Abstract
Wnt/β-catenin signaling is critically involved in metazoan development, stem cell maintenance and human disease. Using Xenopus laevis egg extract to screen for compounds that both stabilize Axin and promote β-catenin turnover, we identified an FDA-approved drug, pyrvinium, as a potent inhibitor of Wnt signaling (EC50 of ~10 nM). We show pyrvinium binds all casein kinase 1 (CK1) family members in vitro at low nanomolar concentrations and pyrvinium selectively potentiates casein kinase 1α (CK1α) kinase activity. CK1α knockdown abrogates the effects of pyrvinium on the Wnt pathway. In addition to its effects on Axin and β-catenin levels, pyrvinium promotes degradation of Pygopus, a Wnt transcriptional component. Pyrvinium treatment of colon cancer cells with mutation of the gene for adenomatous polyposis coli (APC) or β-catenin inhibits both Wnt signaling and proliferation. Our findings reveal allosteric activation of CK1α as an effective mechanism to inhibit Wnt signaling and highlight a new strategy for targeted therapeutics directed against the Wnt pathway.
Control of β-catenin levels is a critical event of Wnt signaling. In the absence of Wnt ligand, cytoplasmic β-catenin is maintained at low levels by its constitutive degradation. β-catenin degradation occurs primarily via its association with a complex consisting of glycogen synthase kinase 3 (GSK3), casein kinase 1α (CK1α), adenomatous polyposis coli (APC) and Axin. Within this destruction complex, β-catenin is phosphorylated by GSK3 and targeted for degradation by the ubiquitin-proteasome pathway. Upon binding of Wnt ligand to Frizzled and low-density lipoprotein-related receptors 5 and 6 (LRP5 and LRP6), β-catenin destruction is inhibited and the scaffold protein Axin is degraded1–4. Thus, Wnt signaling increases cytoplasmic levels of β-catenin, which enters the nucleus and interacts with other factors to activate a TCF/LEF1–mediated transcriptional program5.
Demonstration that loss of APC (the cause of familial adenomatous polyposis, a hereditary cancer syndrome) results in β-catenin accumulation was the first indication that constitutive activation of the Wnt pathway could lead to epithelial cell transformation6. Over 80% of sporadic colon cancers are associated with mutation of APC and 10% with mutation of β-catenin, both of which lead to Wnt pathway activation7.
Xenopus laevis egg extracts have been shown to faithfully recapitulate many events of the Wnt pathway with in vivo reaction kinetics8. In the current report, we describe a new, high-throughput screen for chemical modulators of Wnt signaling using Xenopus egg extract. We identified pyrvinium pamoate (1), an FDA-approved drug, as a potent inhibitor of the Wnt pathway (Fig. 1a and Supplementary Fig. 1). We provide evidence that pyrvinium may represent a new class of compounds that inhibit the Wnt pathway by allosteric activation of CK1α.
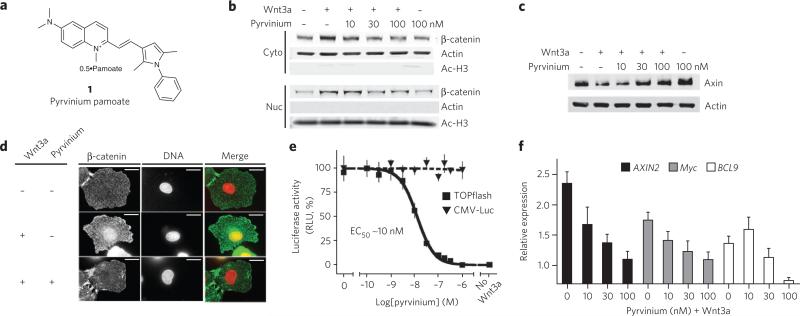
Figure 1. Xenopus egg extract screen identifies pyrvinium as an inhibitor of Wnt signaling.
(a) Chemical structure of pyrvinium pamoate. (b) Pyrvinium decreases cytoplasmic and nuclear β-catenin levels. HEK 293 cells were treated for 16 h as indicated, and fractionated lysates were immunoblotted for β-catenin. Purity of nuclear and cytoplasmic preparations was assessed by immunoblotting for acetylated histone H3 and actin, respectively. (c) Pyrvinium increases cellular Axin levels. Lysates from HEK 293 cells treated for 16 h as indicated were immunoblotted for Axin and actin (loading control). (d) Pyrvinium blocks Wnt-mediated nuclear accumulation of β-catenin. IEC-6 cells treated as indicted were stained for β-catenin and DNA. (e) Pyrvinium inhibits TOPflash activation with an EC50 of ~10 nM. HEK 293 STF (TOPflash) or constitutively expressing luciferase (CMV-Luc) reporter cells were treated as indicated. Graph represents mean ± s.e.m. of TOPflash signal normalized to cell number (performed in quadruplicate). RLU, relative light units. (f) Pyrvinium decreases levels of endogenous Wnt target transcripts. Data shown represent mean of four independent real-time PCR reactions, graphed as relative expression to unstimulated cells and normalized to β-actin. Error bars, RQ (relative quantification) values >95% confidence.
RESULTS
Xenopus extract screen for regulators of the Wnt pathway
Recently, we reconstituted Wnt signaling in Xenopus laevis egg extract using a soluble form of LRP6 (LRP6ICD)4. We adapted this system for high-throughput approaches and performed a small-molecule screen for modulators of Wnt signaling (Supplementary Fig. 2a and Supplementary Table 1). We assessed the status of Wnt activation by monitoring levels of β-catenin–firefly luciferase (FLuc) and Axin–Renilla reniformis luciferase (RLuc) fusion proteins. We reasoned that the reciprocal stability of β-catenin and Axin in response to LRP6-mediated signaling (increased and decreased, respectively) would be a powerful readout to identify specific modulators of the Wnt pathway. Compounds that interfere with energy metabolism should reduce both firefly and Renilla signals. Conversely, general inhibitors of protein degradation (for example, proteasome inhibitors) should enhance both luciferase signals.
Xenopus egg extract used in our screen is transcriptionally and translationally inactive. Thus, the observed effects on the Wnt/β-catenin pathway are due to post-translational events. We titrated LRP6ICD to half-maximal activity in the extract (Supplementary Fig. 2b). We used FDA-approved drug libraries from the National Institute of Neurological Disorders and Stroke custom collection and the Prestwick Chemical Library at the Harvard Medical School Institute for Chemistry and Cell Biology in our screen. From our primary screen, we identified ~20 candidate Wnt pathway activators and inhibitors that altered the β-catenin-FLuc/Axin-RLuc ratio at least three deviations from the mean (Supplementary Fig. 2c; see screen details in Supplementary Table 1).
Pyrvinium pamoate, an antihelminthic drug, inhibited LRP6-mediated Axin–RLuc degradation and β-catenin–FLuc stabilization with the greatest potency in our screen. Pyrvinium is a quinoline-derived cyanine dye previously used in the treatment of pinworm infection (Enterobius vermicularis) that is also efficacious toward animal-like protists such as Plasmodium falciparum and Cryptosporidium parvum (Fig. 1a)9,10. Despite its medicinal use for over 50 years, pyrvinium's mode of action remains unknown. We performed kinetic experiments and found that pyrvinium reversed the effects of LRP6ICD on the turnover rates of β-catenin (increased by pyrvinium) and Axin (decreased by pyrvinium) (Supplementary Fig. 3a–c). Uncropped images for all gels can be found in Supplementary Results.
We also found that pyrvinium modestly promoted the basal rate of β-catenin degradation in the absence of LRP6ICD (Supplementary Fig. 3d). We previously demonstrated using Xenopus egg extract that LRP6ICD inhibits the phosphorylation of β-catenin by GSK3, which promotes its ubiquitin-mediated degradation4. Consistent with these data, we found that pyrvinium reversed the effects of LRP6ICD on β-catenin phosphorylation (Supplementary Fig. 3e).
To assess whether pyrvinium regulates β-catenin and Axin levels in other systems, we tested its effects on cultured mammalian cells. Stimulation of human embryonic kidney (HEK) 293 cells with Wnt3a resulted in elevation of cytoplasmic and nuclear β-catenin levels and reduction of Axin levels. Pyrvinium reversed the effects of Wnt3a in a concentration-dependent manner such that both cytoplasmic and nuclear pools of β-catenin were decreased and Axin levels were increased to levels similar to that observed in the absence of Wnt3a (Fig. 1b,c). Furthermore, pyrvinium caused an increase in Axin levels in cells not treated with Wnt3a (Fig. 1c).
A cellular pool of β-catenin that binds E-cadherin at cell-cell junctions is essential for maintaining epithelial cell polarity and tissue architecture. We assessed whether pyrvinium affects this membrane-bound pool of β-catenin in a nontransformed rat small intestine epithelial cell line, IEC-6. Addition of Wnt3a to IEC-6 cells resulted in accumulation of nuclear β-catenin in approximately 50% of the cells observed (500 cells scored per condition). Treatment of IEC-6 cells with pyrvinium prevented nuclear accumulation of β-catenin (in all cells) with no obvious effect on membrane-bound pools of β-catenin (Fig. 1d).
Wnt signaling leads to changes in transcription of a large set of genes that drive proliferation, growth and cell fate determination11. To evaluate whether pyrvinium inhibits Wnt-mediated transcription, we used a luciferase-based reporter containing TCF/LEF1 binding sites (TOPflash) stably transfected in HEK 293 cells (HEK 293 STF)12. Pyrvinium inhibited Wnt3a-mediated luciferase activity in a dose-dependent manner with an effector concentration for half-maximal response (EC50) of ~10 nM (Fig. 1e). Pyrvinium had no effect on expression of luciferase driven by a constitutive CMV promoter (CMV-Luc) or FOPflash, a scrambled TCF/LEF1 promoter element (Fig. 1e and data not shown). Consistent with the effect of pyrvinium on the TOPflash reporter, activation of endogenous target genes AXIN2, Myc and BCL9 by Wnt3a was inhibited by pyrvinium in a dose-dependent manner to nearly basal (meaning, minus Wnt3a) levels (Fig. 1f)13–16. In contrast, pyrvinium had no discernible effect on the biochemical and/or transcriptional responses of four other major signaling pathways (TGFα, BMP4, IL-4 and Notch), thereby demonstrating specificity of pyrvinium for Wnt signaling (Supplementary Fig. 4a, b).
Chemical specificity was confirmed by experiments showing that the pamoate salt of another antihelminthic compound (pyrantel pamoate, 2) did not inhibit Wnt signaling, whereas the iodide salt of pyrvinium (3) inhibited Wnt signaling with an EC50 essentially identical to that of pyrvinium pamoate (Supplementary Fig. 4c–e and data not shown). As an additional control, a structurally related analog of pyrvinium (VU-WS211, 4) had no effect on TOPflash activity (Supplementary Fig. 4f, g).
The much lower concentration of pyrvinium needed to inhibit Wnt signaling in cultured mammalian cells compared to its effect on β-catenin and Axin turnover in Xenopus egg extracts was not unexpected (Supplementary Fig. 3a–c). These extracts contain high concentrations of proteins and lipids, which can sequester exogenously added compounds; hence, many well-characterized compounds have been shown to be ~1,000-fold less potent in Xenopus egg extract compared to cell-based assays17.
Pyrvinium inhibits Wnt signaling across phyla
To determine whether pyrvinium inhibits Wnt signaling in a developing organism, we investigated its effects on Xenopus laevis. Canonical Wnt/β-catenin signaling leads to accumulation of β-catenin on the dorsal side of the embryo that patterns dorsal-anterior structures18. Experimental activation of the Wnt pathway in ventral blastomeres (for example, via Xwnt8 mRNA injection) induces secondary organizer formation and formation of an ectopic axis (Fig. 2a and Supplementary Fig. 5a). Co-injection of pyrvinium inhibited Xwnt8-mediated secondary axis formation in a dose-dependent manner, whereas co-injection with vehicle control or an inactive analog had no effect (Fig. 2b,c and Supplementary Fig. 5a). Injection of pyrvinium into dorsal blastomeres at the four-cell stage resulted in ventralized embryos, indicating inhibition of endogenous Wnt signaling, whereas an inactive analog had no effect (Supplementary Fig. 5b).
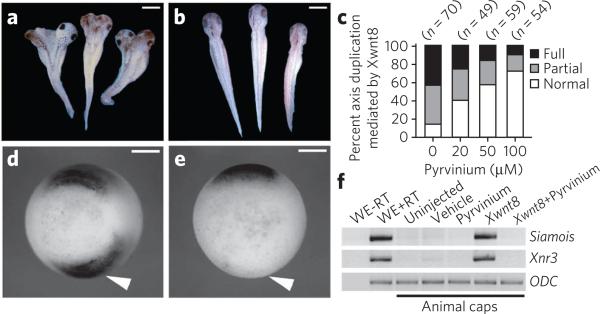
Figure 2. Pyrvinium inhibits Wnt signaling in vivo.
(a–c) Pyrvinium blocks secondary axis induction in Xenopus in a dose-dependent manner. Embryos (four- to eight-cell stage) were injected ventrally with Xwnt8 mRNA (0.5 pg) plus vehicle (a) or pyrvinium pamoate (200 μM) (b), allowed to develop and scored for secondary axis formation (c). n = number of embryos. (d, e) Pyrvinium blocks expression of chordin. Embryos injected with Xwnt8 plus vehicle (d) or pyrvinium pamoate (200 μM) (e) were probed by in situ hybridization (stage 10.5) for chordin (arrowheads). Vegetal view, dorsal side up. Scale bars, 800 μm for a, b and 400 μm for d, e. (f) Pyrvinium inhibits Xwnt8 induction of Wnt target genes Siamois and Xnr3 in Xenopus animal cap explants. RT-PCR of total RNA extracted from animal caps. WE, whole embryo; RT, reverse transcriptase; ODC, ornithine decarboxylase, loading control.
Organizer formation is regulated by canonical Wnt signaling, which directly induces expression of Xnr3, Siamois and other target genes19. Pyrvinium inhibited ectopic expression of chordin (which marks the organizer) in early gastrulae co-injected with Xwnt8 (Fig. 2d, e). Similarly, Xwnt8-induced expression of Siamois and Xnr3 in ectodermal explants was inhibited by co-injection of pyrvinium (Fig. 2f). In contrast, pyrvinium failed to inhibit induction of the myogenic transcription factor XMif5 or the pan-mesodermal marker Xbra in ectodermal explants upon injection of Xnr2 mRNA or addition of bFGF protein (Supplementary Fig. 5c).
We next investigated the effects of pyrvinium using two invertebrate model systems in which Wnt signaling has been extensively characterized. Pyrvinium inhibited Wingless (Wg)-mediated gene transcription in Drosophila melanogaster S2 cells with an EC50 (~38 nM) comparable to that of HEK 293 cells (Supplementary Fig. 5d)20. Furthermore, pyrvinium blocked the increase in Armadillo (β-catenin homolog) levels induced by Wg ligand (Supplementary Fig. 5e).
During Caenorhabditis elegans development, vulval differentiation and Q neuroblast migration depend on Wnt signaling21,22. The QL cell expresses the hox gene MAB-5 in response to a posterior Wnt signal, which is required for its posterior migration. In contrast, the QR cell, which is less sensitive to Wnt, migrates anteri-orly21. Using a weak allele of pop-1 (a TCF homolog) as a sensitized background, pyrvinium treatment resulted in a dose-dependent disruption of posterior QL cell migration (Supplementary Fig. 5f)23. Previous studies have shown that the cuticle and hypodermis of C. elegans is often impermeable to pharmacological agents24. Thus, the high doses of pyrvinium we used to observe an effect may reflect its poor absorption. Alternatively, it may reflect differences in the sequences of the vertebrate and C. elegans genes encoding the cellular target of pyrvinium. Pyrvinium treatment also produced vulval and egg-laying defects, phenotypes consistent with decreased Wnt signaling (Supplementary Fig. 5g–i). The range of phenotypes observed included protruding vulva and vulvaless. No vulval or egg-laying (Egl, data not shown) phenotypes were observed with VU-WS211 treatment. The phenotypic effects of pyrvinium in Xenopus, Drosophila and C. elegans are consistent with inhibition of Wnt-mediated developmental processes. Taken together, our studies suggest that pyrvinium acts as a Wnt pathway inhibitor in vivo and that its target is conserved across metazoan phyla.
CK1α is the target of pyrvinium
We have shown that pyrvinium promotes β-catenin and inhibits Axin turnover (Fig. 1b,c and Supplementary Fig. 3a–d). The simplest model is that pyrvinium regulates the activity of a single target that acts on both proteins. We assembled a complex consisting of purified GSK3, CK1, Axin and β-catenin and tested the effect of pyrvinium on β-catenin phosphorylation, a prerequisite for its degradation. Addition of pyrvinium at low nanomolar concentrations to this purified system resulted in a striking increase in β-catenin phosphorylation, including sites specific for GSK3 and CK1α (Fig. 3a).
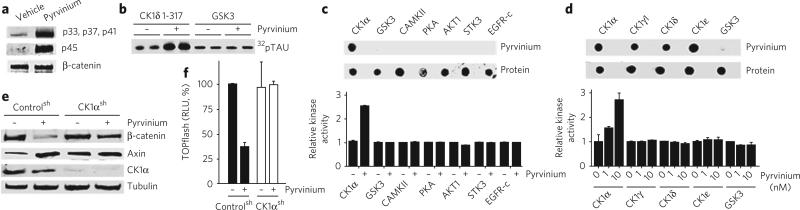
Figure 3. CK1α is the critical target of pyrvinium.
(a) Pyrvinium stimulates β-catenin phosphorylation in vitro. A kinase reaction was assembled in vitro with purified β-catenin, Axin, GSK3 and a constitutively active, truncated form of CK1δ (CK1δ1–317) (100 nM each) plus or minus pyrvinium (10 nM). Phosphorylation of β-catenin on GSK3 sites (p33, p37, p41) and the priming CK1α site (p45) was detected by immunoblotting. (b) Pyrvinium stimulates CK1 activity in vitro. CK1δ1–317 (100 nM) was incubated with recombinant tau (100 nM) plus or minus pyrvinium pamoate (10 nM) in a kinase reaction containing [γ32P]ATP and underwent SDS-PAGE separation and autoradiography. (c, d) Pyrvinium pamoate (10 nM) was incubated with purified recombinant kinases, and binding and kinase activities were assessed. Equivalent amounts (0.5 μg) were spotted for each protein. (c) Pyrvinium binds and activates CK1α but not kinases representative of other major branches of the kinome. (d) Pyrvinium binds all full-length CK1 isoforms tested but only activates CK1α. Graphs for c, d show mean ± s.e.m., performed in triplicate. (e, f) Downregulating CK1α blocks the biochemical and transcriptional responses to pyrvinium. A Jurkat cell line expressing inducible shRNA for CK1α (CK1αsh) was incubated with pyrvinium pamoate (30 nM) for 24 h. Lysates were immunoblotted for β-catenin, Axin and tubulin (loading control) (e) or assayed for TOPflash to assess Wnt signaling (f). For the TOPflash assays, cells were treated with Wnt3a. Graph shows mean ± s.e.m., normalized to cell number and performed in triplicate.
We next tested the four proteins included in our in vitro β-catenin phosphorylation assay for binding to pyrvinium. Using a ligand-binding assay based on the innate fluorescent property of pyrvinium, we observed pyrvinium binding only for CK1 (Supplementary Fig. 6a, b). We next tested whether pyrvinium binding affects CK1 activity toward its substrates (β-catenin, Axin and tau)25–27. Pyrvinium enhanced phosphorylation of all CK1 substrates tested but had no observable effect on GSK3 activity (Fig. 3b and Supplementary Fig. 6c–e). Based on our estimated EC50 for Axin phosphorylation by CK1 (<1 nM), pyrvinium is about tenfold more potent in promoting the activity of purified CK1 compared to its inhibition of Wnt signaling (EC50 of ~10 nM) in cultured cells (Fig. 1e and Supplementary Fig. 6e). A difference in potency between the effects of small molecules in assays using purified components versus cell-based assays is characteristic of small-molecule kinase inhibitors28.
CK1 represents a branch of the family of serine and theronine protein kinases. To demonstrate specificity for CK1, we tested the capacity of pyrvinium to bind and activate representative recombinant, full-length kinases from major branches of the kinase super-family. We observed pyrvinium binding and activation of CK1α alone (Fig. 3c). Previous studies implicated all of the mammalian CK1 isoforms (α, γ1–3, δ and ε) in Wnt signal transduction29. We found that pyrvinium binds all of the CK1 isoforms tested but only activates CK1α (Fig. 3d). This result was surprising because our in vitro kinase assays used a truncated form of CKIδ1–317, lacking the C-terminal regulatory domain (Supplementary Fig. 6f). Compared to other isoforms, CK1α has a minimal C-terminal domain. These findings suggest that the C-terminal regulatory domain of CK1 kinases may interfere with their activation by pyrvinium.
If pyrvinium activates CK1α by inducing a conformational change, we hypothesized that this might be reflected in a change in the proteolytic digestion pattern of CK1α in the presence of pyrvinium. Incubation of purified recombinant CK1α with pyrvinium yielded a tryptic pattern distinct from CK1α alone, suggesting that pyrvinium alters the conformation of CK1α (Supplementary Fig. 6g). In contrast, pyrvinium had no obvious effect on the tryptic pattern of GSK3.
To provide evidence that pyrvinium activates CK1α in vivo, we immunoprecipitated CK1α from cells treated with or without pyrvinium and assessed kinase activity. CK1α isolated from cells treated with pyrvinium had enhanced kinase activity (Supplementary Fig. 7a). To demonstrate interaction between pyrvinium and CK1α within cells, we treated HEK 293 cells expressing HA-tagged CK1α with pyrvinium, immunoprecipitated HA-CK1α and performed mass spectrometry. Significant amounts of pyrvinium were coimmunoprecipitated with HA-CK1α relative to an HA-tagged GSK3 control (Supplementary Fig. 7b).
If Wnt pathway inhibition by pyrvinium were mediated by CK1α activation, a structural analog of pyrvinium that has retained the capacity to inhibit Wnt signaling (VU-WS113, 5) should also activate CK1α. Indeed, we found this to be the case (Supplementary Fig. 7c, d,f). In contrast, a pyrvinium derivative that does not inhibit Wnt signaling failed to activate CK1α (Supplementary Figs. 4f, g and 7e,f).
If pyrvinium inhibits Wnt signaling via CK1α activation, we reasoned that loss of CK1α should abrogate the effects of pyrvinium on Wnt signaling. Using a cell line with significantly reduced CK1α levels owing to inducible expression of short hairpin RNA (shRNA) against CK1α (CK1αsh), we found that, in contrast to the levels in control cells, β-catenin and Axin levels were unaltered by pyrvinium treatment (Fig. 3e)30. Furthermore, pyrvinium failed to inhibit TOPflash activity in CK1αsh cells (Fig. 3f). These results indicate that the effects of pyrvinium on β-catenin and Axin levels and on Wnt signaling are mediated by its activation of CK1α.
Pyrvinium inhibits Wnt signaling downstream of β-catenin
Axin acts as a molecular scaffold to facilitate formation of the β-catenin destruction complex. Our biochemical studies suggest that pyrvinium-mediated inhibition of Wnt signaling could be due to increased levels of the β-catenin destruction complex secondary to Axin stabilization. This type of mechanism has been proposed as the basis for inhibition of Wnt signaling by recently reported compounds31,32. To determine whether pyrvinium acts at the level of the β-catenin destruction complex, we downregulated its components (Axin1 and Axin2 or APC) or treated cells with lithium (GSK3 inhibitor), all of which activate Wnt signaling. Surprisingly, these perturbations were all inhibited by pyrvinium (Fig. 4a). Overexpression of CK1α was similarly sufficient to inhibit lithium activation of Wnt signaling, consistent with inhibition of Wnt signaling downstream of the β-catenin destruction complex by CK1α that is stimulated by pyrvinium (Fig. 4b).
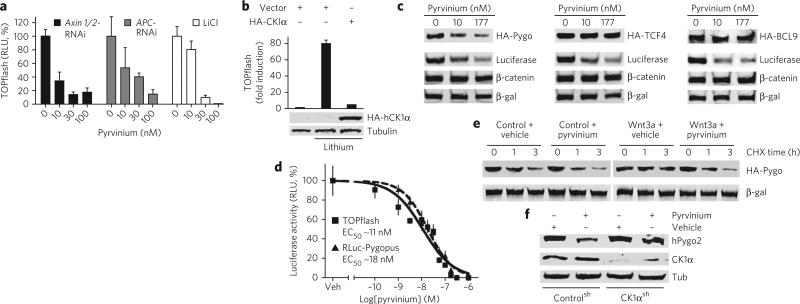
Figure 4. Pyrvinium promotes Pygopus degradation.
(a) Pyrvinium inhibits ligand-independent Wnt activation. HEK 293 STF cells were treated with Axin1- and Axin2-siRNA, APC-siRNA or LiCL (50 mM) and then with pyrvinium pamoate. (b) Overexpression of human CK1α inhibits activation of the Wnt pathway by lithium. HEK 293 STF cells were transfected as indicated and treated with pyrvinium pamoate (100 nM) and lithium (50 mM). Lysates were analyzed by TOPflash assay and immunoblotting for HA. Tubulin, control. Graphs for a, b show mean ± s.e.m. of luciferase signal normalized by cell number (performed in quadruplicate). (c) Pyrvinium promotes turnover of Pygopus. HEK 293 STF cells expressing indicated HA fusions were treated with pyrvinium pamoate. LiCL (50 mM) was added to activate Wnt signaling and inhibit β-catenin degradation. Lysates were immunoblotted for HA, luciferase, β-catenin. β-galactosidase, loading control. (d) Pyrvinium inhibits Wnt signaling and promotes Pygopus turnover to a similar degree. HEK 293 STF cells expressing Renilla-luciferase-Pygopus (RLuc-Pygopus) and β-galactosidase were treated with pyrvinium pamoate. Mean ± s.e.m. of Renilla luciferase signal normalized to β-galactosidase is shown (performed in quadruplicate). Veh, vehicle. (e) Pyrvinium reverses Wnt-mediated inhibition of Pygopus degradation. HEK 293 STF cells expressing HA-Pygopus were treated as indicated, and CHX was added at time = 0. Lysates were prepared as indicated and immunoblotted for HA. β-galactosidase, loading control. (f) Downregulating CK1α blocks pyrvinium-stimulated Pygopus turnover. A Jurkat cell line expressing CK1αsh was incubated with pyrvinium pamoate (30 nM), and lysates were immunoblotted for Pygopus. Tubulin, loading control.
Because our data indicated that pyrvinium could bypass the β-catenin destruction complex, we tested the effect of pyrvinium on SW480 cells, which have two mutant copies of the APC gene. In addition to decreasing β-catenin levels and increasing Axin levels, pyrvinium also inhibited Wnt signaling (Supplementary Figs. 8a, 9 and 10a). Mutations in the GSK3 phosphorylation sites of β-catenin that inhibit its degradation have been found in colon cancers33,34. Pyrvinium had no effect on cytoplasmic β-catenin levels in the colon cancer line HCT-116 WTKO (expresses a nondegradable mutant allele of β-catenin and a knocked-out wild-type allele), yet it potently inhibited Wnt signaling (Supplementary Figs. 8a and 10a). In contrast, treatment with the Axin stabilizer IWR-1 (6) failed to inhibit Wnt signaling in HEK 293 STF cells activated by lithium or in HCT-116 WTKO cells (Supplementary Fig. 8b–f)32.
One possible explanation for inhibition of Wnt signaling by pyrvinium in the context of elevated β-catenin levels is that pyrvinium could block nuclear accumulation of β-catenin. This possibility is ruled out, however, by our finding that pyrvinium had no effect on nuclear entry of β-catenin in lithium-treated cells (Supplementary Fig. 8g, h). Taken together, our results suggest that, in addition to its effects on Axin and β-catenin stability, pyrvinium acts downstream and/or at the level of β-catenin.
Pyrvinium promotes Pygopus degradation
We assessed the effects of pyrvinium on levels of several nuclear factors required for β-catenin–mediated transcription: BCL9, Pygopus and TCF4 (ref. 11). HEK 293 STF cells were treated with lithium (to inhibit β-catenin degradation and activate Wnt signaling) and pyrvinium. In response to pyrvinium, a significant decrease in Pygopus levels was detected that paralleled the decrease in luciferase expressed from the TOPflash reporter; no change in abundance or gel migration, however, was detected for TCF4 or BCL9 (Fig. 4c). Furthermore, pyrvinium treatment caused loss of nuclear GFP-Pygopus signal in HeLa cells and reduced levels of overexpressed and endogenous Pygopus in colon cancer cell lines (Supplementary Fig. 10b,c). This correlation between Wnt signaling and Pygopus levels is supported by our finding that the EC50 values for Wnt inhibition and stimulation of Pygopus degradation by pyrvinium are nearly identical (Fig. 4d).
We found that Wnt signaling inhibits Pygopus degradation (half-life of ~1.5 h in the absence of Wnt and >5 h in the presence of Wnt) and that pyrvinium reverses the effect of Wnt in a post-translational manner (half-life of ~1 h; Fig. 4e). In the absence of Wnt, pyrvinium had only a modest effect on the rate of Pygopus turnover (half-life of ~1 h). Pyrvinium may affect Pygopus degradation via a direct mechanism because CK1α coimmunoprecipitates with Pygopus (Supplementary Fig. 10d). Furthermore, Myc-tagged Pygopus immunoprecipitated from cultured cells can be phosphorylated in a CK1α-dependent manner in an in vitro kinase assay; this effect is enhanced by addition of pyrvinium (Supplementary Fig. 10e). Finally, we show that shRNA against CK1α blocks the effect of pyrvinium on Pygopus levels, confirming that pyrvinium regulates Pygopus stability via CK1α (Fig. 4f).
To determine whether Pygopus is the downstream-most target of pyrvinium in the Wnt pathway, we tested whether pyrvinium could inhibit activation of Wnt signaling by LEFΔN-VP16. The fusion protein LEFΔN-VP16 has been shown to act independently of β-catenin to activate TCF/LEF1–responsive genes35. We found that activation of transcription by LEFΔN-VP16 was sensitive to pyrvinium and CK1α (~50% reduction), indicating that pyrvinium also acts at the level of TCF/LEF1, albeit weakly (Supplementary Fig. 11).
Pyrvinium decreases viability of colon cancer cells
Given the potent effect of pyrvinium on Wnt signaling in a variety of contexts, we next tested its effects on Wnt-mediated cellular transformation and proliferation. Wnt3a induced rounding of HEK 293 cells and formation of colonies in which cells grew on top of each other as well as loss of ZO-1, a tight junction marker; these effects were reversed by pyrvinium treatment (Supplementary Fig. 12a, b). Pyrvinium caused substantial reduction in Ki-67 staining (a cellular marker for proliferation) on HCT-116 cells (Supplementary Fig. 12c). Pyrvinium had no obvious effect, however, on the cell-cycle phasing of SW480 cells as assessed by DNA content (Supplementary Fig. 12d).
At concentrations of pyrvinium that cause significant inhibition of Wnt signaling, we observe no obvious signs of cytotoxicity. Rather, rates of cellular growth and proliferation were substantially reduced (Supplementary Fig. 13). Consistent with lack of cytotoxicity, we found that pyrvinium poorly induced caspase 3/7 activity in SW620 cells, a highly metastatic colon cancer cell line with mutant APC that is resistant to apoptosis (Supplementary Fig. 14). We found, however, that a combination of pyrvinium and 5-fluorouracil (5-FU), a chemotherapeutic agent, synergized to induce a high degree of apoptosis in this cell line.
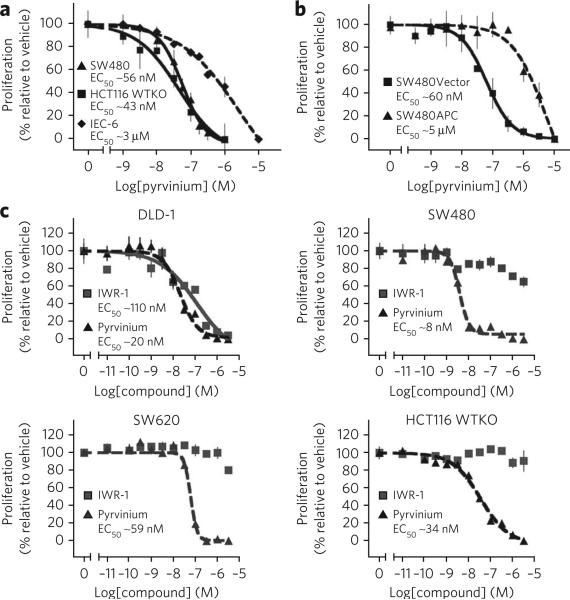
Because pyrvinium inhibits Wnt signaling in the colon cancer lines SW480 and HCT-116 WTKO, we next tested whether pyrvinium affects the viability of these cells. We observed decreased cell viability at pyrvinium concentrations that cause Wnt pathway inhibition (Figs. 1e and 5a). Pyrvinium had relatively less effect on the viability of IEC-6 cells, a nontransformed epithelial cell line (Fig. 5a). To further assess whether pyrvinium selectively decreases viability of cells with Wnt pathway mutations, we compared the sensitivity of the SW480 colon cancer line with truncated APC (SW480Vector) to a line in which wild-type, full-length APC has been introduced so as to restore normal Wnt signaling (SW480APC)36. We found that SW480APC cells are ~80-fold less sensitive to pyrvinium than SW480Vector cells (Fig. 5b).
Figure 5. Pyrvinium selectively decreases cell viability of colon cancer cells with activating mutations in the Wnt pathway.
(a–c) Cell viability assays. Cell viability was determined following treatment with pyrvinium pamoate for 72 h. Mean ± s.e.m. is shown (assays performed in quadruplicate). (a) Colon cancer cell lines (SW480 and HCT116 WTKo) are more sensitive to pyrvinium than a nontransformed epithelial cell line (IEC-6). (b) SW480 cells expressing full-length APC (SW480APC) are more resistant to pyrvinium than SW480 cells transfected with empty vector (SW480vector). (c) Effects of pyrvinium versus IWR-1 on viability of colon cancer cells grown under low serum conditions. Colon cancer lines were treated for 72 h with the indicated concentrations of pyrvinium pamoate or IWR-1 in media with low serum (1% (v/v) FBS) and cell viability determined.
Recently, compounds that inhibit Wnt signaling via Axin stabilization were shown to inhibit viability and proliferation of DLD-1 cells, a colon cancer line mutant for APC31,32. These studies, however, used suboptimal growth conditions (0.5% (v/v) fetal bovine serum (FBS)) for cultured human cells. Under optimal growth conditions (10% (v/v) FBS), we found that pyrvinium potently decreased viability of several commonly studied colon cancer lines; in contrast, the Axin stabilizing compound IWR-1 had no effect (Supplementary Fig. 15). In agreement with previous reports, we detected an effect of IWR-1 on the viability of DLD-1 cells grown under suboptimal growth conditions (1% (v/v) FBS); in contrast to cells treated with pyrvinium, however, other colon cancer lines thought to be driven by Wnt signaling were resistant to IWR-1 under these conditions (Fig. 5c).
DISCUSSION
We propose a model in which pyrvinium activates CK1α so as to regulate the stability of β-catenin and Axin in the cytoplasm and Pygopus and TCF/LEF1 in the nucleus; inhibition of the Wnt pathway at multiple levels occurs as a result (Supplementary Fig. 1). We showed by coimmunoprecipitation that Pygopus exists in a complex with CK1α. Furthermore, Pygopus is predicted to contain multiple CK1 phosphorylation sites, suggesting that its stability could be directly regulated by CK1α. CK1 has previously been shown to phosphorylate LEF1 in vitro37. These phosphorylation sites on LEF1 are present in the LEFΔN-VP16 construct, suggesting that the effect of pyrvinium and CK1α on LEFΔN-VP16 activity may also be direct.
Pygopus was originally identified in Drosophila as a core transcriptional component of the Wnt pathway38–40. In mice, the role of Pygopus in Wnt signaling appears to be more complex in that it is required for a subset of Wnt-mediated developmental processes, suggesting that Pygopus may play a context-dependent role in Wnt signal transduction. Previous studies using colon cancer cell lines with mutations in the genes for β-catenin or APC demonstrated that knockdown of Pygopus is sufficient to inhibit Wnt signaling and decrease cell viability, suggesting that it plays a role in Wnt-driven cancers39. In the current study, we demonstrate that Wnt signaling inhibits Pygopus turnover, a previously uncharacterized mode of regulation.
The CK1 family of serine and threonine kinases is constitutively active, ubiquitously expressed and evolutionarily conserved in eukaryotes41. In addition to Wnt signaling, CK1 family members have been associated with many other cellular processes. CK1 isoforms (α, γ, δ and ε) have a highly conserved catalytic core and differ primarily in their N-terminal and C-terminal domains. CK1 is regulated by its subcellular localization and inhibitory autophosphorylation of its C-terminal domain.
Of the CK1 family members, CK1α is distinct in that it has a minimal C-terminal tail. This suggests that pyrvinium activates CK1α via an allosteric mechanism rather than via relief of autoinhibition. Further support for such a model comes from our observation that, although pyrvinium is incapable of stimulating the activity of full-length CK1δ in our in vitro assays, it promotes activation of a C-terminally truncated form of CK1δ (CK1δ1–317). Finally, we demonstrate that CK1α is differentially sensitive to the action of trypsin proteolysis in the presence or absence of pyrvinium.
Why would having a large C-terminal domain block the capacity of CK1 isoforms to be activated by pyrvinium? One possibility is that the C-terminal domain (which has been postulated to act as a pseudosubstrate) binds to the substrate-binding cavity of CK1 and blocks the activating conformational change induced by pyrvinium. Direct proof of this model will require elucidation of the structure of pyrvinium-bound CK1α in future studies.
Assigning specific biological activities to individual CK1 isoforms has been notoriously difficult (even with RNA interference knockdowns), possibly because of compensatory effects from the various isoforms. Overexpression studies, in contrast, may result in phosphorylation of nonphysiological substrates (for example, due to inappropriate access to substrates normally available only to other CK1 isoforms). Thus, pyrvinium may serve as a useful tool to dissect the biological roles of endogenous CK1α.
Previous studies have implicated pyrvinium in the AKT-mTOR pathway42,43. It is not clear whether these effects are direct, because Wnt signaling has also been shown to activate the mTOR pathway in vitro and in vivo44. In previous xenograft studies, oral doses of pyrvinium were given to mice at levels comparable to those given for its original indication (that is, treatment of humans with pinworms), suggesting bioactivity of pyrvinium at the site of the graft when administered at a safe dose42,45. In these studies, toxicity of the injected pyrvinium was a limiting factor42,45. It is not clear whether toxicity is because of the compound itself, which has an alkylating group, or because of its activation of CK1α, which has other cellular functions. Thus, future studies will be directed toward testing the bioactivity and toxicity of structurally distinct functional derivatives of pyrvinium.
In a previous study, pyrvinium was shown to significantly potentiate the capacity of doxorubicin, a conventional cytotoxic drug, to inhibit tumor growth45. This observation is consistent with our current study demonstrating that pyrvinium synergizes with 5-FU to induce apoptosis in a highly metastatic colorectal cancer line. Another study identified pyrvinium as an inhibitor of androgen receptor activity, and the authors speculated that its mechanism of action may be secondary to inhibition of the Wnt pathway46.
A new functional class of Wnt pathway inhibitors that stabilize Axin by inhibiting tankyrases (poly(ADP-ribose) polymerases) has recently been identified31,32. Given the roles of CK1α and tankyrases in regulating a range of cellular functions, an obvious future direction for experimentation would be to determine the selectivity for the Wnt pathway of CK1α activators and tankyrase inhibitors. It is possible that the Wnt pathway is particularly sensitive to the activities of these two enzymes; thus, modest cellular inhibition of tankyrases or activation of CK1α may be sufficient to inhibit Wnt signaling but not to affect other biological processes mediated by these two enzymes.
Although small-molecule activators of glucokinase have shown great promise for treating type 2 diabetes, the development of small-molecule activators of protein kinases for treating human disease has not been, to our knowledge, aggressively pursued47. It is generally believed that inhibition of tyrosine kinases must be substantial (>90%) and sustained to mediate clinically significant responses (for example, decrease tumor burden); it is unknown whether similar regimen (that is, substantial and sustained activation) will be required for kinase activators to be clinically efficacious. In the case of Wnt signaling, small molecules such as pyrvinium that inhibit the pathway may be useful for treating 90% of all sporadic cases of colon cancer, the second leading cause of cancer-related deaths in the developed world.
METHODS
Chemical compounds and recombinant proteins
Reagents used include pyrvinium pamoate (MP Biomedicals, 95% purity); interleukin-4 (Sigma I4269); EGFR-c (gift from M. Kirschner, Harvard Medical School); TGF-alpha (gift from R. Coffey, Vanderbilt University); BMP4 (Sigma B2680); cycloheximide (Sigma, C7698); CK1–7 (Sigma C0742); CK1 isoforms α, γ1, δ and ε (Invitrogen); GSK3 and CK1δ1–317 (New England Biolabs); CAMKII, PKA, AKT1 and STK3 (SignalChem). LRP6ICD was prepared as previously described with modifications4. Bacterial pellets were denatured with 6 M guanidine HCl, and histidine-tagged LRP6ICD was bound to nickel resin and eluted with imidazole. Eluate was dialyzed to remove guanidine HCl. Pyrvinium iodide, VU-WS211, VU-WS113 and IWR-1 were synthesized by the Vanderbilt Institute of Chemical Biology's medicinal chemistry core. DMSO was used as vehicle for pyrvinium in all experiments unless otherwise stated.
Screen for chemical modulators of Wnt signaling
Radiolabeled β-catenin and Axin were generated in rabbit reticulocyte lysates (Promega). Xenopus egg extract preparation and degradation assays were performed as previously described8. β-catenin–FLuc and Axin-RLuc were generated in TNT SP6 High-Yield Protein Expression System (Promega). In vitro–translated β-catenin–FLuc, Axin-RLuc and LRP6ICD (400 nM) were added to 100 ml of egg extract and rotated end over end at 4 °C for 10 min. Samples were dispensed into 384-well plates (5 μl per well) at 4 °C before pin transfer of compounds. Final concentrations of compounds ranged from ~40–200 μm in 2% (v/v) DMSO. Plates were sealed, vortexed and incubated for 4 h at 25 °C. After incubation, firefly and Renilla luciferase activities were measured using the Dual-Glo Luciferase Assay (Promega) on an EnVision plate reader (Perkin Elmer).
Reporter assays
For cell-based luciferase assays, HEK 293 STF, CMV-Luc and Drosophila S2 cells were seeded into 96-well plates at subconfluency, and luciferase activities were measured by Steady-Glo Luciferase Assay (Promega). Luciferase activities were normalized to viable cell number using the CellTiter-Glo Assay (Promega). TOPflash experiments in HCT116 WTKO and SW480 cells were normalized to cotransfected Renilla gene expression. All graphs were made in Prism 4 (GraphPad Software, Inc.) with nonlinear regression fit to a sigmoidal dose-response curve (variable slope). BRE, SRE and STAT6 reporter assays were performed as previously described48–50. For small interfering RNA (siRNA) silencing, HEK 293 STF cells were transfected with ON-TARGETplus siRNA (Thermo Scientific Dharmacon) against Axin1 (J-009625-06), Axin2 (J-008809-05) or APC (J-003869-09) for 48 h according to manufacturer's protocol. Cells were then treated with pyrvinium for 24 h before we performed luciferase and cell viability assays. For cycloheximide experiment, HEK 293 STF cells expressing HA-Pygopus were treated with Wnt3a and/or pyrvinium pamoate for 30 min before addition of cycloheximide (CHX; 50 μg ml–1). Silencing of CK1α in Jurkat cells has been previously described30. Briefly, CK1αsh or controlsh cells were treated for 48 h with doxycycline, then 24 h with Wnt3a-conditioned media in the presence or absence of pyrvinium pamoate. For TOPflash in Jurkat cells, cells were transfected with TOPflash reporter and Renilla transfection control 24 h after doxycycline treatment. Wnt3a and pyrvinium were added 24 h after transfection for an additional 24 h. For apoptosis assays, SW620 cells were seeded in 96-well plates at 5 × 103 cells per 100 μl well–1, and pyrvinium and/or 5-FU were added after attachment. After 24 h, phase contrast micrographs were obtained, and caspase 3/7 activity was measured by Apo-ONE Homogeneous Caspase-3/7 Assay (Promega) according to manufacturer's instructions.
Cell lines
The following cell lines were gifts: HEK 293 STF (J. Nathans, Johns Hopkins University), HCT116 WTKO (R. Coffey, Vanderbilt University), S2 TOPflash (R. Nusse, Stanford University) and Jurkat CK1αsh and controlsh cells (M. Lenardo, National Institute of Allergy and Infectious Diseases). HEK 293, IEC-6, SW480 and L Wnt3a and L cells were purchased from the American Type Culture Collection. Wg-secreting cells were purchased from the Drosophila Genomics Resource Center. A HEK 293 cell line stably expressing firefly luciferase under the control of the CMV promoter (CMV-Luc) was generated using standard methods. Mammalian cell lines were maintained in DMEM (except SW480 cells, which were maintained in RPMI), 10% (v/v) FBS and antibiotics. Drosophila S2 cells were maintained in Schneider's medium plus 10% (v/v) FBS. Cells were treated with compounds and/or Wnt for 24 h unless otherwise stated.
Dot blots
For ligand dot blot assay, purified protein (0.5 μg) was dotted on nitro-cellulose membranes and blocked for 1 h using 5% (w/v) milk in TBS. Pyrvinium was then added to the blocking solution and incubated for 3 h at 23 °C or overnight at 4 °C. After incubation with pyrvinium, the membrane was washed three times for 5 min in TBS plus 0.1% (v/v) Tween 20. The pyrvinium fluorescence image was acquired on a Xenogen IVIS 200 using excitation 500–550 nm and emission 575–650 nm spectrum fluorescence settings. Replicate dot blots were stained with Colloidal Gold (Bio-Rad) to visualize total protein bound to membrane.
Kinase assays
In vitro kinase assays were performed as previously described4. For CK1 isoforms and GSK3, casein (1 μM; Sigma C8032) was used as substrate. All other kinases were tested with previously characterized and commercially available peptide substrates (400 μM; Invitrogen, SignalChem, New England Biolabs) and diluted to a concentration at which activity was linear with respect to time and enzyme concentration.
Mass spectrometry
HEK 293 cells expressing HA-CK1α, HA-GSK3 or HA (control) were treated for 24 h with pyrvinium (100 nM). HA-tagged proteins were immunoprecipitated from cell lysates and subjected to LC-MS. The amount of pyrvinium within immunoprecipitates (based on relative abundance) was quantified. Nonspecific binding of pyrvinium to beads (HA control) was subtracted from measurements for HA-GSK3 and HA-CK1α samples. LC-MS was performed by the Vanderbilt University Mass Spectrometry Service Laboratory.
Supplementary Material
Acknowledgments
We thank members of the Lee laboratory, K. Gould, V. Siegel and R. Roberts-Galbraith for critical reading of the manuscript; J. Merkle for help with Drosophila S2 cell culture; members of the Institute of Chemistry and Cell Biology–Longwood for assistance with screening; M. Beinz, K. Basler, J. Nathans, R. Nusse, S. Hiebert, R. Coffey, B. Gumbiner and M. Lenardo for reagents. Nematode strains were obtained from the C. elegans Genetics Center, which is supported by the US National Institutes of Health National Center for Research Resources. This work was supported by American Cancer Society Research Scholar Grant RSG-05-126-01, American Cancer Society Institutional Research Grant IRG-58-009-46, National Cancer Institute Grant GI SPORE P50 CA95103, Mouse Models of Human Cancers Consortium (US National Institutes of Health–National Cancer Institute) 5U01 CA084239, US National Institutes of Health Grant 1 R01 GM081635-01 (E.L.); US National Institutes of Health Grant 1 R01 NS26115 (D.M.M.); American Heart Association Predoctoral Fellowship 0615279B, Molecular Endocrinology Training Grant 5 T 32 DK007563, Training Program in Developmental Biology 5 T32 HD007502 (National Institute of Child Health and Human Development) (C.A.T.); US National Institute of General Medical Studies Medical-Scientist Training Grant 5 T32 GM007347 (C.S.C. and A.J.H.); American Heart Association Predoctoral Fellowships 0615162B, US National Institutes of Health Cancer Biology Training Grant T32 CA09592 (K.K.J.). E.L. is a recipient of a Pew Scholarship in the Biomedical Sciences.
Footnotes
Author contributions
C.A.T. designed, performed and analyzed biochemical, extract and cell culture experiments. C.A.T. and E.T. performed screen under E.L. and A.S.'s guidance. J.S. designed and performed C. elegans experiments under D.M.M.'s guidance. E.T. and A.J.H. performed Xenopus embryo experiments. B.L. provided bioinformatics support. D.O., A.G.W., K.K., B.M., V.P.G. and G.A.S. designed and performed chemical synthesis. C.S.C., K.K.J., A.J.H., B.I.H. and L.A.L. provided essential reagents and discussions. K.C.M. provided technical assistance. C.A.T., E.L. and L.A.L. wrote the manuscript with advice from all authors. E.L. guided all aspects of study.
Additional information
Supplementary information and chemical compound information is available online at http://www.nature.com/naturechemicalbiology/. Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/. Correspondence and requests for materials should be addressed to E.L.
Competing financial interests
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/naturechemicalbiology/.
References
- 1.Yamamoto H, et al. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J. Biol. Chem. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- 2.Tolwinski NS, et al. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev. Cell. 2003;4:407–418. doi: 10.1016/s1534-5807(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 3.Kofron M, et al. Wnt11/beta-catenin signaling in both oocytes and early embryos acts through LRP6-mediated regulation of axin. Development. 2007;134:503–513. doi: 10.1242/dev.02739. [DOI] [PubMed] [Google Scholar]
- 4.Cselenyi CS, et al. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3's phosphorylation of beta-catenin. Proc. Natl. Acad. Sci. USA. 2008;105:8032–8037. doi: 10.1073/pnas.0803025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat. Rev. Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 6.Kinzler KW, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 7.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 8.Salic A, Lee E, Mayer L, Kirschner MW. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol. Cell. 2000;5:523–532. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- 9.Hempelmann E. Hemozoin biocrystallization in Plasmodium falciparum and the antimalarial activity of crystallization inhibitors. Parasitol. Res. 2007;100:671–676. doi: 10.1007/s00436-006-0313-x. [DOI] [PubMed] [Google Scholar]
- 10.Downey AS, Chong CR, Graczyk TK, Sullivan DJ. Efficacy of pyrvinium pamoate against Cryptosporidium parvum infection in vitro and in a neonatal mouse model. Antimicrob. Agents Chemother. 2008;52:3106–3112. doi: 10.1128/AAC.00207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Xu Q, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 13.Lustig B, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jho EH, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de la Roche M, Worm J, Bienz M. The function of BCL9 in Wnt/beta-catenin signaling and colorectal cancer cells. BMC Cancer. 2008;8:199. doi: 10.1186/1471-2407-8-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He TC, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto DT, Perlman ZE, Burbank KS, Groen AC, Mitchison TJ. The kinesin Eg5 drives poleward microtubule flux in Xenopus laevis egg extract spindles. J. Cell Biol. 2004;167:813–818. doi: 10.1083/jcb.200407126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larabell CA, et al. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway. J. Cell Biol. 1997;136:1123–1136. doi: 10.1083/jcb.136.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhanot P, et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 21.Whangbo J, Kenyon C. A Wnt signaling system that specifies two patterns of cell migration in C. elegans. Mol. Cell. 1999;4:851–858. doi: 10.1016/s1097-2765(00)80394-9. [DOI] [PubMed] [Google Scholar]
- 22.Gleason JE, Korswagen HC, Eisenmann DM. Activation of Wnt signaling bypasses the requirement for RTK/Ras signaling during C. elegans vulval induction. Genes Dev. 2002;16:1281–1290. doi: 10.1101/gad.981602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korswagen HC, et al. The Axin-like protein PRY-1 is a negative regulator of a canonical Wnt pathway in C. elegans. Genes Dev. 2002;16:1291–1302. doi: 10.1101/gad.981802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis JA, Wu CH, Berg H, Levine JH. The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics. 1980;95:905–928. doi: 10.1093/genetics/95.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 26.Gao ZH, Seeling JM, Hill V, Yochum A, Virshup DM. Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex. Proc. Natl. Acad. Sci. USA. 2002;99:1182–1187. doi: 10.1073/pnas.032468199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierre M, Nunez J. Multisite phosphorylation of tau proteins from rat brain. Biochem. Biophys. Res. Commun. 1983;115:212–219. doi: 10.1016/0006-291x(83)90991-9. [DOI] [PubMed] [Google Scholar]
- 28.Knight ZA, Shokat KM. Features of selective kinase inhibitors. Chem. Biol. 2005;12:621–637. doi: 10.1016/j.chembiol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Price MA. CKI, there's more than one: casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev. 2006;20:399–410. doi: 10.1101/gad.1394306. [DOI] [PubMed] [Google Scholar]
- 30.Bidère N, et al. Casein kinase 1alpha governs antigen-receptor-induced NF-kappaB activation and human lymphoma cell survival. Nature. 2009;458:92–96. doi: 10.1038/nature07613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang SM, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 32.Chen B, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–1134. [PubMed] [Google Scholar]
- 34.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 35.Aoki M, Hecht A, Kruse U, Kemler R, Vogt PK. Nuclear endpoint of Wnt signaling: neoplastic transformation induced by transactivating lymphoid-enhancing factor 1. Proc. Natl. Acad. Sci. USA. 1999;96:139–144. doi: 10.1073/pnas.96.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faux MC, et al. Restoration of full-length adenomatous polyposis coli (APC) protein in a colon cancer cell line enhances cell adhesion. J. Cell Sci. 2004;117:427–439. doi: 10.1242/jcs.00862. [DOI] [PubMed] [Google Scholar]
- 37.Hämmerlein A, Weiske J, Huber O. A second protein kinase CK1-mediated step negatively regulates Wnt signalling by disrupting the lymphocyte enhancer factor-1/beta-catenin complex. Cell. Mol. Life Sci. 2005;62:606–618. doi: 10.1007/s00018-005-4507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kramps T, et al. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell. 2002;109:47–60. doi: 10.1016/s0092-8674(02)00679-7. [DOI] [PubMed] [Google Scholar]
- 39.Thompson B, Townsley F, Rosin-Arbesfeld R, Musisi H, Bienz M. A new nuclear component of the Wnt signalling pathway. Nat. Cell Biol. 2002;4:367–373. doi: 10.1038/ncb786. [DOI] [PubMed] [Google Scholar]
- 40.Parker DS, Jemison J, Cadigan KM. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development. 2002;129:2565–2576. doi: 10.1242/dev.129.11.2565. [DOI] [PubMed] [Google Scholar]
- 41.Knippschild U, et al. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell. Signal. 2005;17:675–689. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Esumi H, Lu J, Kurashima Y, Hanaoka T. Antitumor activity of pyrvinium pamoate, 6-(dimethylamino)-2-[2-(2,5-dimethyl-1-phenyl-1H-pyrrol-3-yl) ethenyl]-1-me thyl-quinolinium pamoate salt, showing preferential cytotoxicity during glucose starvation. Cancer Sci. 2004;95:685–690. doi: 10.1111/j.1349-7006.2004.tb03330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenbluth JM, Mays DJ, Pino MF, Tang LJ, Pietenpol JA. A gene signature-based approach identifies mTOR as a regulator of p73. Mol. Cell. Biol. 2008;28:5951–5964. doi: 10.1128/MCB.00305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inoki K, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 45.Yu DH, et al. Pyrvinium targets the unfolded protein response to hypoglycemia and its anti-tumor activity is enhanced by combination therapy. PLoS ONE. 2008;3:e3951. doi: 10.1371/journal.pone.0003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones JO, et al. Non-competitive androgen receptor inhibition in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2009;106:7233–7238. doi: 10.1073/pnas.0807282106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat. Rev. Drug Discov. 2009;8:399–416. doi: 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- 48.Mithani SK, et al. Smad3 has a critical role in TGF-beta-mediated growth inhibition and apoptosis in colonic epithelial cells. J. Surg. Res. 2004;117:296–305. doi: 10.1016/S0022-4804(03)00335-4. [DOI] [PubMed] [Google Scholar]
- 49.Gille H, et al. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 1995;14:951–962. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goenka S, Boothby M. Selective potentiation of Stat-dependent gene expression by collaborator of Stat6 (CoaSt6), a transcriptional cofactor. Proc. Natl. Acad. Sci. USA. 2006;103:4210–4215. doi: 10.1073/pnas.0506981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.