Abstract
Minimal acceptable global intelligence is often a determinant for entry into studies utilizing children’s self-reported health-related quality of life (HRQL) or symptoms’ appraisal. However, most measures of cognitive functioning are lengthy and require a trained psychologist for administration. We used the Peabody Picture Vocabulary Test (third edition; PPVT-III) to assess adequacy of verbal comprehension and language flexibility before entry into a pilot pharmacologic intervention trial in pediatric BT survivors who were >1 year from treatment, and received >23.4 gray as part of therapy. Participation included the ability to complete self-reported measures of HRQL. Among thirteen BT survivors who were screened, twelve proceeded to the full intervention trial and then underwent a detailed baseline neurocognitive assessment including the Wechsler Abbreviated Scale of Intelligence (WASI), administered by a neuropsychologist. Correlation of PPVT-III with WASI was 0.90 for full scale IQ (P < 0.0001), 0.89 for verbal IQ (P = 0.0001) and 0.75 for performance IQ (P = 0.0004) The PPVT-III is easy to administer by trained clinical staff and is a reliable clinic-based screening tool for research studies. While it is not designed to replace in depth neuropsychological evaluation of potential areas of cognitive dysfunction, it provides an estimation of minimal global cognitive functioning for entry into studies that rely on self-report in childhood BT survivors and other cancer survivors who have received central nervous system-directed therapy.
Keywords: Childhood brain tumor survivor, Intelligence, Screening, PPVT-III
Introduction
With the recognition of the long-term impact of cancer related cognitive dysfunction in the child, urgent efforts to introduce preventive and remediative interventions have emerged [1–3]. A recent meta-analysis of 39 studies in brain tumor survivors reported deficits with effect sizes (compared to normative data) ranging from −0.45 for reading achievement to −1.43 for psychomotor skill [4]. The most commonly detected losses are in the areas of vigilant attention, processing speed, visual-spatial skills, and working memory, across-domain achievement, verbal and non-verbal learning and recall, and language [5]. These findings, and the urgent need for a better understanding of the moderating variables involved in treatment and long-term patient care, have resulted in recommendations for screening and monitoring of all BT survivors for potential deficits in these areas. Recommendations for preventive and remedial therapy, school and legal advocacy-related support, and planning for school and vocational transitioning have also emerged [6]. However, measurement of all vulnerable domains can be lengthy, and recent efforts have focused on gaining the maximum amount of information in the minimum amount of time [7, 8].
To date the evaluation of behavioral or pharmacologic interventions has relied on measurement with extensive neuropsychologic batteries. These batteries assess the overall intelligence and more carefully characterize domains that contribute to cognitive functioning. The most commonly used tests of IQ in children are the Wechsler Intelligence Scale for Children, 4th Edition which takes around 90 min to administer and the Stanford Binet Intelligence Scales, 5th Edition taking about 60 min [9, 10]. These instruments are considered the “gold standard” for measuring intelligence in clinical and educational settings. A shorter test, often used by researchers, is the Wechsler Abbreviated Scale of Intelligence (WASI), taking approximately 30 min to give all four subtests in a developmentally typical population.[11] All three intelligence tests are highly reliable with average internal consistency composite reliability as high as 98% for Verbal, Nonverbal and Full Scale IQ scores. However, as instruments of inclusion, all these are costly in vulnerable childhood populations in terms of administration time, the high degree of examiner training and practice required, and the necessity for access to a trained psychologist for interpretation. Added to these drawbacks is that children whose cognition has been compromised for health reasons take longer than is typical to complete standardized tests.
Recently, screening measures of intelligence have been used to determine the capacity of a child rater to provide self-report outcomes (PROs) of functioning and well-being. These screeners, which are compiled from several subtests from larger batteries, have been used principally to assess verbal comprehension [12]. However, most require the expertise of a trained psychologist for administration and/or interpretation, thereby limiting their general application.
In contrast, the Peabody Picture Vocabulary Test (third edition; PPVT-III) is an individually administered, untimed, norm-referenced, wide-range test, which assesses receptive oral vocabulary. It provides one total standard score (mean 100, s.d. 15), and can be used as an estimate of general verbal ability in persons aged 2.5 years to 90 + years [13]. It has two parallel forms for repeat testing. It usually takes 10–15 min to administer. The individual must select one picture from among four to match a word orally presented by the examiner, and only one answer per item is correct. For example, when the examiner presents a stimulus word like “scissors,” the individual selects the illustration depicting “scissors” from among four pictures on a test plate. The respondent can acknowledge their choice by the number (1–4) associated with the frame, or by pointing to the picture. Consequently, subjective judgment is not required and examiner training requirements are minimal. The mean split-half reliability coefficient has been reported to be 0.94 [13]. The PPVT-III has been shown to be strongly correlated with the Oral and Written Language Scales (r = 0.66 to 0.83) and with indices from the Weschler Intelligence Scale for Children—Third Edition (r = 0.82 to 0.91) for general population samples of children aged 7 years 11 months through 14 years 4 months [13].
We report on the PPVT-III as a screening tool in evaluating eligibility for a pilot intervention study among a childhood brain tumor (BT) survivor population. Our principal goal with this test was to determine if the child had an adequate level of cognitive capacity to understand and complete self-report measures of HRQL and follow instructions in other study assessments.
Patients and methods
Study overview
Childhood BT patients who were >1 year from end of cancer therapy that included >23.4 Gy cranial RT were invited for pre-screening into an intervention trial using a pharmacologic agent hypothesized to improve cognitive function. Following parent consent and child assent, patients were assessed in a quiet room in the pediatric oncology clinic by the principal investigator of the study or a study coordinator. All participants were required to have their vision and hearing aids in use as needed. Eligibility requirements precluded any subject who was already receiving a cognitive enhancing agent or a psychoactive agent. The study was approved by the Institutional Review board of Wake Forest University Health Sciences. The parent consent for the PPVT-III pre-screening was the first of a two-part consent, anticipating that some subjects would not go on to receive the intervention.
Examiner training included familiarity with the test manual and practice sessions prior to the initiation of the study. Training involved establishing patient rapport and comprehension of instructions, understanding of selecting the appropriate start item (based on chronologic age of the subject), establishing the basal item set and ceiling item set for an individual, and calculating standard scores.
Conduct of test
The PPVT-III picture easel was set up between the child and the examiner, and practice questions were initiated in a quiet room in the clinic per the publisher’s guidelines. Picture plates were presented in accordance with test instructions to attain basal and ceiling thresholds. Raw scores were converted to age-referenced standard scores [13].
Participants who proceeded to the trial then underwent a complete cognitive assessment battery including the WASI. This was usually carried out at a separate appointment, within 2 weeks following PPVT-III pre-screening. The WASI was the first in a series of tests carried out by a licensed psychologist in the child neuropsychology clinic, thereby providing a second tier for establishing intellectual eligibility.
Baseline neurocognitive characteristics were scored and normalized to age appropriate references according to individual test manufacturer’s guidelines. Descriptive statistics were used to summarize patient demographic and clinical characteristics, and PPVT-III and WASI scores. Pearson correlation was carried out for PPVT-III with full scale IQ, verbal IQ, and performance IQ. A Wilcoxon signed rank test was used to compare differences in the medians of the PPVT-III and WASI scores. All statistical tests were two-sided and performed in SAS (v. 9.1, SAS Institute, Cary, NC).
Results
Thirteen BT patients with median age 11.8 years (range 9.3–17.9 years) were screened with the PPVT-III, with detailed patient and treatment characteristics in Table 1. The presenting site of disease was the posterior fossa in 10 patients (77%). Treatment involved combined modality therapy for most; only three study participants received surgery and radiation without chemotherapy (n = 3).
Table 1.
Baseline characteristics of childhood brain tumor (BT) survivors (n = 13) screened for intervention
| Patient characteristics | |
|---|---|
| Gender | |
| Male N, (%) | 7 (54.8%) |
| Race | |
| White | 11 |
| Non-white | 2 |
| Age at BT diagnosis (median; range) | 4.2 (0.6–12.9) |
| Age at screening (median; range) | 11.8 years (9.3–17.9) |
| Disease/treatment characteristic | |
| BT histology | |
| Medulloblastoma | 9 |
| Craniopharyngioma | 1 |
| Pilocytic astrocytoma | 1 |
| Ependymoma | 1 |
| Pineoblastoma | 1 |
| Cranial radiation dose (median; range) | 55.8 Gray (50.4–60) |
| Age at cranial radiation (median; range) | 5.5 years (2.8–13.2) |
| Time since radiation (median; range) | 4.9 years (1.9–15.2) |
| VP shunt present | 3 |
| Chemotherapy treatment | |
| Y | 10 |
| N | 3 |
| Lansky/Karnofsky performance status | 90 (70–100) |
BT brain tumor, VP ventriculoperitoneal
Although the exact testing times were not captured, the test was administered with ease within 20 min for all subjects. One subject who attained a PPVT-III score of 72 did not proceed to full study testing due to a medical ineligibility for the pharmacologic intervention. The mean of the PPVT-III scores among 12 subjects who were screened and proceeded to the next phase of the study was 92.8 (SD 16.9; range 61–117).
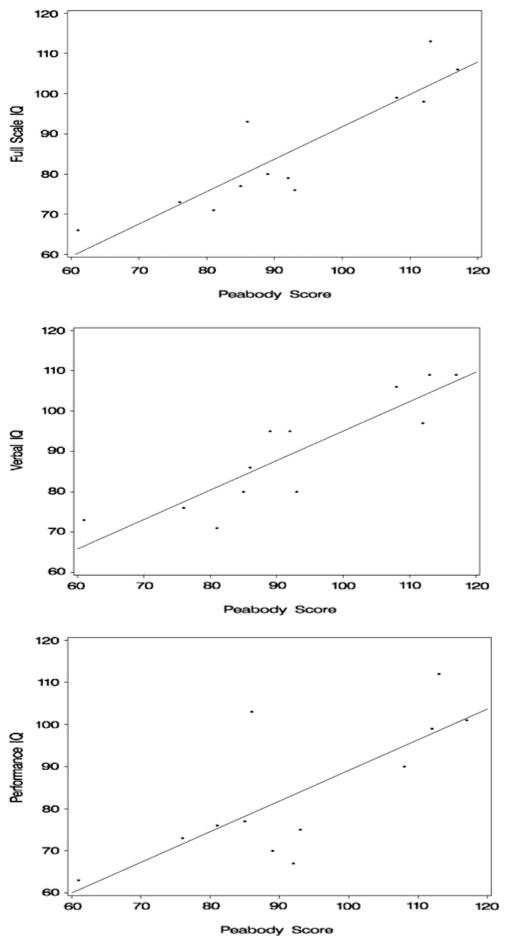
Among these 12 patients who proceeded to full study testing the mean full scale IQ was 85.9 (s.d. 15.2) on the WASI. The median time to completion of the WASI with a trained psychologist was 42 min (range 40–52 min). Sub-scale scores were a mean verbal IQ of 89.7 (s.d. 13.9) and performance IQ of 83.8 (s.d. 16.3). The PPVT-III slightly overestimated IQ scores with a median difference of 3 points higher than the verbal IQ (P = 0.23), 10.5 points higher than the performance IQ (P = 0.02), and 9 points higher than the full scale IQ (P = 0.01) in this BT population. Correlation of PPVT-III with WASI was 0.90 for full scale IQ (P < 0.0001), 0.89 for verbal IQ (P = 0.0001) and 0.75 for performance IQ (P = 0.0004) (Fig. 1).
Fig. 1.
The correlation between the PPVT-III and verbal (r = 0.89; P = 0.0001) performance (r = 0.75; P = 0.0004) and full scale (r = 0.90; P < 0.0001) intelligence quotient (WASI) in a childhood BT population is robust. Regression equations: Full Scale IQ = 11.2 + 0.81 × PPVT-III ± 7.1 (R2 = 0.80).Verbal IQ = 21.9 + 0.73 × PPVT-III ± 6.8 (R2 = 0.79); Performance IQ = 16.3 + 0.73 × PPVT-III ± 11.3 (R2 = 0.57)
Discussion
We demonstrate that the PPVT-III is a reliable screening tool for the estimation of adequate cognitive capacity in BT survivors, particularly in the verbal domain, given the strong correlation with verbal and full-scale IQ. Its application in research studies of childhood BT survivors who received intensive therapy including cranial radiation is warranted for two reasons. First, altered cognitive functioning has direct bearing on the interpretation of HRQL or other outcomes, which rely on self-report [14]. In addition, the validity of neuropsychological assessments, even those that do not require a verbal response, relies heavily on subjects’ ability to understand and follow instructions. To our knowledge this is the first application of this instrument as a study screening device in a childhood BT survivor cohort.
The PPVT-III is a measure of receptive vocabulary with excellent psychometric properties in the general population, including strong reliability and substantial correlations with more lengthy intelligence tests. We demonstrate it is also appealing as an initial tool in research on pediatric BT survivors, due to ease and convenience of administration by trained clinic staff, obviating the requirement for a neuropsychologist at the screening stage. Our findings of a higher correlation of the PPVT-III with verbal and full scale IQ, is consistent with the performance of this instrument in the general population where the original measure (PPVT-R) demonstrated higher correlations with verbal ability and full scale IQ on the Wechsler IQ evaluation (0.68–0.74) than with performance IQ (0.50–0.64).
The use of cognitive skills for detailed neuropsychology testing and PROs as study outcomes in our trial underscores the need for a minimal level of verbal comprehension and flexibility of language in the enrolled subjects. The proclivity of the PPVT-III to overestimate IQ in this population speaks to the balance of sensitivity versus specificity in a screening tool for the study purposes. In this case, the potential error is more likely to screen in a participant who might not be eligible based on IQ by the Wechsler evaluation. However, the consequence of that error is less egregious than failure to capture self-reported HRQL from this population because their known risk of cognitive dysfunction. It is important to note that the difference between the Wechsler and PPVT-III score was not significant for the verbal subscale. This and the fact that the PPVT-III strongly correlated with verbal IQ, affirms its use as a screener which does not unduly restrict or preclude children from this population.
The PPVT (version R or III) has been applied to characterize language skills in several vulnerable populations wherein global and domain-specific neurocognitive deficits overlap with those of childhood cancer survivors. These include children with traumatic brain injury, autism, HIV or a history of prematurity.[15–18] The PPVT-R has also been used to evaluate global function in childhood cancer populations while on treatment, and to evaluate the trajectory of the domain of language function in a brain tumor cohort following treatment [19–21]. However, these studies did not examine the performance characteristics of the PPVT compared to the more extensive measures of IQ, and were limited in subject number, similar to our study.
In summary, the PPVT-III is a feasible screening tool and an indicator of minimal global intelligence in pediatric BT survivors off therapy, who have received cranial radiation at >23.4 Gy. Our findings should not be interpreted as an endorsement of this measure as a substitute for full neurocognitive evaluation in this at risk population. However, with the increasing call for use of PROs and long term cognitive studies in cancer studies [22], the PPVT-III may be an effective means to evaluate if a pediatric BT survivor can reliably and validly respond and participate when detailed neuropsychological testing and self-reported PROs are the outcomes. This instrument should be considered for inclusion as a clinic-based screening method for future research studies in this population, and fully validated in a larger population of childhood brain tumor survivors to confirm our findings.
Acknowledgments
We thank the patients and families who participated in this pilot study. We also thank Dr. Kevin McMullen for facilitating patient accrual and Dr. Edward Shaw for senior mentor-ship. All authors read and approved the final version of the article. Funding for this work was provided by the Lance Armstrong Foundation (Young Investigator Award to S.M.C.), and the National Institutes of Health (R25 CA122061 to S.M.C; P30CA12197J.A.T.).
Footnotes
Conflict of Interest None declared.
Contributor Information
Sharon M. Castellino, Email: scastell@wfubmc.edu, Department of Pediatrics, Wake Forest University Health Sciences, Winston-Salem, NC, USA. Department of Pediatrics, Section Hematology/Oncology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA
Janet A. Tooze, Department of Public Health Sciences, Wake Forest University Health Sciences, Winston-Salem, NC, USA
Lynn Flowers, Department of Neuropsychology, Wake Forest University Health Sciences, Winston-Salem, NC, USA.
Susan K. Parsons, Department of Medicine, Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Tufts University School of Medicine, Boston, MA, USA. Department of Pediatrics, Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Tufts University School of Medicine, Boston, MA, USA
References
- 1.Duffner PK. Long-term effects of radiation therapy on cognitive and endocrine function in children with leukemia and brain tumors. Neurologist. 2004;10:293–310. doi: 10.1097/01.nrl.0000144287.35993.96. [DOI] [PubMed] [Google Scholar]
- 2.Butler RW, Mulhern RK. Neurocognitive interventions for children and adolescents surviving cancer. J Pediatr Psychol. 2005;30:65–78. doi: 10.1093/jpepsy/jsi017. [DOI] [PubMed] [Google Scholar]
- 3.Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 4.Robinson KE, Kuttesch JF, Champion JE, et al. A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatr Blood Cancer. 2010;55:525–531. doi: 10.1002/pbc.22568. [DOI] [PubMed] [Google Scholar]
- 5.Butler RW, Sahler OJ, Askins MA, et al. Interventions to improve neuropsychological functioning in childhood cancer survivors. Dev Disabil Res Rev. 2008;14:251–258. doi: 10.1002/ddrr.33. [DOI] [PubMed] [Google Scholar]
- 6.Nathan PC, Patel SK, Dilley K, et al. Guidelines for identification of, advocacy for, and intervention in neurocognitive problems in survivors of childhood cancer: a report from the Children’s Oncology Group. Arch Pediatr Adolesc Med. 2007;161:798–806. doi: 10.1001/archpedi.161.8.798. [DOI] [PubMed] [Google Scholar]
- 7.Lageman SK, Cerhan JH, Locke DE, Anderson SK, Wu W, Brown PD. Comparing neuropsychological tasks to optimize brief cognitive batteries for brain tumor clinical trials. J Neurooncol. 2010;96:271–276. doi: 10.1007/s11060-009-9960-y. [DOI] [PubMed] [Google Scholar]
- 8.Krull KR, Okcu MF, Potter B, et al. Screening for neuro-cognitive impairment in pediatric cancer long-term survivors. J Clin Oncol. 2008;26:4138–4143. doi: 10.1200/JCO.2008.16.8864. [DOI] [PubMed] [Google Scholar]
- 9.Weschler D. Weschler intelligence scale for children. 4. The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- 10.Roid GH. Stanford Binet intelligence scales. 5. Riverside Publishing; Itaska, IL: 2003. [Google Scholar]
- 11.Wechsler D. Wechsler abbreviated scale of intelligence (WASI(TM)) Harcourt Assessment Inc; San Antonio, TX: 1999. [Google Scholar]
- 12.Garvie PA, Tao ML, Schum LN, Rey-Casserly CM, Mulhern RK, Parsons SK. Use of a verbal language screening tool prior to pediatric quality of life (QOL) questionnaire administration. Neuro Oncol. 2004;6:445. [Google Scholar]
- 13.Dunn L, Dunn L, Williams K, Wang J. Peabody picture vocabulary test III. American Guidance Service Inc; Circle Pines, MN: 1997. [Google Scholar]
- 14.Tao ML, Parsons SK. Editorial: quality of life assessment in pediatric brain tumor patients and survivors: lessons learned and challenges to face. J Clin Oncol. 2005;23:5424–5426. doi: 10.1200/JCO.2005.05.906. [DOI] [PubMed] [Google Scholar]
- 15.Condouris K, Meyer E, Tager-Flusberg H. The relationship between standardized measures of language and measures of spontaneous speech in children with autism. Am J Speech Lang Pathol Am Speech Lang Hear Assoc. 2003;12:349–358. doi: 10.1044/1058-0360(2003/080). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donders J, Warschausky S. Neurobehavioral outcomes after early versus late childhood traumatic brain injury. J Head Trauma Rehabil. 2007;22:296–302. doi: 10.1097/01.HTR.0000290974.01872.82. [DOI] [PubMed] [Google Scholar]
- 17.Myers EH, Hampson M, Vohr B, et al. Functional connectivity to a right hemisphere language center in prematurely born adolescents. NeuroImage. 2010;51:1445–1452. doi: 10.1016/j.neuroimage.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brackis-Cott E, Kang E, Dolezal C, Abrams EJ, Mellins CA. The impact of perinatal HIV infection on older school-aged children’s and adolescents’ receptive language and word recognition skills. AIDS Patient Care STDs. 2009;23:415–421. doi: 10.1089/apc.2008.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacLean WE, Jr, Noll RB, Stehbens JA, et al. Neuropsychological effects of cranial irradiation in young children with acute lymphoblastic leukemia 9 months after diagnosis. The Children’s Cancer Group. Arch Neurol. 1995;52:156–160. doi: 10.1001/archneur.1995.00540260060017. [DOI] [PubMed] [Google Scholar]
- 20.Crisp J, Ungerer JA, Goodnow JJ. The impact of experience on children’s understanding of illness. J Pediatr Psychol. 1996;21:57–72. doi: 10.1093/jpepsy/21.1.57. [DOI] [PubMed] [Google Scholar]
- 21.Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22:706–713. doi: 10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]
- 22.Sloan JA, Berk L, Roscoe J, et al. Integrating patient-reported outcomes into cancer symptom management clinical trials supported by the National Cancer Institute-sponsored clinical trials networks. J Clin Oncol. 2007;25:5070–5077. doi: 10.1200/JCO.2007.12.7670. [DOI] [PubMed] [Google Scholar]