoofer, Chrissie Matiki (deceased), Douglas Mayers, Isabel Mayuni, Marita McDonough, Joyce Meme, Ceppie Merry, Khama Mita, Chimwemwe Mkomawanthu, Gertrude Mndala, Ibrahim Mndala, Agnes Moses, Albans Msika, Wezi Msungama, Beatrice Mtimuni, Jane Muita, Noel Mumba, Bonface Musis, Charles Mwansambo, Gerald Mwapasa, Jacqueline Nkhoma, Megan Parker, Richard Pendame, Ellen Piwoz, Byron Raines, Zane Ramdas, John Rublein, Mairin Ryan, Ian Sanne, Christopher Sellers, Diane Shugars, Dorothy Sichali, Wendy Snowden, Alice Soko, Allison Spensley, Jean-Marc Steens, Gerald Tegha, Martin Tembo, Roshan Thomas, Hsiao-Chuan Tien, Beth Tohill, Charles van der Horst, Esther Waalberg, Elizabeth Widen, Jeffrey Wiener, Cathy Wilfert, Patricia Wiyo, Innocent Zgambo, and Chifundo Zimba. M.E.B., D.K., C.S.C., D.J.J., M.T., A.S., A.P.K., S.R.E., C.M.v.d.H., and L.S.A. designed and conducted the study; E.M.W. performed data analysis and interpretation; L.S.A. and V.L.F. contributed to interpretation of data analysis; and E.M.W. wrote the paper and has primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ARV, antiretroviral drug; BAN, Breastfeeding, Antiretrovirals, and Nutrition; LNS, lipid-based nutrient supplement.
Literature Cited
- 1.Rasmussen KM. The influence of maternal nutrition on lactation. Annu Rev Nutr. 1992;12:103–17. [DOI] [PubMed] [Google Scholar]
- 2.Winkvist A, Rasmussen KM. Impact of lactation on maternal body weight and body composition. J Mammary Gland Biol Neoplasia. 1999;4:309–18. [DOI] [PubMed] [Google Scholar]
- 3.Rogers IS, Golding J, Emmett PM. The effects of lactation on the mother. Early Hum Dev. 1997;49 Suppl:S191–203. [DOI] [PubMed] [Google Scholar]
- 4.Brown KH, Akhtar NA, Robertson AD, Ahmed MG. Lactational capacity of marginally nourished mothers: relationships between maternal nutritional status and quantity and proximate composition of milk. Pediatrics. 1986;78:909–19. [PubMed] [Google Scholar]
- 5.Daly SE, Hartmann PE. Infant demand and milk supply. Part 1: infant demand and milk production in lactating women. J Hum Lact. 1995;11:21–6. [DOI] [PubMed] [Google Scholar]
- 6.Dewey KG. Effects of maternal caloric restriction and exercise during lactation. J Nutr. 1998;128:S386–9. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Guidelines on HIV and infant feeding: principles and recommendations for infant feeding in the context of HIV and a summary of evidence. Geneva: WHO Press; 2010. [PubMed] [Google Scholar]
- 8.WHO. Nutrient requirements for people living with HIV/AIDS: report of a technical consultation. Geneva: WHO Press; 2003. [Google Scholar]
- 9.Novotny R, Haas JD. Maternal anthropometry and infant growth with exclusive breast feeding in La Paz, Bolivia. J Trop Pediatr. 1987;33:309–14. [DOI] [PubMed] [Google Scholar]
- 10.Kulkarni B, Shatrugna V, Nagalla B, Rani KU. Regional body composition changes during lactation in Indian women from the low-income group and their relationship to the growth of their infants. J Am Coll Nutr. 2011;30:57–62. [DOI] [PubMed] [Google Scholar]
- 11.Gewa CA, Oguttu M, Yandell NS. Maternal nutrition in rural Kenya: health and socio-demographic determinants and its association with child nutrition. Matern Child Nutr. 2012;8:275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kayira D, Bentley ME, Wiener J, Mkhomawanthu C, King CC, Chitsulo P, Chigwenembe M, Ellington S, Hosseinipour MC, Kourtis AP, et al. A lipid-based nutrient supplement mitigates weight loss among HIV-infected women in a factorial randomized trial to prevent mother-to-child transmission during exclusive breastfeeding. Am J Clin Nutr. 2012;95:759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alam DS, Van Raaij JM, Hautvast JG, Yunus M, Fuchs GJ. Energy stress during pregnancy and lactation: consequences for maternal nutrition in rural Bangladesh. Eur J Clin Nutr. 2003;57:151–6. [DOI] [PubMed] [Google Scholar]
- 14.Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, Kourtis AP, Martinson F, Tegha G, Knight RJ, Ahmed YI, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Horst C, Chasela C, Ahmed Y, Hoffman I, Hosseinipour M, Knight R, Fiscus S, Hudgens M, Kazembe P, Bentley M, et al. Modifications of a large HIV prevention clinical trial to fit changing realities: a case study of the Breastfeeding, Antiretroviral, and Nutrition (BAN) protocol in Lilongwe, Malawi. Contemp Clin Trials. 2009;30:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamieson DJ, Chasela CS, Hudgens MG, King CC, Kourtis AP, Kayira D, Hosseinipour MC, Kamwendo DD, Ellington SR, Wiener JB, et al. Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48-week follow-up of the BAN randomised controlled trial. Lancet. 2012;379:2449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flax VL, Bentley ME, Chasela CS, Kayira D, Hudgens MG, Knight RJ, Soko A, Jamieson DJ, van der Horst CM, Adair LS. Use of lipid-based nutrient supplements by HIV-infected Malawian women during lactation has no effect on infant growth from 0 to 24 weeks. J Nutr. 2012;142:1350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferguson YO, Eng E, Bentley M, Sandelowski M, Steckler A, Randall-David E, Piwoz EG, Zulu C, Chasela C, Soko A, et al. Evaluating nurses’ implementation of an infant-feeding counseling protocol for HIV-infected mothers: The BAN Study in Lilongwe, Malawi. AIDS Educ Prev. 2009;21:141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cogill B. Anthropometric indicators measurement guide. Washington, DC: Food and Nutrition Technical Assistance Project, Academy for Educational Development; 2003. [Google Scholar]
- 20.Hartikainen H, Maleta K, Kulmala T, Ashorn P. Seasonality of gestational weight gain and foetal growth in rural Malawi. East Afr Med J. 2005;82:294–9. [DOI] [PubMed] [Google Scholar]
- 21.Marshall SW. Power for tests of interaction: effect of raising the Type I error rate. Epidemiol Perspect Innov. 2007;4:4. [DOI] [PMC free article] [PubMed]
- 22.Brown KH, Dewey KG. Relationships between maternal nutritional status and milk energy outuput of women in developing countries. In: Picciano MF, Lonnerdal B, editors. Mechanisms regulating lactation and infant nutrient utilization. New York: Wiley-Liss; 1992. p. 77–99. [Google Scholar]
- 23.Parker ME, Tembo M, Adair L, Chasela C, Piwoz EG, Jamieson DJ, Ellington S, Kayira D, Soko A, Mkhomawanthu C, et al. The health of \HIV-exposed children after early weaning. Matern Child Nutr. 2013;9:217–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson SE, Rogers RR, Ziegler EE, Fomon SJ. Gain in weight and length during early infancy. Early Hum Dev. 1989;19:223–39. [DOI] [PubMed] [Google Scholar]
- 25.Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. Am J Hum Biol. 2010;22:330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita M, Roth E, Lo YJ, Hurst C, Vollner J, Kendell A. In poor families, mothers’ milk is richer for daughters than sons: a test of Trivers-Willard hypothesis in agropastoral settlements in Northern Kenya. Am J Phys Anthropol. 2012;149:52–9. [DOI] [PubMed] [Google Scholar]
- 27.Powe CE, Knott CD, Conklin-Brittain N. Infant sex predicts breast milk energy content. Am J Hum Biol. 2010;22:50–4. [DOI] [PubMed] [Google Scholar]
- 28.Hinde K. Richer milk for sons but more milk for daughters: sex-biased investment during lactation varies with maternal life history in rhesus macaques. Am J Hum Biol. 2009;21:512–9. [DOI] [PubMed] [Google Scholar]
- 29.Brown KH, Robertson AD, Akhtar NA. Lactational capacity of marginally nourished mothers: infants’ milk nutrient consumption and patterns of growth. Pediatrics. 1986;78:920–7. [PubMed] [Google Scholar]
- 30.Prentice A, Paul A, Prentice A, Black A, Cole T, Whitehead R. Cross-cultural differences in lactation performance. In: Hamosh M GAS, editor. Human lactation 2: maternal and environmental factors. New York: Plenum Press; 1986. p. 13–44.
- 31.Murnane PM, Arpadi SM, Sinkala M, Kankasa C, Mwiya M, Kasonde P, Thea DM, Aldrovandi GM, Kuhn L. Lactation-associated postpartum weight changes among HIV-infected women in Zambia. Int J Epidemiol. 2010;39:1299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butte NF, Hopkinson JM. Body composition changes during lactation are highly variable among women. J Nutr. 1998;128:S381–5. [DOI] [PubMed] [Google Scholar]
- 33.Adair LS, Popkin BM. Prolonged lactation contributes to depletion of maternal energy reserves in Filipino women. J Nutr. 1992;122:1643–55. [DOI] [PubMed] [Google Scholar]
- 34.Wegzyn CM, Fredrick LM, Stubbs RO, Woodward WC, Norton M. Diarrhea associated with lopinavir/ritonavir-based therapy: results of a meta-analysis of 1469 HIV-1-infected participants. J Int Assoc Physicians AIDS Care (Chic). 2012;11:252–9. [DOI] [PubMed] [Google Scholar]
- 35.WHO. HIV and infant feeding: revised principles and recommendations: rapid advice. Geneva: WHO; 2009. [Google Scholar]