Abstract
MicroRNAs (miRNAs) have prognostic and therapeutic value for colorectal cancers (CRCs). Although formalin-fixed paraffin-embedded (FFPE) tissues are available for biomarker studies, the stability of miRNAs in these tissues stored for long periods (>20 years) is unknown. The present effort involved analysis of 345 FFPE CRC tissues, stored for 6 to 28 years (1982-2004), for the expression of six miRNAs (miR-20a, miR-21, miR-106a, miR-181b, miR-203, and miR-324-5p) using TaqMan® microRNA assays and quantitative real-time PCR (qRT-PCR). Evaluation, by linear regression analysis, of miRNA expression among archived CRC tissues found similar levels of all six miRNAs in tissues stored over this period (correlation coefficients, R2, ranged from <0.0001-0.009; and t-test p-values were ≥ 0.05). Thus, miRNAs are stable in FFPE tissues stored for long periods of time, and such samples can be used for discovery of biomarkers.
Keywords: miRNA, Colorectal Cancer, FFPE, Stability, Biomarkers
INTRODUCTION
MicroRNAs (miRNAs) are a family of small (20 to 24-nucleotide) non-coding RNAs that regulate gene expression at the post-transcriptional level (1, 2). Abnormal expression of miRNAs is involved in the development of various human malignancies (1, 2), thus several recent studies are utilizing miRNAs to understand biology and pathology of cancers. Also, miRNA expression profiles have potential prognostic and therapeutic values for evaluating malignancies, including colorectal cancers (CRCs) (3). Schetter et al. demonstrated that miR-20a, miR-21, miR-106a, miR-181b, and miR-203 were upregulated and had the highest differential expression between colon adenocarcinomas and their matching normal tissues; whereas, miR-324-5p was down-regulated in CRCs compared to their corresponding normals (3). Furthermore, this study has established the prognostic value of miR-21 in CRCs.
For several molecular studies fresh frozen samples are ideal; however, they are not readily available. Furthermore, it is difficult to archive a large set of fresh frozen samples with a long period of follow-up to perform survival or predictive analyses for assessing the clinical value of miRNAs in a retrospective setting. Therefore, studies related to biomarker discovery rely on formalin-fixed paraffin-embedded (FFPE) tissues. Although FFPE tissues are commonly available for analyses, the stability of miRNAs from these tissues stored for long periods (>20 years) is not known. As reported for RNA, (4) use of archival tissues to assess the clinical utility of miRNAs may be problematic due to questions about the quality of miRNA extracted from FFPE tissues. Thus, the goal of the present study was to determine whether these differentially expressed miRNAs are stable in FFPE CRC tissues stored over long periods of time.
MATERIALS AND METHODS
Patient Tissue Collection
This investigation was performed with the approval of the Institutional Review Board and Bioethics Committee of the University of Alabama at Birmingham (UAB). We obtained 345 FFPE tumor and their corresponding normal (benign colonic epithelial) tissues of CRCs that were stored for 6 to 28 years (1982 through 2004) from the Anatomic Pathology Division of UAB.
RNA Isolation and Quality Control
Two 10-μm thick sections were cut from the tumor and corresponding normal tissue blocks of each case. Tissue sections were first de-paraffinized in 4M guanidine isothiocyanate (GITC) solution (Invitrogen, CA) at 92 °C for 30 min, then total RNA was extracted with TRIZOL™ reagent (Invitrogen, CA). In each case, the integrity of the total RNA quality was measured using a NanoDrop 2000 spectrophotometer (Fisher Scientific, PA).
miRNA Real-time PCR
Quantitative RT-PCR of miRNAs was performed using TaqMan® MicroRNA Assays (Applied Biosystems, CA) by two-step RT-PCR. Briefly, cDNA synthesis was first carried out using the TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems, CA). The template consisted of 20 ng of total RNA and microRNA-specific primers. This PCR reaction was carried out in a 15 μL reaction mixture and was performed on the iQ™5 Real-Time PCR Detection System (Bio-Rad, CA) for 30 min at 16 °C, 30 min at 42 °C, and 5 min at 85 °C.
Next, the target cDNA product was amplified using sequence-specific primers supplied with the miRNA-specific TaqMan® MicroRNA Assay kits. This PCR reaction was performed in a 20 μL reaction mixture (1.33 μL template cDNA) and run for 10 min at 95°C, 15 sec at 95°C, 1 min at 60°C for 40 cycles. The fluorescent signal was collected at the endpoint of every cycle. TaqMan miRNA assays are highly sensitive and specific to detect and quantify mature miRNAs. RNU6B (an endogenous reference miRNA) was used for normalizing miRNA expression. The expression of miRNAs was calculated using cycle threshold (Ct) values, then Δ Ct (dCt = CtmiRNA - CtRNU6B), and ΔΔ Ct (ddCt = dCttumor - dCtnormal) values were calculated for each miRNA. All experiments were performed in triplicate.
Statistical Analysis
Pearson’s linear correlation analysis was used to determine any significant association between miRNA expression and tissue storage time. To determine the linear relationship between ddCt and length of storage, the equation y=β0+β1x+ε was used, where y denotes the ddCt of the miRNA, x denotes the year in which the sample was collected, β0 denotes the intercept, β1 denotes the slope, and ε denotes the error term. P-values ≤ 0.05 were considered to be statistically significant. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
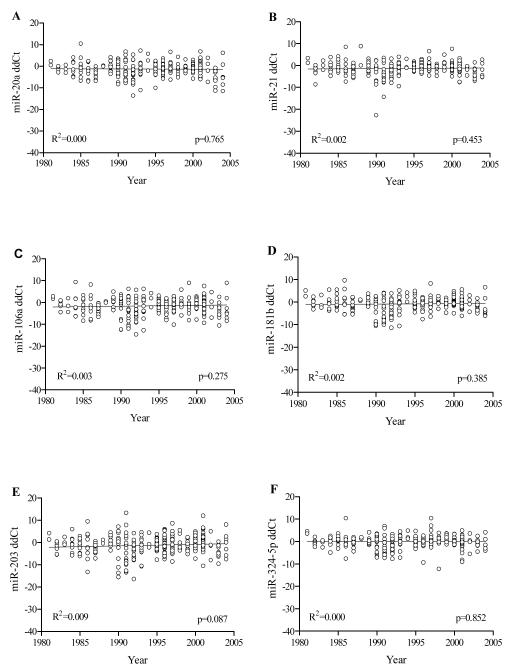
The expression levels of miRNAs, measured in FFPE archival tissues were plotted against the acquisition year of the tissue block (Figure 1). Regression analysis was conducted where; normalized ΔΔ Ct values of detected miRNAs of all cases are shown on the ‘y-axis’ and the year of diagnosis is shown on the ‘x-axis’. Regression analyses showed that there was no correlation between the levels of miRNAs and the acquisition year (i.e., there was no statistically significant difference in the expression levels for each miRNA in FFPE tissues over several years of storage). For our panel of six miRNAs (miR-20a, miR-21, miR-106a, miR-181b, miR-203, and miR-324-5p), the correlation coefficient values (R2) were <0.0001, 0.002, 0.003, 0.002, 0.009, and <0.0001, respectively and all p-values (t-test) were greater than 0.05 (Figure 1A-1F). Hence, our results demonstrate that miRNA expression levels and stability are not significantly altered in FFPE tissues of CRCs stored for 6 to 28 years.
Figure 1. miRNA Expression in FFPE samples of CRCs.
Expression of miRNAs based on ddCt values derived by qRT-PCR analysis of FFPE CRC tissue sections in paraffin blocks stored for 6-28 years (1982-2004). A) miR-20a, B) miR-21, C) miR-106a, D) miR-181b, E) miR-203, and F) miR-324-5p. Linear regression analyses demonstrate that expression of the six miRNAs is not significantly different in tissues stored over this period.
DISCUSSION
Our evaluation of miRNA expression in archived CRC tissues found similar levels of all six examined miRNAs in tissues stored for over 28 years. An earlier evaluation by Xi et al. (5) involved analysis of the stability of miR-181b in 40 FFPE CRC samples, and suggested that the expression of miR-181b was stable over a 10 year period. This report, as well as ours, indicates that miRNAs are stable in FFPE tissues stored for long periods of time. Another study by Siebolts et al. (6) evaluated expression of miR-16 and miR-122a in only three CRC (among 88 different types of malignancies) of frozen and their corresponding FFPE tissues and found no significant differences in the expression levels of these miRNAs. Additionally, there are studies reporting similar yields and stability of miRNAs from matched frozen and FFPE samples (4, 5). However, the study by Siebolts et al. suggested that specifically the miRNA-16 levels in FFPEs samples of lymph nodes (n=40) stored up to 27 years were decreased nearly by 50%. In contrast, the present investigation is the first to establish the stability of six differentially expressed and potentially clinically relevant miRNAs (miR-20a, miR-21, miR-106a, miR-181b, miR-203, and miR-324-5p) measured by qRT-PCR in a large series of FFPE CRC specimens (n=345) stored for more than 28 years.
Although RNAs in fresh or frozen tissues are relatively stable, there are substantial logistical problems involved in the acquisition of these samples. Conversely, FFPE tissues are a readily available source of samples for retrospective studies to demonstrate correlations between molecular markers and clinical outcomes (7). Therefore, it would be advantageous to use such tissues for investigations on the clinical value of miRNAs. The lack of sufficient data on the quality of the RNA derived from FFPE tissues due to fixation-related issues, however, is impeding preclinical translational studies of miRNA in cancer. In the fixation process, RNA can form cross-links with protein, and longer RNA molecules are more likely to have cross-links than smaller molecules, such as miRNAs (4); therefore, miRNAs may not be affected by the fixation. As observed in our study, similar expression levels of the miRNAs in FFPE tissues stored over long periods also support this premise. Currently, we are correlating the miRNA expression profiles with other molecular markers of CRCs and with tumor progression and clinical outcomes. In summary, the findings of the present investigation suggest that miRNAs are stable in FFPE CRC tissues stored for long periods and provide a rationale for using FFPE tissues in investigations involving miRNA profiling.
ACKNOWLEDGMENT
We thank Donald L. Hill, Ph.D., Division of Preventive Medicine, University of Alabama at Birmingham, for his critical review of this manuscript. This work was supported by the NCI/NIH grants, P50 CA089019, 2U54-CA118948, and R03-CA139629.
References
- 1.Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, Iuliano R, Palumbo T, Pichiorri F, Roldo C, Garzon R, Sevignani C, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 2.Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev. 2005;15:563–8. doi: 10.1016/j.gde.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Schetter AJ, Leung SY, Sohn JJ, Zanetti KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK, Liu CG, Calin GA, Croce CM, Harris CC. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. Jama. 2008;299:425–36. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Smyth P, Flavin R, Cahill S, Denning K, Aherne S, Guenther SM, O’Leary JJ, Sheils O. Comparison of miRNA expression patterns using total RNA extracted from matched samples of formalin-fixed paraffin-embedded (FFPE) cells and snap frozen cells. BMC Biotechnol. 2007;7:36. doi: 10.1186/1472-6750-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xi Y, Nakajima G, Gavin E, Morris CG, Kudo K, Hayashi K, Ju J. Systematic analysis of microRNA expression of RNA extracted from fresh frozen and formalin-fixed paraffin-embedded samples. RNA. 2007;13:1668–74. doi: 10.1261/rna.642907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siebolts U, Varnholt H, Drebber U, Dienes HP, Wickenhauser C, Odenthal M. Tissues from routine pathology archives are suitable for microRNA analyses by quantitative PCR. J Clin Pathol. 2009;62:84–8. doi: 10.1136/jcp.2008.058339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrahamsen HN, Steiniche T, Nexo E, Hamilton-Dutoit SJ, Sorensen BS. Towards quantitative mRNA analysis in paraffin-embedded tissues using real-time reverse transcriptase-polymerase chain reaction: a methodological study on lymph nodes from melanoma patients. J Mol Diagn. 2003;5:34–41. doi: 10.1016/S1525-1578(10)60449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]